Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review
Abstract
1. Introduction
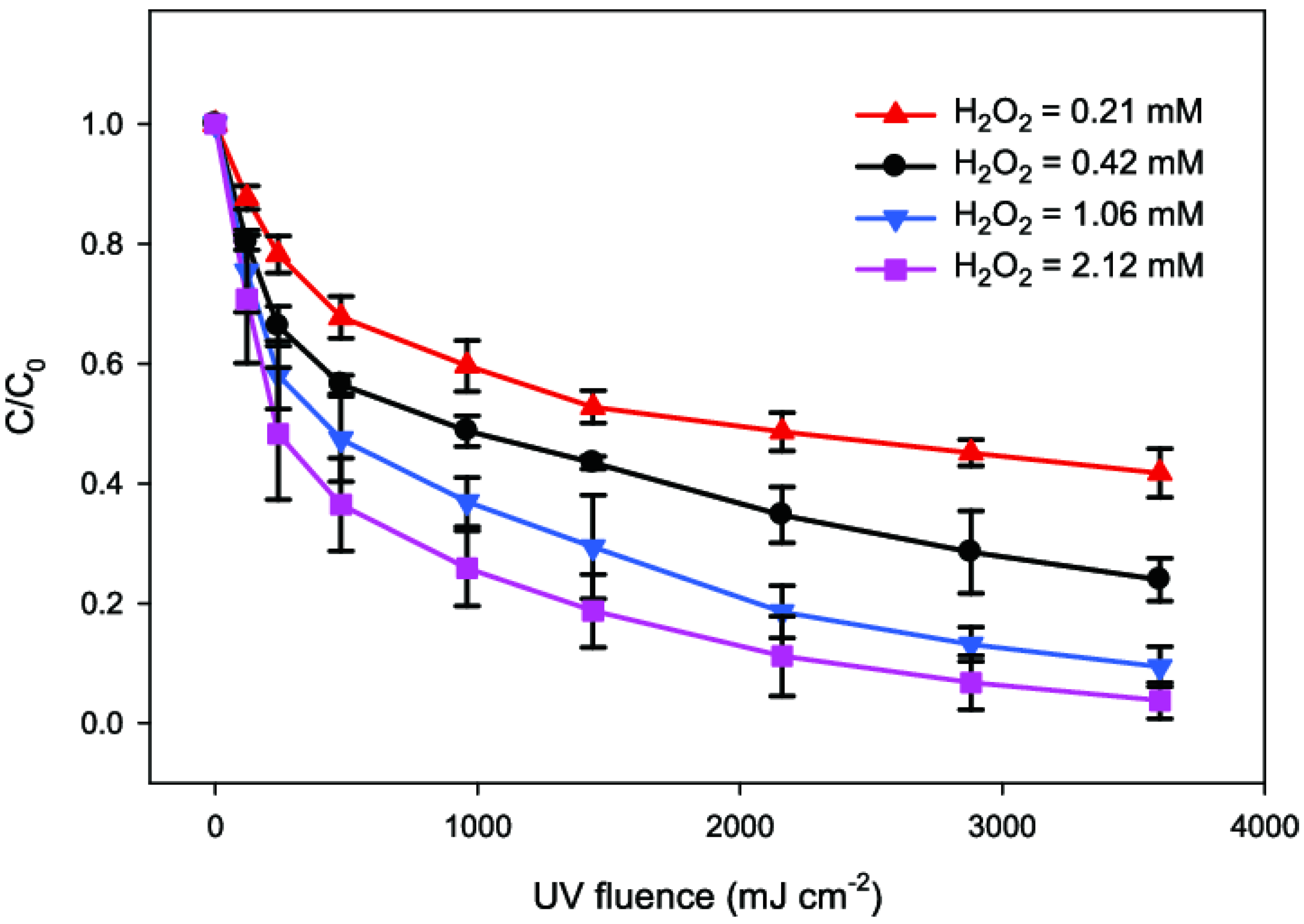
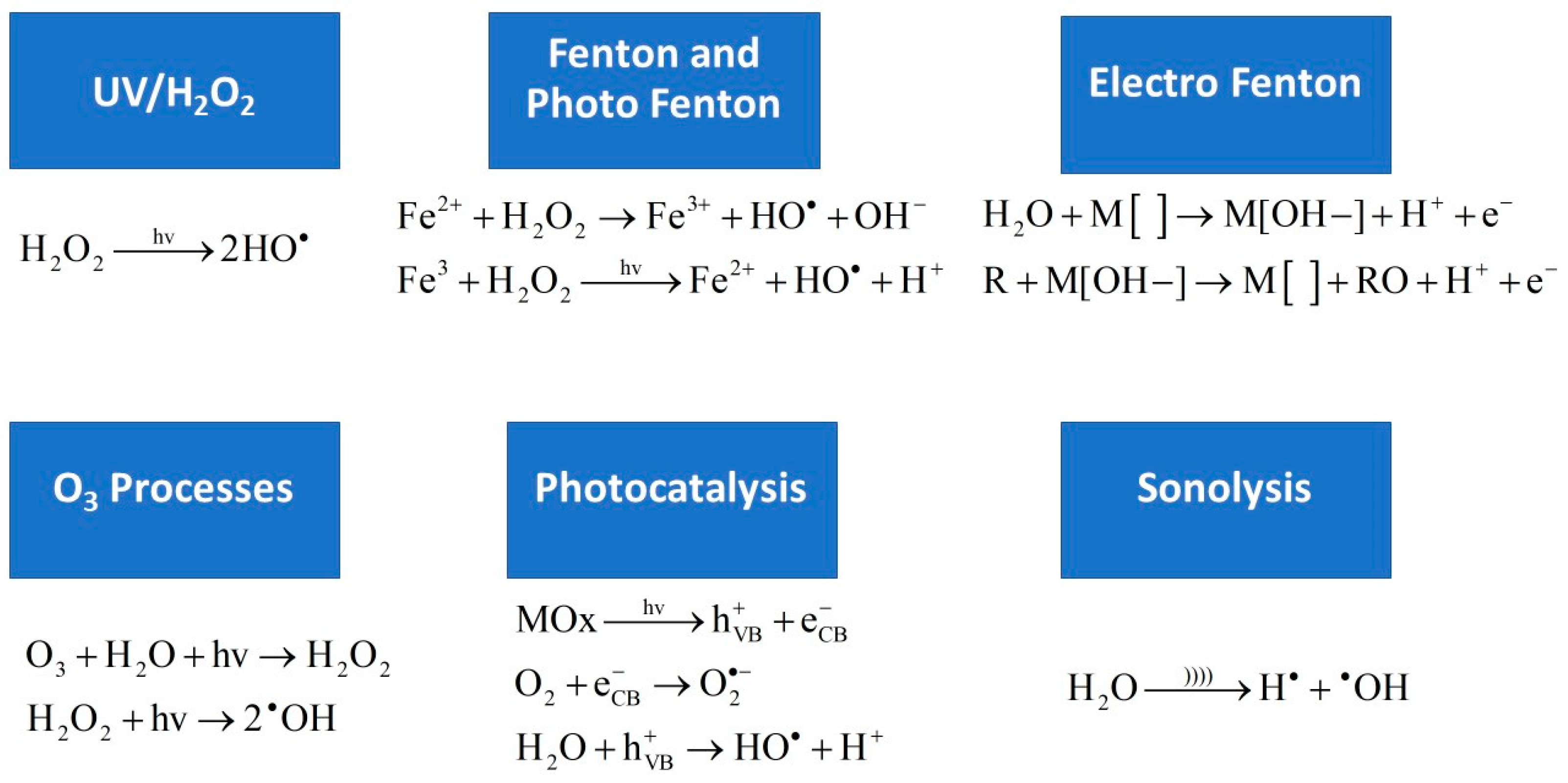
2. UV/H2O2 (Ultraviolet/Hydrogen Peroxide Processes)
3. Fenton and Photo-Fenton
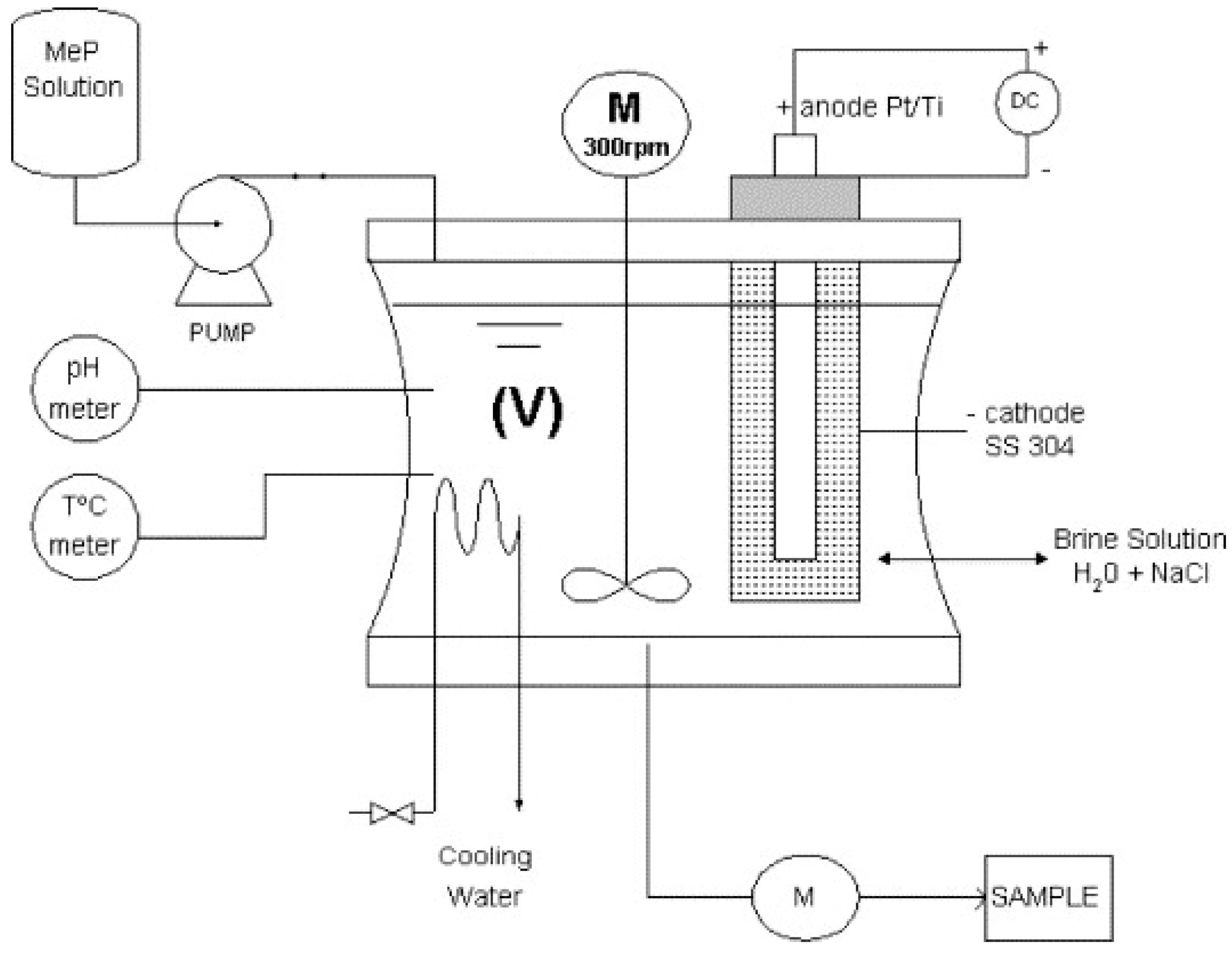
4. Electro-Fenton
5. Ozone-Based (O3) Processes
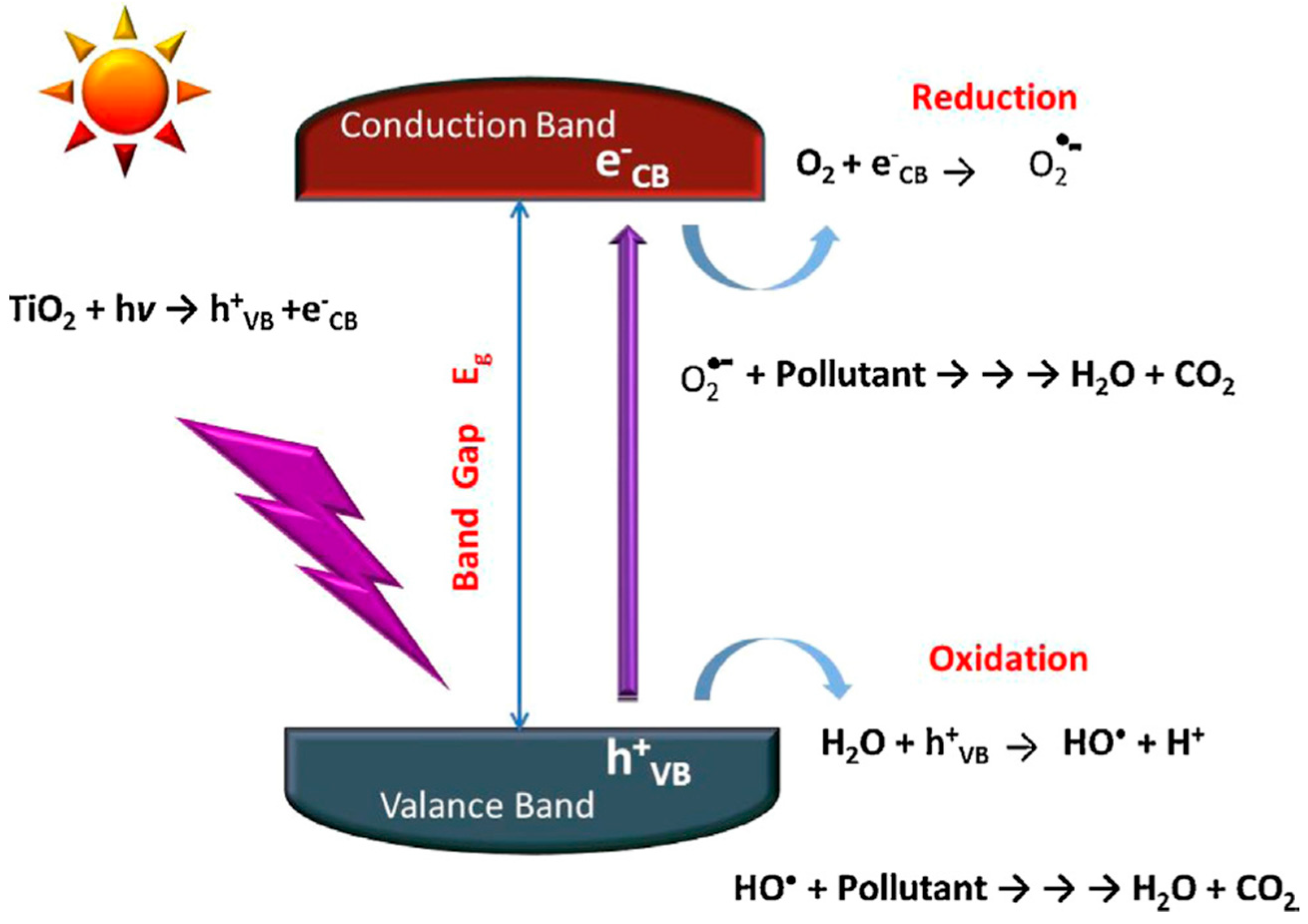
6. Photocatalysis
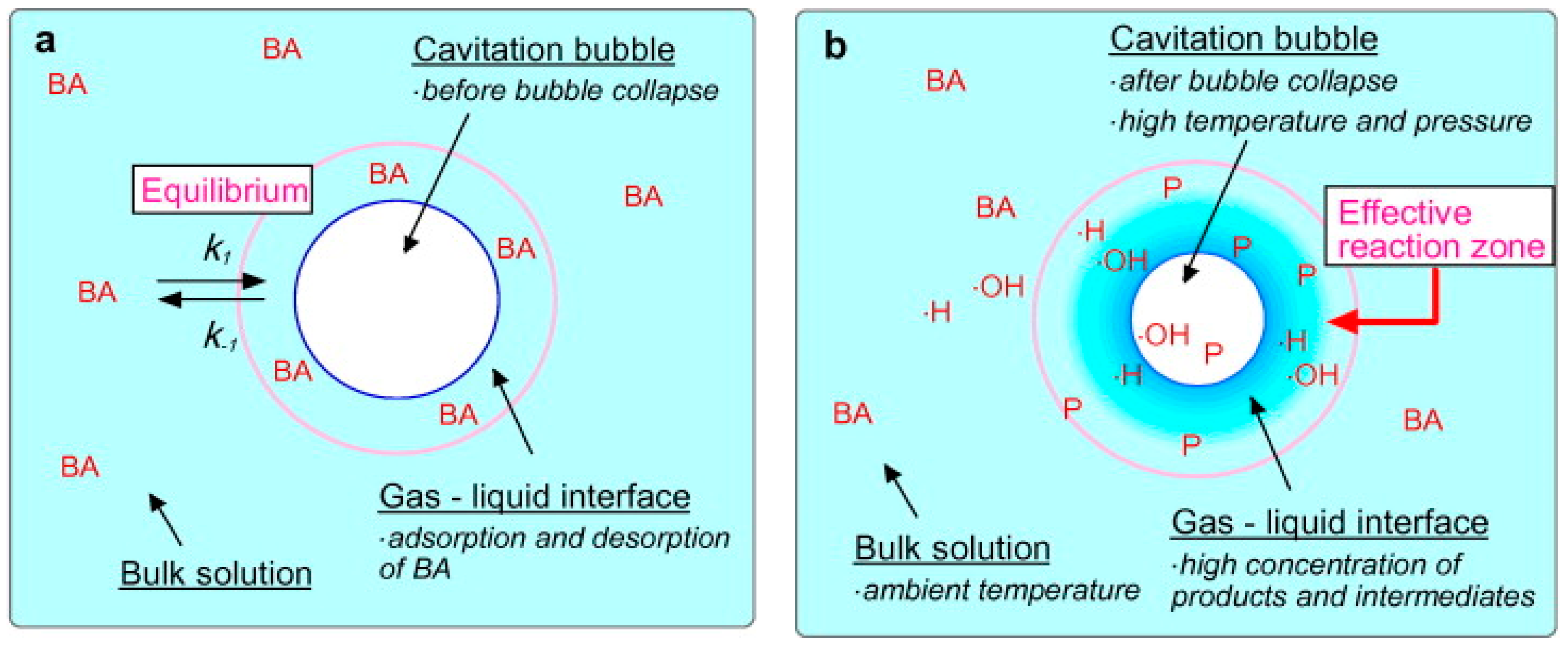
7. Sonolysis (Ultrasound (US) Radiation)
8. Single and Combined AOPs
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Covinich, L.G.; Bengoechea, D.I.; Fenoglio, R.J.; Area, M.C. Advanced Oxidation Processes for Wastewater Treatment in the Pulp and Paper Industry: A Review. Am. J. Environ. Eng. 2014, 4, 56–70. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A. Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review. J. Environ. Manag. 2018, 208, 56–76. [Google Scholar] [CrossRef]
- Mota, A.L.N.; Albuquerque, L.F.; Beltrame, L.T.C.; Chiavone-Filho, O.; Machulek, A., Jr.; Nascimento, C.A.O. Advanced oxidation Processes and Their Application in The Petroleum Industry: A Review. Braz. J. Pet. Gas 2008, 2, 122–142. [Google Scholar]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Phugare, S.S.; Kalyani, D.C.; Surwase, S.N.; Jadhav, J.P. Ecofriendly degradation, decolorization and detoxification of textile effluent by a developed bacterial consortium. Ecotoxicol. Environ. Saf. 2011, 74, 1288–1296. [Google Scholar] [CrossRef]
- Keen, O.S.; McKay, G.; Mezyk, S.P.; Linden, K.G.; Rosario-Ortiz, F.L. Identifying the factors that influence the reactivity of effluent organic matter with hydroxyl radicals. Water Res. 2014, 50, 408–419. [Google Scholar] [CrossRef]
- Krishnan, S.; Rawindran, H.; Sinnathambi, C.M.; Lim, J.W. Comparison of various advanced oxidation processes used in remediation of industrial wastewater laden with recalcitrant pollutants. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 206, p. 012089. [Google Scholar]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes—A review. Chemosphere 2017, 174, 665–688. [Google Scholar] [CrossRef]
- Tian, J.; Wu, C.; Yu, H.; Gao, S.; Li, G.; Cui, F.; Qu, F. Applying ultraviolet/persulfate (UV/PS) pre-oxidation for controlling ultra-filtration membrane fouling by natural organic matter (NOM) in surface water. Water Res. 2018, 132, 190–199. [Google Scholar] [CrossRef]
- Zorpas, A.A.; Lasaridi, K. Measuring waste prevention. Waste Manag. 2013, 33, 1047–1056. [Google Scholar] [CrossRef]
- Lasaridi, K.; Chroni, C.; Antonis, A.; Zorpas, K.; Abeliotis, K. Chapter 4: Waste Prevention. In Sustainable Solid Waste Management; Harokopio University: Athens, Greece, 2016; pp. 53–92. [Google Scholar]
- Perez-Gimeno, A.; Navarro-Pedreno, J.; Almendro-Candel, M.B.; Gomez, I.; Zorpas, A.A. The use of wastes (organic and inorganic) in land restoration in relation to their characteristics and cost. Waste Manag. Res. J.-Ternational Solid Wastes Public Clean. Assoc. ISWA 2019, 37, 502–507. [Google Scholar] [CrossRef]
- Prousek, J. Advanced Oxidation Processes for water treatment, Chemical Processes. Chem. Listy 1996, 90, 229–237. [Google Scholar]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation by ozone to remove recalcitrance from waste waters—A review. Environ. Technol. 1999, 22, 51–59. [Google Scholar]
- Gil, L.A.G.A.; Vicente, M.Á. The Handbook of Environmental Chemistry—Applications of Advanced Oxidation Processes (AOPs) in Drinking Water; Springer International Publishing: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen per-oxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Matafonova, G.; Batoev, V. Recent advances in application of UV light-emitting diodes for degrading organic pollutants in water through advanced oxidation processes: A review. Water Res. 2018, 132, 177–189. [Google Scholar] [CrossRef]
- Macías-Quiroga, I.F.; Henao-Aguirre, P.A.; Marín-Flórez, A.; Arredondo-López, S.M.; Sanabria-González, N.R. Bibliometric analysis of advanced oxidation processes (AOPs) in wastewater treatment: Global and Ibero-American research trends. Environ. Sci. Pollut. Res. Int. 2021, 28, 23791–23811. [Google Scholar] [CrossRef]
- Garcia-Costa, A.L.; Alves, A.; Madeira, L.M.; Santos, M.S. Oxidation processes for cytostatic drugs elimination in aqueous phase: A critical review. J. Environ. Chem. Eng. 2021, 9, 104709. [Google Scholar] [CrossRef]
- Bobu, M.; Yediler, A.; Siminiceanu, I.; Zhang, F.; Schulte-Hostede, S. Comparison of different advanced oxidation processes for the degradation of two fluoroquinolone antibiotics in aqueous solutions. J. Environ. Sci. Heal Part A Toxic Hazard. Subst. Environ. Eng. 2013, 48, 251–262. [Google Scholar] [CrossRef]
- Jamil, T.S.; Roland, H.; Michael, H.; Jens-Uwe, R. Homogeneous photocatalytic processes for degradation of some endocrine disturbing chemicals under UV irradiation. J. Water Process. Eng. 2017, 18, 159–168. [Google Scholar] [CrossRef]
- Verma, S.; Sillanpää, M. Degradation of anatoxin-a by UV-C LED and UV-C LED/H2O2 advanced oxidation processes. Chem. Eng. J. 2015, 274, 274–281. [Google Scholar] [CrossRef]
- Imoberdorf, G.; Mohseni, M. Kinetic study and modeling of the vacuum-UV photoinduced degradation of 2,4-D. Chem. Eng. J. 2012, 187, 114–122. [Google Scholar] [CrossRef]
- Saritha, P.; Aparna, C.; Himabindu, V.; Anjaneyulu, Y. Comparison of various advanced oxidation processes for the degradation of 4-chloro-2 nitrophenol. J. Hazard. Mater. 2007, 149, 609–614. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Canonica, S.; von Gunten, U. Efficiency and energy requirements for the transformation of organic micropollutants by ozone, O3/H2O2 and UV/H2O2. Water Res. 2011, 45, 3811–3822. [Google Scholar] [CrossRef]
- Bagheri, M.; Mohseni, M. Impact of hydrodynamics on pollutant degradation and energy efficiency of VUV/UV and H2O2/UV oxidation processes. J. Environ. Manag. 2015, 164, 114–120. [Google Scholar] [CrossRef]
- Ike, I.A.; Karanfil, T.; Cho, J.; Hur, J. Oxidation byproducts from the degradation of dissolved organic matter by advanced oxidation processes—A critical review. Water Res. 2019, 164, 114929. [Google Scholar] [CrossRef]
- Azbar, N.; Yonar, T.; Kestioglu, K. Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent. Chemosphere 2004, 55, 35–43. [Google Scholar] [CrossRef]
- Kusvuran, E.; Gulnaz, O.; Irmak, S.; Atanur, O.M.; Yavuz, H.I.; Erbatur, O. Comparison of several advanced oxidation processes for the decolorization of Reactive Red 120 azo dye in aqueous solution. J. Hazard. Mater. 2004, 109, 85–93. [Google Scholar] [CrossRef]
- Rueda-Márquez, J.J.; Pintado-Herrera, M.G.; Martín-Díaz, M.L.; Acevedo-Merino, A.; Manzano, M.A. Combined AOPs for potential wastewater reuse or safe discharge based on multi-barrier treatment (microfiltration-H2O2/UV-catalytic wet per-oxide oxidation). Chem. Eng. J. 2015, 270, 80–90. [Google Scholar] [CrossRef]
- Crapulli, F.; Santoro, D.; Sasges, M.R.; Ray, A.K. Mechanistic modeling of vacuum UV advanced oxidation process in an annular photoreactor. Water Res. 2014, 64, 209–225. [Google Scholar] [CrossRef]
- Rosenfeldt, E.; Boal, A.K.; Springer, J.; Stanford, B.; Rivera, S.; Kashinkunti, R.D.; Metz, D.H. Comparison of UV-mediated Advanced Oxidation. J.-Am. Water Work. Assoc. 2013, 105, 29–33. [Google Scholar] [CrossRef]
- Zoschke, K.; Dietrich, N.; Bornick, H.; Worch, E. UV-based advanced oxidation processes for the treatment of odour compounds: Efficiency and by-product formation. Water Res. 2012, 46, 5365–5373. [Google Scholar] [CrossRef]
- Oppenlaender, T. Photochemical Purification of Water and Air: Advanced Oxidation Processes (AOPs): Principles, Reaction Mechanisms, Reactor Concepts; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Edalatmanesh, M.; Dhib, R.; Mehrvar, M. Kinetic modeling of aqueous phenol degradation by UV/H2O2 process. Int. J. Chem. Kinet. 2008, 40, 34–43. [Google Scholar] [CrossRef]
- Ali, F.; Khan, J.A.; Shah, N.S.; Sayed, M.; Khan, H.M. Carbamazepine degradation by UV and UV-assisted AOPs: Kinetics, mechanism and toxicity investigations. Process. Saf. Environ. Prot. 2018, 117, 307–314. [Google Scholar] [CrossRef]
- Fenton, H.J.H. Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Haber, R.W.F. Unpaarigkeit und Radikalketten im Reaktionsmechanismus organischer und enzymatischer Vorgänge. Ber. Dtsch. Chem. Ges. 1931, 64, 2844–2856. [Google Scholar] [CrossRef]
- Cañizares, P.; Lobato, J.; Paz, R.; Rodrigo, M.; Sáez, C. Advanced oxidation processes for the treatment of olive-oil mills wastewater. Chemosphere 2007, 67, 832–838. [Google Scholar] [CrossRef]
- Blanco, J.; Torrades, F.; de la Varga, M.; García-Montaño, J. Fenton and biological-Fenton coupled processes for textile wastewater treatment and reuse. Desalination 2012, 286, 394–399. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. The feasibility of using combined Fenton-SBR for antibiotic wastewater treatment. Desalination 2012, 285, 14–21. [Google Scholar] [CrossRef]
- Cortez, S.; Teixeira, P.; Oliveira, R.; Mota, M. Evaluation of Fenton and ozone-based advanced oxidation processes as mature landfill leachate pre-treatments. J. Environ. Manag. 2011, 92, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Zorpas, A.A.; Costa, C.N. Combination of Fenton oxidation and composting for the treatment of the olive solid residue and the olive mile wastewater from the olive oil industry in Cyprus. Bioresour. Technol. 2010, 101, 7984–7987. [Google Scholar] [CrossRef] [PubMed]
- Zorpas, A.A. Alternative treatment of urban wastewater using electrochemical oxidation. Desalination Water Treat. 2011, 27, 268–276. [Google Scholar] [CrossRef]
- Zorpas, A.A. Chemical oxidation and Membrane Bioreactor for the treatment of Household heating wastewater. Desalination Water Treat. 2013, 51, 6952–6960. [Google Scholar] [CrossRef]
- Huang, C.P.; Dong, C.; Tang, Z. Advanced Chemical Oxidation: Its Present role and Potential Future in Hazardous waste treatment. Waste Manag. 1993, 13, 361–377. [Google Scholar] [CrossRef]
- Meric, S.; Selcuk, H.; Belgiorno, V. Acute toxicity removal in textile finishing wastewater by Fenton’s oxidation, ozone and coagulation-flocculation processes. Water Res. 2005, 39, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.W.; Hwang, K.Y. Effects on Reaction Conditions on the Oxidation Efficiency in the Fenton Process. Wat. Res. 2000, 34, 2786–2790. [Google Scholar] [CrossRef]
- Xu, X.R.; Zhao, Z.Y.; Li, X.Y.; Gu, J.D. Chemical oxidative degradation of methyl tert-butyl ether in aqueous solution by Fenton’s reagent. Chemosphere 2004, 55, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Zorpas, A.A.; Inglezakis, V.J. Intergraded Applied Methodology for the Treatment of Heavy Polluted Waste Waters from Olive Oil Industries. Appl. Environ. Soil Sci. 2011, 2011, 1–14. [Google Scholar] [CrossRef][Green Version]
- Hsueh, C.L.; Huang, Y.H.; Wang, C.C.; Chen, C.Y. Degradation of azo dyes using low iron concentration of Fenton and Fenton-like system. Chemosphere 2005, 58, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, D.; Tang, Q. The synthesis of NdFeB magnetic activated carbon and its application in degradation of azo dye methyl orange by Fenton-like process. J. Taiwan Inst. Chem. Eng. 2014, 45, 2584–2589. [Google Scholar] [CrossRef]
- Mahdad, F.; Younesi, H.; Bahramifar, N.; Hadavifar, M. Optimization of Fenton and photo-Fenton-based advanced oxidation processes for post-treatment of composting leachate of municipal solid waste by an activated sludge process. KSCE J. Civ. Eng. 2016, 20, 2177–2188. [Google Scholar] [CrossRef]
- Punzi, M.; Anbalagan, A.; Börner, R.A.; Svensson, B.-M.; Jonstrup, M.; Mattiasson, B. Degradation of a textile azo dye using biological treatment followed by photo-Fenton oxidation: Evaluation of toxicity and microbial community structure. Chem. Eng. J. 2015, 270, 290–299. [Google Scholar] [CrossRef]
- Fiorentino, A.; Esteban, B.; Garrido-Cardenas, J.A.; Kowalska, K.; Rizzo, L.; Aguera, A.; Pérez, J.A.S. Effect of solar photo-Fenton process in raceway pond reactors at neutral pH on antibiotic resistance determinants in secondary treated urban wastewater. J. Hazard. Mater. 2019, 378, 120737. [Google Scholar] [CrossRef]
- Wang, W.; Qu, Y.; Yang, B.; Liu, X.; Su, W. Lactate oxidation in pyrite suspension: A Fenton-like process in situ generating H2O. Chemosphere 2012, 86, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Arapoglou, D.; Vlyssides, A.; Israilides, C.; Zorpas, A.; Karlis, P. Detoxification of methyl-parathion pesticide in aqueous solutions by electrochemical oxidation. J. Hazard. Mater. 2003, 98, 191–199. [Google Scholar] [CrossRef]
- Lyssides, A.G.V.; Israelides, C.J.; Mourafeti, V.N.; Karvouni, G. Olive Oil Wastewater treatment with the use of An Electrolysis System. Bioresour. Technol. 1997, 61, 163–170. [Google Scholar]
- Vlyssides, A.G.; Loizidou, M.; Karlis, P.K.; Zorpas, A.A.; Papaioannou, D. Electrochemical Oxidation of a Textile Dye Wastewater using a Ptr/Ti electrode. J. Hazard. Mater. 1999, 70, 41–52. [Google Scholar] [CrossRef]
- Vlyssides, A.; Papaioannou, D.; Loizidoy, M.; Karlis, P.; Zorpas, A. Testing an electrochemical method for treatment of textile dye wastewater. Waste Manag. 2000, 20, 569–574. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.; Yao, B.; Yang, J.; Zhi, D. Current progress in electrochemical anodic-oxidation of pharmaceuticals: Mechanisms, influencing factors, and new technique. J. Hazard. Mater. 2021, 418, 126313. [Google Scholar] [CrossRef]
- Chang, X.; Meyer, M.; Liu, X.; Zhao, Q.; Chen, H.; Chen, J.-A.; Qiu, Z.; Yang, L.; Cao, J.; Shu, W. Determination of antibiotics in sewage from hospitals, nursery and slaughter house, wastewater treatment plant and source water in Chongqing region of Three Gorge Reservoir in China. Environ. Pollut. 2010, 158, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, C.; Wu, J.; Niu, J. Insights into the electrochemical degradation of sulfamethoxazole and its metabolite by Ti/SnO2-Sb/Er-PbO2 anode. Chin. Chem. Lett. 2020, 31, 2673–2677. [Google Scholar] [CrossRef]
- Ciríaco, L.; Anjo, C.; Correia, J.; Pacheco, M.; Lopes, A. Electrochemical degradation of Ibuprofen on Ti/Pt/PbO2 and Si/BDD electrodes. Electrochim. Acta 2009, 54, 1464–1472. [Google Scholar] [CrossRef]
- Beltran, F.J. Ozone Reaction Kinetics for Water and Waste water Systems; Lewis Publishers: Boca Raton, FL, USA, 2003. [Google Scholar]
- Krasner, S.W.; Mitch, W.; McCurry, D.L.; Hanigan, D.; Westerhoff, P. Formation, precursors, control, and occurrence of nitrosamines in drinking water: A review. Water Res. 2013, 47, 4433–4450. [Google Scholar] [CrossRef]
- Rakness, K.L. Process Design, Operation, and Optimization, Ozone in Drinking Water Treatment; American Water Works Association: Denver, CO, USA, 2005. [Google Scholar]
- Almomani, F.A.; Shawaqfah, M.; Bhosale, R.R.; Kumar, A. Removal of emerging pharmaceuticals from wastewater by ozone-based advanced oxidation processes. Environ. Prog. Sustain. Energy 2016, 35, 982–995. [Google Scholar] [CrossRef]
- Patel, S.; Mondal, S.; Majumder, S.K.; Das, P.; Ghosh, P. Treatment of a Pharmaceutical Industrial Effluent by a Hybrid Process of Advanced Oxidation and Adsorption. ACS Omega 2020, 5, 32305–32317. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Comparison of different advanced oxidation processes for treatment of antibiotic aqueous solution. Desalination 2010, 256, 43–47. [Google Scholar] [CrossRef]
- Vaitsis, C.; Mechili, M.; Argirusis, N.; Kanellou, E.; Pandis, P.K.; Sourkouni, G.; Zorpas, A.; Argirusis, C. Ultrasound-Assisted Preparation Methods of Nanoparticles for Energy-Related Applications. In Nanotechnology and the Environment; IntechOpen: London, UK, 2020. [Google Scholar]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.B.D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Karunakaran, C.; Senthilvelan, S. Photocatalysis with ZrO2: Oxidation of aniline. J. Mol. Catal. A Chem. 2005, 233, 1–8. [Google Scholar] [CrossRef]
- Chan, S.H.S.; Wu, T.Y.; Juan, J.C.; Teh, C.Y. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. J. Chem. Technol. Biotechnol. 2011, 86, 1130–1158. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Nyamukamba, P.; Tichagwa, L.; Mamphweli, S.; Petrik, L. Silver/Carbon Codoped Titanium Dioxide Photocatalyst for Improved Dye Degradation under Visible Light. Int. J. Photoenergy 2017, 2017, 3079276. [Google Scholar] [CrossRef]
- Stucchi, M.; Bianchi, C.; Pirola, C.; Vitali, S.; Cerrato, G.; Morandi, S.; Argirusis, C.; Sourkouni, G.; Sakkas, P.; Capucci, V. Surface decoration of commercial micro-sized TiO2 by means of high energy ultrasound: A way to enhance its photocatalytic activity under visible light. Appl. Catal. B Environ. 2015, 178, 124–132. [Google Scholar] [CrossRef]
- Stucchi, M.; Bianchi, C.; Argirusis, C.; Pifferi, V.; Neppolian, B.; Cerrato, G.; Boffito, D. Ultrasound assisted synthesis of Ag-decorated TiO2 active in visible light. Ultrason. Sonochem. 2018, 40, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R.N. To Study the Effect of the Concentration of Carbon on Ultraviolet and Visible Light Photo Catalytic Activity and Characterization of Carbon Doped TiO2. J. Nanomed. Nanotechnol. 2015, 6, 1. [Google Scholar]
- Poulia, A.; Sakkas, P.; Kanellopoulou, D.; Sourkouni, G.; Legros, C.; Argirusis, C. Preparation of metal–ceramic composites by sonochemical synthesis of metallic nano-particles and in-situ decoration on ceramic powders. Ultrason. Sonochem. 2016, 31, 417–422. [Google Scholar] [CrossRef]
- Stucchi, M.; Bianchi, C.L.; Pirola, C.; Cerrato, G.; Morandi, S.; Argirusis, C.; Sourkouni, G.; Naldoni, A.; Capucci, V. Copper NPs decorated titania: A novel synthesis by high energy US with a study of the photocatalytic activity under visible light. Ultrason. Sonochem. 2016, 31, 295–301. [Google Scholar] [CrossRef]
- Pekakis, P.A.; Xekoukoulotakis, N.; Mantzavinos, D. Treatment of textile dyehouse wastewater by TiO2 photocatalysis. Water Res. 2006, 40, 1276–1286. [Google Scholar] [CrossRef]
- Elmolla, E.; Chaudhuri, M. Optimization of Fenton process for treatment of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution. J. Hazard. Mater. 2009, 170, 666–672. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Degradation of the antibiotics amoxicillin, ampicillin and cloxacillin in aqueous solution by the photo-Fenton process. J. Hazard. Mater. 2009, 172, 1476–1481. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination 2010, 252, 46–52. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. J. Hazard. Mater. 2010, 173, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, N.; Salari, D.; Khataee, A. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Ameta, S.C.; Ameta, R. Advance Oxidation Processes for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Lee, W.C.H. Photocatalytic Oxidation of Arsenite in TiO2 Suspension: Kinetics and Mechanisms. Environ. Sci. Technol 2002, 36, 3872–3878. [Google Scholar] [CrossRef]
- Buschmann, J.; Canonica, S.; Lindauer, U.; Hug, S.J.; Sigg, L. Photoirradiation of Dissolved Humic Acid Induces Arsenic(III) Oxidation. Environ. Sci. Technol. 2005, 39, 9541–9546. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, W.P.I.; Kocar, D. Photochemical Oxidation of As(III) in Ferrioxalate Solutions. Environ. Sci. Technol. 2003, 37, 1581–1588. [Google Scholar]
- Fei, H.; Leng, W.; Li, X.; Cheng, X.; Xu, Y.; Zhang, J.; Cao, C. Photocatalytic Oxidation of Arsenite over TiO2: Is Superoxide the Main Oxidant in Normal Air-Saturated Aqueous Solutions? Environ. Sci. Technol. 2011, 45, 4532–4539. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, Y.; Felix, N. Visible-Light Induced Water Detoxification Catalyzed by PtII Dye Sensitized Titania. J. Am. Chem. Soc. 2008, 130, 12566–12567. [Google Scholar]
- Stepnowski, P.; Zaleska, A. Comparison of different advanced oxidation processes for the degradation of room temperature ionic liquids. J. Photochem. Photobiol. A Chem. 2005, 170, 45–50. [Google Scholar] [CrossRef]
- Hunge, Y.M. Basics and advanced developments in photocatalysis—A review (Mini review). Int. J. Hydrol. 2018, 2, 539–540. [Google Scholar] [CrossRef]
- Pestman, J.M.; Engberts, J.B.F.N.; De Jong, F. Sonochemistry: Theory and applications. Recl. Des Trav. Chim. Des Pays-Bas 2010, 113, 533–542. [Google Scholar] [CrossRef]
- Adewuyi, Y.G. Sonochemistry: Environmental Science and Engineering Applications. Ind. Eng. Chem. Res. 2001, 40, 4681–4715. [Google Scholar] [CrossRef]
- Pdtrier, C.; Francony, A. Ultrasonic waste-water treatment: Incidence of ultrasonic frequency on the rate of phenol and carbon tetrachloride degradation. Ultrason. Sonochem. 1997, 4, 295–300. [Google Scholar] [CrossRef]
- Sakkas, P.M.; Schneider, O.; Sourkouni, G.; Argirusis, C. Sonochemistry in the service of SOFC research. Ultrason. Sonochem. 2014, 21, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Gogate, P.R. Cavitational reactors for process intensification of chemical processing applications: A critical review. Chem. Eng. Process. Process Intensif. 2008, 47, 515–527. [Google Scholar] [CrossRef]
- Vaitsis, C.; Sourkouni, G.; Argirusis, C. Metal Organic Frameworks (MOFs) and ultrasound: A review. Ultrason. Sonochem. 2018, 52, 106–119. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Gagol, M.; Soltani, R.D.C.; Przyjazny, A.; Boczkaj, G. Effective degradation of sulfide ions and organic sulfides in cavitation-based advanced oxidation processes (AOPs). Ultrason. Sonochem. 2019, 58, 104610. [Google Scholar] [CrossRef] [PubMed]
- Okitsu, K.; Nanzai, B.; Kawasaki, K.; Takenaka, N.; Bandow, H. Sonochemical decomposition of organic acids in aqueous solution: Understanding of molecular behavior during cavitation by the analysis of a heterogeneous reaction kinetics model. Ul-Trasonics Sonochem. 2009, 16, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S. The Chemical Effects of Ultrasound. Sci. Am. 1989, 260, 80–86. [Google Scholar] [CrossRef]
- Suslick, K.; Didenko, Y.; Fang, M.M.; Hyeon, T.; Kolbeck, K.J.; McNamara, W.B.; Mdleleni, M.M.; Wong, M. Acoustic cavitation and its chemical consequences. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 1999, 357, 335–353. [Google Scholar] [CrossRef]
- Saharan, V.K.; Rizwani, M.A.; Malani, A.A.; Pandit, A.B. Effect of geometry of hydrodynamically cavitating device on degradation of orange-G. Ultrason. Sonochem. 2013, 20, 345–353. [Google Scholar] [CrossRef]
- Bashir, T.A.; Soni, A.G.; Mahulkar, A.V.; Pandit, A.B. The CFD driven optimisation of a modified venturi for cavitational activity. Can. J. Chem. Eng. 2011, 89, 1366–1375. [Google Scholar] [CrossRef]
- Madhavan, J.; Grieser, F.; Ashokkumar, M. Degradation of orange-G by advanced oxidation processes. Ultrason. Sonochem. 2010, 17, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraju, D.; Glass, B.D.; Oelgemoeller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Jose, J.; Philip, L. Continuous flow pulsed power plasma reactor for the treatment of aqueous solution containing volatile organic compounds and real pharmaceutical wastewater. J. Environ. Manag. 2021, 286, 112202. [Google Scholar] [CrossRef] [PubMed]
- De Bel, E.; Janssen, C.; De Smet, S.; Van Langenhove, H.; Dewulf, J. Sonolysis of ciprofloxacin in aqueous solution: Influence of operational parameters. Ultrason. Sonochem. 2011, 18, 184–189. [Google Scholar] [CrossRef]
- Naddeo, V.; Landi, M.; Scannapieco, D.; Belgiorno, V. Sonochemical degradation of twenty-three emerging contaminants in urban wastewater. Desalination Water Treat. 2013, 51, 6601–6608. [Google Scholar] [CrossRef]
- Lianou, A.; Frontistis, Z.; Chatzisymeon, E.; Antonopoulou, M.; Konstantinou, I.; Mantzavinos, D. Sonochemical oxidation of piroxicam drug: Effect of key operating parameters and degradation pathways. J. Chem. Technol. Biotechnol. 2017, 93, 28–34. [Google Scholar] [CrossRef]
- Ziylan-Yavas, A.; Ince, N.H. Single, simultaneous and sequential applications of ultrasonic frequencies for the elimination of ibuprofen in water. Ultrason. Sonochem. 2018, 40, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Serna-Galvis, E.A.; Silva-Agredo, J.; Giraldo-Aguirre, A.L.; Florez-Acosta, O.A.; Torres-Palma, R.A. High frequency ultrasound as a selective advanced oxidation process to remove penicillinic antibiotics and eliminate its antimicrobial activity from water. Ultrason. Sonochem. 2016, 31, 276–283. [Google Scholar] [CrossRef]
- Güyer, G.T.; Ince, N.H. Degradation of diclofenac in water by homogeneous and heterogeneous sonolysis. Ultrason. Sonochem. 2011, 18, 114–119. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Ike, I.A.; Linden, K.G.; Orbell, J.D.; Duke, M. Critical review of the science and sustainability of persulphate advanced oxidation processes. Chem. Eng. J. 2018, 338, 651–669. [Google Scholar] [CrossRef]
- Wei, Z.; Villamena, F.A.; Weavers, L.K. Kinetics and Mechanism of Ultrasonic Activation of Persulfate: An in Situ EPR Spin Trapping Study. Environ. Sci. Technol. 2017, 51, 3410–3417. [Google Scholar] [CrossRef]
- Abu Amr, S.S.; Aziz, H.A. New treatment of stabilized leachate by ozone/Fenton in the advanced oxidation process. Waste Manag. 2012, 32, 1693–1698. [Google Scholar] [CrossRef]
- Adityosulindro, S.; Barthe, L.; González-Labrada, K.; Haza, U.J.J.; Delmas, H.; Julcour, C. Sonolysis and sono-Fenton oxidation for removal of ibuprofen in (waste)water. Ultrason. Sonochem. 2017, 39, 889–896. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, H.; Xue, X. Ultrasound enhanced heterogeneous activation of peroxydisulfate by magnetite catalyst for the degradation of tetracycline in water. Sep. Purif. Technol. 2012, 84, 147–152. [Google Scholar] [CrossRef]
- Chandak, S.; Ghosh, P.K.; Gogate, P.R. Treatment of real pharmaceutical wastewater using different processes based on ultra-sound in combination with oxidants. Process Saf. Environ. Prot. 2020, 137, 149–157. [Google Scholar] [CrossRef]
- Naddeo, V.; Uyguner-Demirel, C.S.; Prado, M.; Cesaro, A.; Belgiorno, V.; Ballesteros, F. Enhanced ozonation of selected pharmaceutical compounds by sonolysis. Environ. Technol. 2015, 36, 1876–1883. [Google Scholar] [CrossRef]
- Mendez-Arriaga, F.; Torres-Palma, R.A.; Petrier, C.; Esplugas, S.; Gimenez, J.; Pulgarin, C. Ultrasonic treatment of water contaminated with ibuprofen. Water Res. 2008, 42, 4243–4248. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Kim, S.; Yoon, Y.; Jung, Y.; Hwang, T.-M.; Lee, J.; Kang, J.-W. Comparative evaluation of ibuprofen removal by UV/H2O2 and UV/S2O82− processes for wastewater treatment. Chem. Eng. J. 2015, 269, 379–390. [Google Scholar] [CrossRef]
- Tokumura, M.; Sugawara, A.; Raknuzzaman, M.; Mamun, H.-A.-; Masunaga, S. Comprehensive study on effects of water matrices on removal of pharmaceuticals by three different kinds of advanced oxidation processes. Chemosphere 2016, 159, 317–325. [Google Scholar] [CrossRef]
- Maroudas, A.; Pandis, P.K.; Chatzopoulou, A.; Davellas, L.-R.; Sourkouni, G.; Argirusis, C. Synergetic decolorization of azo dyes using ultrasounds, photocatalysis and photo-fenton reaction. Ultrason. Sonochem. 2021, 71, 105367. [Google Scholar] [CrossRef] [PubMed]
- Faouzi, M.; Cañizares, P.; Gadri, A.; Lobato, J.; Nasr, B.; Paz, R.; Rodrigo, M.; Saez, C. Advanced oxidation processes for the treatment of wastes polluted with azoic dyes. Electrochim. Acta 2006, 52, 325–331. [Google Scholar] [CrossRef]
- Voukkali, I.; Loizia, P.; Pedreño, J.N.; Zorpas, A.A. Urban strategies evaluation for waste management in coastal areas in the framework of area metabolism. Waste Manag. Res. J. Int. Solid Wastes Public Clean. Assoc. ISWA 2021, 39, 448–465. [Google Scholar] [CrossRef] [PubMed]
- Zorpas, A.A.; Coumi, C.; Drtil, M.; Voukalli, I. Municipal sewage sludge characteristics and waste water treatment plant effectiveness under warm climate conditions. Desalination Water Treat. 2011, 36, 319–333. [Google Scholar] [CrossRef]
- Zorpas, A.A.; Vlyssides, A.G.; Loizidou, M. Dewatered anaerobically-stabilized primary sewage sludge composting: Metal leachability and uptake by natural Clinoptilolite. Commun. Soil Sci. Plant Anal. 1999, 30, 1603–1613. [Google Scholar] [CrossRef]
- Zorpas, A.A.; Coumi, C.; Drtil, M.; Voukalli, I.; Samaras, P. Operation description and physicochemical characteristics of influent, effluent and the tertiary treatment from a sewage treatment plant of the Eastern Region of Cyprus under warm climates. Desalination Water Treat. 2010, 22, 244–257. [Google Scholar] [CrossRef]
- Gągol, M.; Przyjazny, A.; Boczkaj, G. Highly effective degradation of selected groups of organic compounds by cavitation based AOPs under basic pH conditions. Ultrason. Sonochem. 2018, 45, 257–266. [Google Scholar] [CrossRef]
- Agarkoti, C.; Gogate, P.R.; Pandit, A.B. Coupling of acoustic/hydrodynamic cavitation with ozone (O3), hydrogen peroxide (H2O2), magnesium oxide (MgO) and manganese dioxide (MnO2) for the effective treatment of CETP effluent. Sep. Purif. Technol. 2021, 284, 120281. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Qian, J. Degradation of roxarsone in UV-based advanced oxidation processes: A comparative study. J. Hazard. Mater. 2021, 410, 124558. [Google Scholar] [CrossRef] [PubMed]
- Cotton, C.; Dotson, A.; Jousset, S.; Linden, K.; Collins, J. Applying UVAOP at an existing WTP: Effects on disinfection strategy and DBP formation. In Proceedings of the Water Quality Technology Conference and Exposition, Savannah, Georgia, 14–18 November 2010; pp. 3008–3013. [Google Scholar]
- Li, W.; Zhang, M.; Wang, H.; Lian, J.; Qiang, Z. Removal of recalcitrant organics in reverse osmosis concentrate from coal chemical industry by UV/H2O2 and UV/PDS: Efficiency and kinetic modeling. Chemosphere 2021, 287, 131999. [Google Scholar] [CrossRef]
- Meng, T.; Sun, W.; Su, X.; Sun, P. The optimal dose of oxidants in UV-based advanced oxidation processes with respect to primary radical concentrations. Water Res. 2021, 206, 117738. [Google Scholar] [CrossRef]
- Tang, Y.; Lee, C.S.; Walker, H.; Gobler, C.; Apul, O.; Venkatesan, A.K.; Mao, X. Effect of residual H2O2 on the removal of advanced oxidation byproducts by two types of granular activated carbon. J. Environ. Chem. Eng. 2021, 9, 106838. [Google Scholar] [CrossRef]
- Wang, H.; Hasani, M.; Wu, F.; Warriner, K. Pre-oxidation of spent lettuce wash water by continuous Advanced Oxidation Process to reduce chlorine demand and cross-contamination of pathogens during post-harvest washing. Food Microbiol. 2021, 103, 103937. [Google Scholar] [CrossRef]
- Ahmed, N.; Vione, D.; Rivoira, L.; Carena, L.; Castiglioni, M.; Bruzzoniti, M. A Review on the Degradation of Pollutants by Fenton-Like Systems Based on Zero-Valent Iron and Persulfate: Effects of Reduction Potentials, pH, and Anions Occurring in Waste Waters. Molecule 2021, 26, 4584. [Google Scholar] [CrossRef]
- Ahmed, Y.; Zhong, J.; Yuan, Z.; Guo, J. Simultaneous removal of antibiotic resistant bacteria, antibiotic resistance genes, and micropollutants by a modified photo-Fenton process. Water Res. 2021, 197, 117075. [Google Scholar] [CrossRef]
- Butt, A.L.; Mpinga, J.K.; Tichapondwa, S.M. Photo-Fenton Oxidation of Methyl Orange Dye Using South African Ilmenite Sands as a Catalyst. Catalysts 2021, 11, 1452. [Google Scholar] [CrossRef]
- Gorozabel-Mendoza, M.L.; Filho, O.A.E.; Zambrano-Intriago, L.A.; Baquerizo-Crespo, R.J.; Giler-Molina, J.M.; Rodríguez-Díaz, J.M. Degradation of Blue 1 and Yellow 6 Dyes in Binary Mixture Using Photo-Fenton/Sunlight System: Optimization by Factorial Designs. Water Air Soil Pollut. 2021, 232, 500. [Google Scholar] [CrossRef]
- Rodrigues-Silva, F.; Lemos, C.R.; Naico, A.A.; Fachi, M.M.; Amaral, B.D.; de Paula, V.C.; Rampon, D.S.; Beraldi-Magalhães, F.; Prola, L.D.; Pontarolo, R.; et al. Study of isoniazid degradation by Fenton and photo-Fenton processes, by-products analysis and toxicity evaluation. J. Photochem. Photobiol. A Chem. 2021, 425, 113671. [Google Scholar] [CrossRef]
- Vieira, M.M.; Dornelas, A.S.P.; Carlos, T.D.; Pallini, A.; Gravato, C.; Pereira, D.H.; Sarmento, R.A.; Cavallini, G.S. When treatment increases the contaminant’s ecotoxicity: A study of the Fenton process in the degradation of methylene blue. Chemosphere 2021, 283, 131177. [Google Scholar] [CrossRef]
- Widyastuti, N.; Hidayat, M.; Purnomo, C.W. Enhanced Biogas Production from Sugarcane Vinasse using Electro-Fenton as Pre-treatment Method. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 830. [Google Scholar]
- Brillas, E. A critical review on ibuprofen removal from synthetic waters, natural waters, and real wastewaters by advanced oxidation processes. Chemosphere 2021, 286, 131849. [Google Scholar] [CrossRef] [PubMed]
- Dutschke, M.; Schnabel, T.; Schütz, F.; Springer, C. Degradation of chlorinated volatile organic compounds from contaminated ground water using a carrier-bound TiO2/UV/O3-system. J. Environ. Manag. 2021, 304, 114236. [Google Scholar] [CrossRef]
- Gautam, P.; Popat, A.; Lokhandwala, S. Advances & Trends in Advance Oxidation Processes and Their Applications. In Advanced Industrial Wastewater Treatment and Reclamation of Water; Springer: Berlin/Heidelberg, Germany, 2022; pp. 45–69. [Google Scholar]
- Vieira, W.T.; de Farias, M.B.; Spaolonzi, M.P.; da Silva, M.G.C.; Vieira, M.G.A. Latest advanced oxidative processes applied for the removal of endocrine disruptors from aqueous media—A critical report. J. Environ. Chem. Eng. 2021, 9, 105748. [Google Scholar] [CrossRef]
- Bolujoko, N.B.; Unuabonah, E.I.; Alfred, M.O.; Ogunlaja, A.; Ogunlaja, O.O.; Omorogie, M.O.; Olukanni, O.D. Toxicity and removal of parabens from water: A critical review. Sci. Total Environ. 2021, 792, 148092. [Google Scholar] [CrossRef]
- Cardoso, I.M.F.; Cardoso, R.M.F.; da Silva, J.C.G.E. Advanced Oxidation Processes Coupled with Nanomaterials for Water Treatment. Nanomaterial 2021, 11, 2045. [Google Scholar] [CrossRef]
- Fosso-Kankeu, E.; Pandey, S.; Ray, S.S. Photocatalysts in Advanced Oxidation Processes for Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Evgenidou, E.; Ofrydopoulou, A.; Malesic-Eleftheriadou, N.; Nannou, C.; Ainali, N.M.; Christodoulou, E.; Bikiaris, D.N.; Kyzas, G.Z.; Lambropoulou, D.A. New insights into transformation pathways of a mixture of cytostatic drugs using Polyester-TiO2 films: Identification of intermediates and toxicity assessment. Sci. Total Environ. 2020, 741, 140394. [Google Scholar] [CrossRef]
- Kusuma, T.D.; Jyothi, M.S.V.N.; Rao, C.P.; Maliyekkal, S.M. Advanced Oxidation Processes: A Promising Route for Abatement of Emerging Contaminants in Water. In Nanomaterials and Nanocomposites for Environmental Remediation; Springer: Berlin/Heidelberg, Germany, 2021; pp. 275–305. [Google Scholar]
- Liu, H.; Li, C.; Zhang, T.; Xu, Z.; Li, Y.; Li, B.; Tian, S. UV facilitated synergistic effects of polymetals in ore catalyst on peroxy-monosulfate activation: Implication for the degradation of bisphenol S. Chem. Eng. J. 2022, 431, 133989. [Google Scholar] [CrossRef]
- Kaus, N.H.M.; Imam, S.S.; Aziz, A.W.; Lee, H.L.; Adnan, R.; Ibrahim, M.L. Controlled growth of BiFeO3 nano-particles in the presence of alginate template for adsorptive removal of different dyes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126294. [Google Scholar] [CrossRef]
- Kaus, N.H.M.; Rithwan, A.F.; Adnan, R.; Ibrahim, M.L.; Thongmee, S.; Yusoff, S.F.M. Effective Strategies, Mechanisms, and Photocatalytic Efficiency of Semiconductor Nanomaterials Incorporating rGO for Environmental Contaminant Degradation. Catalysts 2021, 11, 302. [Google Scholar] [CrossRef]
- Zare, E.N.; Iftekhar, S.; Park, Y.; Joseph, J.; Srivastava, V.; Khan, M.A.; Makvandi, P.; Sillanpaa, M.; Varma, R.S. An overview on non-spherical semiconductors for heterogeneous photocatalytic degradation of organic water contaminants. Chemosphere 2021, 280, 130907. [Google Scholar] [CrossRef]
- Liu, P.; Wu, Z.; Abramova, A.V.; Cravotto, G. Sonochemical processes for the degradation of antibiotics in aqueous solutions: A review. Ultrason. Sonochem. 2021, 74, 105566. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Xu, J.; Li, J.; Wu, F.; Zhou, D. Strengthening arsenite oxidation in water using metal-free ultrasonic activation of sulfite. Chemosphere 2021, 281, 130860. [Google Scholar] [CrossRef] [PubMed]
- Poblete, R.; Cortes, E.; Salihoglu, G.; Salihoglu, N.K. Ultrasound and heterogeneous photocatalysis for the treatment of vinasse from pisco production. Ultrason. Sonochem. 2020, 61, 104825. [Google Scholar] [CrossRef] [PubMed]
- Rayaroth, M.P.; Aravindakumar, C.T.; Shah, N.S.; Boczkaj, G. Advanced oxidation processes (AOPs) based wastewater treatment—Unexpected nitration side reactions—A serious environmental issue: A review. Chem. Eng. J. 2022, 430, 133002. [Google Scholar] [CrossRef]
- Rossi, G.; Mainardis, M.; Aneggi, E.; Weavers, L.K.; Goi, D. Combined ultrasound-ozone treatment for reutilization of primary effluent—A preliminary study. Environ. Sci. Pollut. Res. 2021, 28, 700–710. [Google Scholar] [CrossRef]
- Sourkouni, G.; Kalogirou, C.; Moritz, P.; Gödde, A.; Pandis, P.K.; Höfft, O.; Vouyiouka, S.; Zorpas, A.A.; Argirusis, C. Study on the influence of advanced treatment processes on the surface properties of polylactic acid for a bio-based circular economy for plastics. Ultrason. Sonochem. 2021, 76, 105627. [Google Scholar] [CrossRef] [PubMed]
- Sunasee, S.; Leong, K.H.; Wong, K.T.; Lee, G.; Pichiah, S.; Nah, I.W.; Jeon, B.H.; Yoon, Y.; Jang, M. Sonophotocatalytic degradation of bisphenol A and its intermediates with graphitic carbon nitride. Environ. Sci. Pollut. Res. 2019, 26, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Toxicity changes of wastewater during various advanced oxidation processes treatment: An overview. J. Clean. Prod. 2021, 315, 128202. [Google Scholar] [CrossRef]

| Semiconductor | Bandgap (eV @300K) |
|---|---|
| ZnS (Wurtzite) | 3.91 |
| ZnS (Zinc blende) | 3.54 |
| SnO2 | 3.60 |
| TiO2 | 3.20 |
| ZnO | 3.03 |
| WO3 | 2.60 |
| CdS | 2.42 |
| Fe2O3 | 2.20 |
| CdO | 2.10 |
| Cu2O | 2.10 |
| CdSe | 1.70 |
| AlSb | 1.58 |
| CdTe | 1.56 |
| GaAs | 1.42 |
| Experimental Parameters | |||||||
|---|---|---|---|---|---|---|---|
| AOP | Waste/ Contaminant | pH | Time [min] | Other (Volume, Concentration, Power, Frequencies, etc.) | COD Reduction [%] | Waste Degradation [%] * | Ref. |
| O3/Fenton | Leachate | 7 | 90 | 1700 mg/L | 65 | n/a | [127] |
| O3/Fenton | Leachate | 7 | 60 | 400 mg L | 72 | n/a | [48] |
| Fenton | Leachate | 3 | 40 | 46 | n/a | ||
| Fenton | Leachate | 4.5 | 80 | 740 mg/L | 56 | n/a | [59] |
| UV/Fenton | Leachate | 4.5 | 80 | 84 | n/a | ||
| UV/Fenton | Amoxicillin | 2 | 50 | 6 W | 81 | n/a | [92] |
| Fenton | Amoxicillin | 3 | 50 | 104 mg/L, | 80 | n/a | [76] |
| UV/Fenton | Amoxicillin | 3 | 50 | 104 mg/L, | 81 | n/a | |
| UV/TiO2/H2O2 | Amoxicillin | 5 | 300 | 600 mL, 6 W,100 mg/L, | 26 | n/a | |
| UV/TiO2 | Amoxicillin | 3 | 300 | 600 mL, 6 W, 50 mg/L, | 12 | n/a | |
| UV/TiO2 | Amoxicillin | 3 | 30 | 600 mL, 6 W, 50 mg/L | 12 | n/a | [91] |
| UV/TiO2/H2O2 | Amoxicillin | 5 | 30 | 600 mL, 6 W,100 mg/L | 26 | n/a | |
| O3/UV/H2O2 | Enrofloxacin | 11 | 20 | 1.4 L, 15 W | 88 | n/a | [26] |
| O3/UV | Enrofloxacin | 11 | 30 | 1.4 L, 15 W | 75 | n/a | |
| O3/UV/H2O2 Fenton | Enrofloxacin | 11 | 15 | 1.4 L, 15 W | 100 | n/a | |
| Sonolysis | ibuprofen | 4.3 | 180 | 20 mg/L, 12 kHz, 1 L | n/a | 10 | [128] |
| Sonolysis | Ibuprofen | 4.3 | 180 | 20 mg/L, 20 kHz, 1 L | n/a | 40 | |
| Sonolysis | Ibuprofen | 4.3 | 180 | 20 mg/L, 862 kHz | n/a | 80 | |
| Fenton | Ibuprofen | 2.6 | 180 | 20 mg/L | n/a | 80 | |
| Sonolysis/Fenton | Ibuprofen | 2.6 | 120 | 20 mg/L, 12 kHz, 1 L | n/a | 95 | |
| Sonolysis/Fenton | Ibuprofen | 2.6 | 120 | 20 mg/L, 20 kHz, 1 L | n/a | 97 | |
| Sonolysis/Fenton | ibuprofen | 2.6 | 120 | 20 mg/L, 862 kHz | n/a | 100 | |
| Sonolysis/Fenton | Diclofenac | n/a | 40 | 20 mg/L, 20 kHz, 200 mL | n/a | 73 | [129,130,131] |
| Sonolysis | Ibuprofen | n/a | 180 | 14.6μg/L, 45 kHz | n/a | 60 | [119] |
| Sonolysis | Diclofenac | 5.3 | 180 | 16.6 μg/L, 200 mL, 45 kHz | n/a | 60 | |
| Sonolysis | Ibuprofen | 3 | 30 | 21 mg/L–300 mL, 300 kHz | n/a | 98 | [132] |
| UV/H2O2 | Ibuprofen | 7 | <30 min | 11 W | n/a | 60 | [133] |
| AOP | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| UV/H2O2 |
|
| [142,143,144,145,146,147,148] |
| Fenton-based |
|
| [149,150,151,152,153,154,155] |
| Ozone-based |
|
| [156,157,158,159] |
| Photocatalysis |
|
| [160,161,162,163,164,165,166,167,168] |
| Sonolysis |
|
| [169,170,171,172,173,174,175,176] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. ChemEngineering 2022, 6, 8. https://doi.org/10.3390/chemengineering6010008
Pandis PK, Kalogirou C, Kanellou E, Vaitsis C, Savvidou MG, Sourkouni G, Zorpas AA, Argirusis C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. ChemEngineering. 2022; 6(1):8. https://doi.org/10.3390/chemengineering6010008
Chicago/Turabian StylePandis, Pavlos K., Charalampia Kalogirou, Eirini Kanellou, Christos Vaitsis, Maria G. Savvidou, Georgia Sourkouni, Antonis A. Zorpas, and Christos Argirusis. 2022. "Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review" ChemEngineering 6, no. 1: 8. https://doi.org/10.3390/chemengineering6010008
APA StylePandis, P. K., Kalogirou, C., Kanellou, E., Vaitsis, C., Savvidou, M. G., Sourkouni, G., Zorpas, A. A., & Argirusis, C. (2022). Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. ChemEngineering, 6(1), 8. https://doi.org/10.3390/chemengineering6010008










