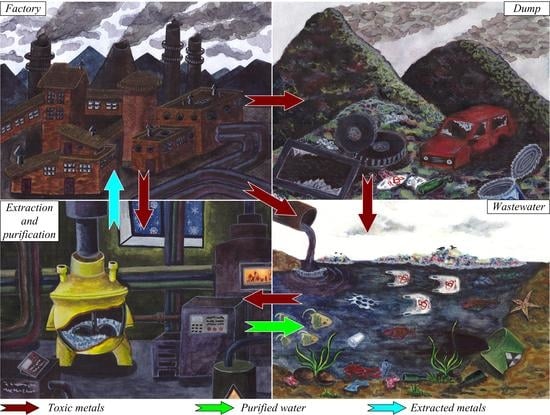
Ionic Liquids as Components of Systems for Metal Extraction
Abstract
:1. Introduction
2. Ionic Liquids as Extractants in Extraction Systems
2.1. Ionic Liquids Based on Phosphonium Cations for Metal Extraction
2.2. Ionic Liquids Based on Ammonium Cations for Metal Extraction
2.2.1. Group 5 and 6 Metals of the Periodic Table of Elements
2.2.2. Transition Metals of Periods 3 and 4 of the Periodic Table of Elements
2.2.3. Noble Metals
2.2.4. Lithium
2.2.5. Rare Earth Elements
2.3. Ionic Liquids Based on Imidazolium Cations for Metal Extraction
2.3.1. Transition Metals
2.3.2. Metals of Period 5 of the Periodic Table of Elements
2.3.3. Noble Metals and Platinum-Group Metals
2.4. Ionic Liquids Based on Pyridinium, Piperidinium, Pyrrolidinium, Morpholinium, Benzimidazolium, Guanidinium, and Betainium Cations
2.4.1. Heavy Metals
2.4.2. Noble Metals
2.4.3. Rare Earth Elements and Actinides
2.4.4. Group 5 Metals of the Periodic Table of Elements
3. Ionic Liquids as Diluents in Extraction Systems
3.1. Ionic Liquids Based on the Phosphonium Cation as Diluents
3.2. Ionic Liquids Based on the Imidazolium Cation as Diluents
4. Solid-Phase Extraction of Metals with Ionic Liquids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | |
| [A336][Br] | trialkylmethylammonium bromide |
| [A336][CA-12] | trialkylmethylammonium sec-octylphenoxy acetic acid |
| [A336][CA-100] | trialkylmethylammonium sec-nonylphenoxy acetic acid |
| [A336][Cy272] | trialkylmethylammonium bis(2,4,4-trimethylpentyl)phosphinate |
| [A336][I] | trialkylmethylammonium iodide |
| Aliquat 336 | a mixture of trioctylmethylammonium and tridecylmethylammonium chlorides |
| [A336][MTBA] | trialkylmethylammonium 2-(methylthio)benzoate |
| [A336][NO3] | trialkylmethylammonium nitrate |
| [A336][oleate] | trialkylmethylammonium oleate |
| [A336][P204] | trialkylmethylammonium di-2-ethylhexyl phosphate |
| [A336][P507] | trialkylmethylammonium 2-ethylhexyl 2-ethylhexylphosphonate |
| [A336][SCN] | trioctylmethylammonium thiocyanate |
| B | |
| [bmim][NTf2] | 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| [bmim][PF6] | 1-butyl-3-methylimidazolium hexafluorophosphate |
| C | |
| Cyanex 272 | di(2,4,4,-trimethylpentyl)phosphinic acid |
| Cyphos 101 | trihexyl(tetradecyl)phosphonium chloride |
| Cyphos 104 | trihexyl(tetradecyl)phosphonium bis(2,4,4-trimethylpentyl)phosphinate |
| [C4C1im][OAc] | 1-butyl-3-methylimidazolium acetate |
| [C4C1im][OTf] | 1-butyl-3-methylimidazolium trifluoromethanesulfonate |
| [C4C1im][Me2PO4] | 1-butyl-3-methylimidazolium dimethyl phosphate |
| [C4C1im][MeSO4] | 1-butyl-3-methylimidazolium methyl sulfate |
| [C8C1im][Cl] | 1-octyl-3-methylimidazolium chloride |
| [C8C1im][OTf] | 1-octyl-3-methylimidazolium trifluoromethanesulfonate |
| [C2mim][NTf2] | 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| [C4mim][Br] | 1-butyl-3-methylimidazolium bromide |
| [C6mim][NTf2] | 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| [C6mim][Br] | 1-hexyl-3-methylimidazolium bromide |
| [C8mim][Br] | 1-octyl-3-methylimidazolium bromide |
| [C16mim][Cl] | 1-hexadecyl-3-methylimidazolium chloride |
| D | |
| [DiOcAPmim][NTf2] | 1-methyl-3-dioctylaminopropylimidazolium bis(trifluoromethylsulfonyl)imide |
| D2EHPA | di(2-ethylhexyl) phosphonic acid |
| DMDODGA | N, N′-dimethyl-N, N′-dioctyl-3-oxadiglycolamide |
| E | |
| [EBPiP][NTf2] | N-butyl-N-ethyl-piperidinium bis(trifluoromethylsulfonyl)imide |
| [EMIM]-DEP | 1-ethyl-3-methylimidazolium diethyl phosphate |
| [EOPiP][NTf2] | N-octyl-N-ethyl-piperidinium bis(trifluoromethylsulfonyl)imide |
| [EPipMIBK][NTf2] | N-ethyl-N-(4-methyl-2-oxopentyl)-piperidinium bis(trifluoromethylsulfonyl)imide |
| H | |
| [Hbet][NTf2] | betainium bis(trifluoromethylsulfonyl)imide |
| I | |
| IL | ionic liquid |
| K | |
| K-3PC10-PrBr | 1-propyl-3-undecanoylpyridinium bromide |
| M | |
| [MBCNPIP]+ | 1-butyronitrile-1-methylpiperidinium cation |
| [4MBCNPYR]+ | 1-butyronitrile-4-methylpyridinium cation |
| [MBCNPYRRO]+ | 1-butyronitrile-1-methylpyrrolidinium cation |
| [MImMIBK][NTf2] | N-methyl-N-(4-methyl-2-oxopentyl)imidazolium bis(trifluoromethylsulfonyl)imide |
| [MOPIP]+ | 1-methyl-1-octylpiperidinium cation |
| [MOPYRRO]+ | 1-methyl-1-octylpyrrolidinium cation |
| [MPS2PIP]+ | 1-methyl-1-[4,5-bis(methyl sulfide)]pentylpiperidinium cation |
| [MPS2PYRRO]+ | 1-methyl-1-[4,5-bis(methyl sulfide)]pentylpyrrolidinium cation |
| [MPTPIP]+ | 1-methyl-2-pentenepiperidinium cation |
| [MPTPYRRO]+ | 1-methyl-2-pentenepyrrolidinium cation |
| N | |
| [N4444][DEHP] | tetrabutylammonium bis(2-ethylhexyl)phosphate |
| [N4444][EHPMEH] | tetrabutylammonium mono-2-ethylhexyl 2-ethylhexyl phosphate |
| N8888C18:1 | tetraoctylammonium oleate |
| N8888C18:2 | tetraoctylammonium linoleate |
| [N8888][DEHP] | tetraoctylammonium bis(2-ethylhexyl) phosphate |
| [N8888][DEHOX] | tetraoctylammonium di(2-ethylhexyl)oxamate |
| [N8888][DODGA] | tetraoctylammonium dioctyl diglycolamate |
| O | |
| [OdMIM][NTf2] | 1,2-dimethyl-3-octylimidazolium bis(trifluoromethylsulfonyl)imide |
| [omim][NTf2] | 1-octyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| [OMIM][NTf2] | 1-methyl-3-octylimidazolium bis(trifluoromethylsulfonyl)imide |
| [OPy][BF4] | N-octylpyridinium tetrafluoroborate |
| [OPy][Cl] | N-octylpyridinium chloride |
| Ox-3PC10-PrBr | 3-[1-(hydroxyimine)undecyl]-1-propylpyridinium bromide |
| Ox-3PC10-PrCl | 3-[1-(hydroxyimine)undecyl]-1-propylpyridinium chloride |
| P | |
| [P1888] [C6SAc] | methyltrioctylphosphonium S-hexyl thioglycolate |
| [P2225][NTf2] | triethylpentylphosphonium bis(trifluoromethylsulfonyl)imide |
| [P2226][NTf2] | triethylhexylphosphonium bis(trifluoromethylsulfonyl)imide |
| [P4444][BTMPP] | tetrabutylphosphonium bis(2,4,4-trimethylpentyl)phosphinate |
| [P66614][BTB] | trihexyl(tetradecyl)phosphonium 2-(benzylthio)benzoate |
| [P66614][hfac] | trihexyl(tetradecyl)phosphonium 1,1,1,5,5,5-hexafluoro-2,4-pentanedionate |
| [P66614][MA] | trihexyl(tetradecyl)phosphonium N,N,N′,N′-tetra(2-ethylhexyl)malonate |
| [P66614][NO3] | trihexyl(tetradecyl)phosphonium nitrate |
| [P66614][PTB] | trihexyl(tetradecyl)phosphonium 2-(propylthio)benzoate |
| [P8888][oleate] | tetraoctylphosphonium oleate |
| 3PC10 | 1-(3-pyridyl)undecan-1-one oxime |
| R | |
| REE | rare earth elements |
| S | |
| Solvesso 100 | a mixture of aromatic hydrocarbons obtained by pyrolysis of oil fractions |
| T | |
| TBAH2P | tetrabutyl ammonium dihydrogen phosphate |
| THN+ | tetrahexylammonium cation |
| TON+ | tetraoctylammonium cation |
| [THN][Br] | tetrahexylammonium bromide |
| [THN][Dca] | tetrahexylammonium dicyanamide |
| [THN][SCN] | tetrahexylammonium thiocyanate |
| [THN][Tf2N] | tetrahexylammonium bis(trifluoromethylsufonyl)imide |
| [TOMA] [D2EHP] | trioctylmethylammonium bis(2-ethylhexyl)phosphinate |
| [TON][Br] | tetraoctylammonium bromide |
| [TON][Dca] | tetraoctylammonium dicyanamide |
| [TON][Tf2N] | tetraoctylammonium bis(trifluoromethylsufonyl)imide |
References
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Nasirpour, N.; Mohammadpourfard, M.; Heris, S.Z. Ionic liquids: Promising compounds for sustainable chemical processes and applications. Chem. Eng. Res. Des. 2020, 160, 264–300. [Google Scholar] [CrossRef]
- Yavir, K.; Konieczna, K.; Marcinkowski, Ł.; Kloskowski, A. Ionic liquids in the microextraction techniques: The influence of ILs structure and properties. TrAC Trends Anal. Chem. 2020, 130, 115994. [Google Scholar] [CrossRef]
- Feng, J.; Loussala, H.M.; Han, S.; Ji, X.; Li, C.; Sun, M. Recent advances of ionic liquids in sample preparation. TrAC Trends Anal. Chem. 2020, 125, 115833. [Google Scholar] [CrossRef]
- Pirkwieser, P.; López-López, J.A.; Kandioller, W.; Keppler, B.K.; Moreno, C.; Jirsa, F. Novel 3-hydroxy-2-naphthoate-based task-specific ionic liquids for an efficient extraction of heavy metals. Front. Chem. 2018, 6, 172. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Kumar, J.R.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Sun, T.; Xu, C.; Fu, J.; Chen, Q.; Chen, J.; Shen, X. Extraction of U (VI) by the ionic liquid hexyltributylphosphonium bis (trifluoromethylsulfonyl) imides: An experimental and theoretical study. Sep. Purif. Technol. 2017, 188, 386–393. [Google Scholar] [CrossRef]
- Yudaev, P.A.; Kolpinskaya, N.A.; Chistyakov, E.M. Organophosphorous extractants for metals. Hydrometallurgy 2021, 201, 105558. [Google Scholar] [CrossRef]
- Ghosh, A.; Datta, D.; Uslu, H.; Bamufleh, H.S.; Kumar, S. Separation of copper ion (Cu2+) from aqueous solution using tri-n-butyl phosphate and di-2-ethylhexyl phosphoric acid as extractants. J. Mol. Liq. 2018, 258, 147–154. [Google Scholar] [CrossRef]
- Navarro-González, M.; Ortega-López, V.; Lópéz-Fernández, J.I.; Amo-Salas, M.; González-Carcedo, S. Heavy-metal extraction from sewage sludge using phosphorous-based salts: Optimization process with Na2H2P2O7. Environ. Technol. 2017, 38, 2305–2313. [Google Scholar] [CrossRef]
- Wehbie, M.; Arrachart, G.; Sukhbaatar, T.; Le Goff, X.F.; Karamé, I.; Pellet-Rostaing, S. Extraction of uranium from sulfuric acid media using amino-diamide extractants. Hydrometallurgy 2021, 200, 105550. [Google Scholar] [CrossRef]
- Masmoudi, A.; Zante, G.; Trébouet, D.; Barillon, R.; Boltoeva, M. Solvent extraction of lithium ions using benzoyltrifluoroacetone in new solvents. Sep. Purif. Technol. 2021, 255, 117653. [Google Scholar] [CrossRef]
- Asrami, M.R.; Tran, N.N.; Nigam, K.D.P.; Hessel, V. Solvent Extraction of Metals: Role of Ionic Liquids and Microfluidics. Sep. Purif. Technol. 2021, 262, 118289. [Google Scholar] [CrossRef]
- Paiva, A.P.; Nogueira, C.A. Ionic Liquids in the Extraction and Recycling of Critical Metals from Urban Mines. Waste Biomass Valorization 2021, 12, 1725–1747. [Google Scholar] [CrossRef]
- Rajadurai, V.; Anguraj, B.L. Ionic liquids to remove toxic metal pollution. Environ. Chem. Lett. 2020, 19, 1173–1203. [Google Scholar] [CrossRef]
- Prusty, S.; Pradhan, S.; Mishra, S. Ionic liquid as an emerging alternative for the separation and recovery of Nd, Sm and Eu using solvent extraction technique—A review. Sustain. Chem. Pharm. 2021, 21, 100434. [Google Scholar] [CrossRef]
- Danso, I.K.; Cueva-Sola, A.B.; Masaud, Z.; Lee, J.Y.; Jyothi, R.K. Ionic Liquids for the Recovery of Rare Earth Elements from Coal Combustion Products. In Clean Coal Technologies; Jyothi, R.K., Parhi, P.K., Eds.; Springer: Cham, Switzerland, 2021; Chapter 25. [Google Scholar] [CrossRef]
- Atanassova, M. Solvent extraction of metallic species in ionic liquids: An overview of s-, p-and d-elements. J. Chem. Technol. Metall. 2021, 56, 443. [Google Scholar]
- Rout, A.; Binnemans, K. Influence of the ionic liquid cation on the solvent extraction of trivalent rare-earth ions by mixtures of Cyanex 923 and ionic liquids. Dalton Trans. 2015, 44, 1379–1387. [Google Scholar] [CrossRef] [Green Version]
- Quijada-Maldonado, E.; Olea, F.; Sepúlveda, R.; Castillo, J.; Cabezas, R.; Merlet, G.; Romero, J. Possibilities and Challenges for ionic liquids in hydrometallurgy. Sep. Purif. Technol. 2020, 251, 117289. [Google Scholar] [CrossRef]
- Alguacil, F.J. Liquid-Liquid Extraction of Indium (III) from the HCl Medium by Ionic Liquid A327H+ Cl− and Its Use in a Supported Liquid Membrane System. Molecules 2020, 25, 5238. [Google Scholar] [CrossRef] [PubMed]
- Alguacil, F.J.; Lopez, F.A. Insight into the Liquid–Liquid Extraction System AuCl4−/HCl/A327H+ Cl− Ionic Liquid/Toluene. Processes 2021, 9, 608. [Google Scholar] [CrossRef]
- Quijada-Maldonado, E.; Sánchez, F.; Pérez, B.; Tapia, R.; Romero, J. Task-specific ionic liquids as extractants for the solvent extraction of molybdenum (VI) from aqueous solution using different commercial ionic liquids as diluents. Ind. Eng. Chem. Res. 2018, 57, 1621–1629. [Google Scholar] [CrossRef]
- Micheau, C.; Lejeune, M.; Arrachart, G.; Draye, M.; Turgis, R.; Michel, S.; Legeai, S.; Pellet-Rostaing, S. Recovery of tantalum from synthetic sulfuric leach solutions by solvent extraction with phosphonate functionalized ionic liquids. Hydrometallurgy 2019, 189, 105107. [Google Scholar] [CrossRef]
- Jin, C.; Chen, M.; Fan, M.; Luo, G.; Shao, M.; Huang, Z.; Xie, X. Hydrophobic phosphonium-based ionic liquids as novel extractants for palladium (II) recovery from alkaline cyanide solutions. J. Mol. Liq. 2021, 336, 116358. [Google Scholar] [CrossRef]
- Nayak, S.; Devi, N. Studies on extraction of gallium (III) from chloride solution using Cyphos IL 104 and its removal from photodiodes and red mud. Hydrometallurgy 2017, 171, 191–197. [Google Scholar] [CrossRef]
- Dhiman, S.; Gupta, B. Cyphos IL 104 assisted extraction of indium and recycling of indium, tin and zinc from discarded LCD screen. Sep. Purif. Technol. 2020, 237, 116407. [Google Scholar] [CrossRef]
- Mishra, B.B.; Devi, N. Solvent extraction and separation of europium (III) using a phosphonium ionic liquid and an organophosphorus extractant-A comparative study. J. Mol. Liq. 2018, 271, 389–396. [Google Scholar] [CrossRef]
- Hoogerstraete, T.V.; Binnemans, K. Highly efficient separation of rare earths from nickel and cobalt by solvent extraction with the ionic liquid trihexyl (tetradecyl) phosphonium nitrate: A process relevant to the recycling of rare earths from permanent magnets and nickel metal hydride batteries. Green Chem. 2014, 16, 1594–1606. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Yu, Z.; Liu, H. Extraction of rare earths by undiluted [P666, 14] [NO3] and DEHEHP, and the recovery of rare earths from lamp phosphors. J. Mater. Cycles Waste Manag. 2019, 21, 1518–1525. [Google Scholar] [CrossRef]
- Mahandra, H.; Faraji, F.; Ghahreman, A. Novel Extraction Process for Gold Recovery from Thiosulfate Solution Using Phosphonium Ionic Liquids. ACS Sustain. Chem. Eng. 2021, 9, 8179–8185. [Google Scholar] [CrossRef]
- Ilyas, S.; Kim, H.; Srivastava, R.R. Separation of platinum group metals from model chloride solution using phosphonium-based ionic liquid. Sep. Purif. Technol. 2021, 278, 119577. [Google Scholar] [CrossRef]
- Rout, A.; Binnemans, K. Liquid–liquid extraction of europium (III) and other trivalent rare-earth ions using a non-fluorinated functionalized ionic liquid. Dalton Trans. 2014, 43, 1862–1872. [Google Scholar] [CrossRef] [Green Version]
- Parmentier, D.; Paradis, S.; Metz, S.J.; Wiedmer, S.K.; Kroon, M.C. Continuous process for selective metal extraction with an ionic liquid. Chem. Eng. Res. Des. 2016, 109, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Jing, Y.; Xiao, J.; Wang, X.; Jia, Y. Liquid-liquid extraction of lithium using novel phosphonium ionic liquid as an extractant. Hydrometallurgy 2017, 169, 314–320. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Lan, Z.; Xu, H. Unique extraction of U (VI), Pu (IV), Am (III) and Eu (III) from nitric acid medium using a phosphonium based ionic liquid consisting of novel anion, 1,1,1,5,5,5-hexafluoro-2, 4-pentanedionate–Dual solvent behavior. Sep. Purif. Technol. 2021, 262, 118309. [Google Scholar] [CrossRef]
- Platzer, S.; Leyma, R.; Wolske, S.; Kandioller, W.; Heid, E.; Schröder, C.; Schagerl, M.; Krachler, R.; Jirsa, F.; Keppler, B.K. Thioglycolate-based task-specific ionic liquids: Metal extraction abilities vs acute algal toxicity. J. Hazard. Mater. 2017, 340, 113–119. [Google Scholar] [CrossRef]
- Platzer, S.; Kar, M.; Leyma, R.; Chib, S.; Roller, A.; Jirsa, F.; Krachler, R.; MacFarlane, D.R.; Kandioller, W.; Keppler, B.K. Task-specific thioglycolate ionic liquids for heavy metal extraction: Synthesis, extraction efficacies and recycling properties. J. Hazard. Mater. 2017, 324, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Leyma, R.; Platzer, S.; Jirsa, F.; Kandioller, W.; Krachler, R.; Keppler, B.K. Novel thiosalicylate-based ionic liquids for heavy metal extractions. J. Hazard. Mater. 2016, 314, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, G.; Guan, W.; Zeng, L.; Zhou, Q.; Xia, Y.; Wang, Q.; Li, Q.; Cao, Z. Complete removal of trace vanadium from ammonium tungstate solutions by solvent extraction. Hydrometallurgy 2018, 179, 268–273. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, J.; Zhang, Y.; Liu, T.; Hu, P.; Liu, H.; Luo, D. Separation and recovery of vanadium and aluminum from oxalic acid leachate of shale by solvent extraction with Aliquat 336. Sep. Purif. Technol. 2020, 249, 116867. [Google Scholar] [CrossRef]
- Imam, D.M.; El-Nadi, Y.A. Recovery of molybdenum from alkaline leach solution of spent hydrotreating catalyst by solvent extraction using methyl tricaprylammonium hydroxide. Hydrometallurgy 2018, 180, 172–179. [Google Scholar] [CrossRef]
- Devi, N. Solvent extraction and separation of copper from base metals using bifunctional ionic liquid from sulfate medium. Trans. Nonferrous Met. Soc. 2016, 26, 874–881. [Google Scholar] [CrossRef]
- Castillo, J.; Coll, M.T.; Fortuny, A.; Donoso, P.N.; Sepúlveda, R.; Sastre, A.M. Cu (II) extraction using quaternary ammonium and quaternary phosphonium based ionic liquid. Hydrometallurgy 2014, 141, 89–96. [Google Scholar] [CrossRef]
- Foltova, S.S.; Hoogerstraete, T.V.; Banerjee, D.; Binnemans, K. Samarium/cobalt separation by solvent extraction with undiluted quaternary ammonium ionic liquids. Sep. Purif. Technol. 2019, 210, 209–218. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Raiguel, S.; Binnemans, K. Separation of transition metals from rare earths by non-aqueous solvent extraction from ethylene glycol solutions using Aliquat 336. Sep. Purif. Technol. 2018, 201, 318–326. [Google Scholar] [CrossRef]
- Alguacil, F.J.; García-Díaz, I.; Escudero, E. Extraction of indium (III) from sulphuric acid medium by the ionic liquid (PJMTH+ HSO4−). Sep. Purif. Technol. 2019, 211, 764–767. [Google Scholar] [CrossRef]
- Parmentier, D.; Metz, S.J.; Kroon, M.C. Tetraalkylammonium oleate and linoleate based ionic liquids: Promising extractants for metal salts. Green Chem. 2013, 15, 205–209. [Google Scholar] [CrossRef]
- Parmentier, D.; Hoogerstraete, T.V.; Banerjee, D.; Valia, Y.A.; Metz, S.J.; Binnemans, K.; Kroon, M.C. A mechanism for solvent extraction of first row transition metals from chloride media with the ionic liquid tetraoctylammonium oleate. Dalton Trans. 2016, 45, 9661–9668. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Cho, C.-W.; Kim, S.; Song, M.-H.; Bediako, J.K.; Yun, Y.-S. Selective recovery of Au (III), Pt (IV), and Pd (II) from aqueous solutions by liquid–liquid extraction using ionic liquid Aliquat-336. J. Mol. Liq. 2016, 216, 18–24. [Google Scholar] [CrossRef]
- Vereycken, W.; Riaño, S.; Gerven, T.V.; Binnemans, K. Extraction Behavior and Separation of Precious and Base Metals from Chloride, Bromide, and Iodide Media Using Undiluted Halide Ionic Liquids. ACS Sustain. Chem. Eng. 2020, 8, 8223–8234. [Google Scholar] [CrossRef]
- Boudesocque, S.; Mohamadou, A.; Conreux, A.; Marin, B.; Dupont, L. The recovery and selective extraction of gold and platinum by novel ionic liquids. Sep. Purif. Technol. 2019, 210, 824–834. [Google Scholar] [CrossRef]
- Shi, C.; Jing, Y.; Xiao, J.; Wang, X.; Yao, Y.; Jia, Y. Solvent extraction of lithium from aqueous solution using non-fluorinated functionalized ionic liquids as extraction agents. Sep. Purif. Technol. 2017, 172, 473–479. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Duan, M.; Hao, X.; Yang, Q.; Zhang, Q.; Huang, X. Liquid-liquid extraction of lithium from aqueous solution using novel ionic liquid extractants via COSMO-RS and experiments. Fluid Phase Equilib. 2018, 459, 129–137. [Google Scholar] [CrossRef]
- Obon, E.; Fortuny, A.; Coll, M.T.; Sastre, A.M. Mathematical modelling of neodymium, terbium and dysprosium solvent extraction from chloride media using methyl-tri (octyl/decyl) ammonium oleate ionic liquid as extractant. Hydrometallurgy 2017, 173, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Obón, E.; Fortuny, A.; Coll, M.T.; Sastre, A.M. Experimental and modelling studies of neodymium solvent extraction from chloride media with methyl-tri (octyl/decyl) ammonium oleate ionic liquid diluted in kerosene. Hydrometallurgy 2017, 174, 216–226. [Google Scholar] [CrossRef]
- Rout, A.; Binnemans, K. Solvent extraction of neodymium (III) by functionalized ionic liquid trioctylmethylammonium dioctyl diglycolamate in fluorine-free ionic liquid diluent. Ind. Eng. Chem. Res. 2014, 53, 6500–6508. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, W.; Deng, Y.; Zhang, J.; Zhao, H.; Chen, J. Extraction and recovery of cerium (IV) and fluorine (I) from sulfuric solutions using bifunctional ionic liquid extractants. Chem. Eng. J. 2012, 179, 19–25. [Google Scholar] [CrossRef]
- Guo, L.; Chen, J.; Shen, L.; Zhang, J.; Zhang, D.; Deng, Y. Highly selective extraction and separation of rare earths (III) using bifunctional ionic liquid extractant. ACS Sustain. Chem. Eng. 2014, 2, 1968–1975. [Google Scholar] [CrossRef]
- Wang, W.; Yang, H.; Cui, H.; Zhang, D.; Liu, Y.; Chen, J. Application of bifunctional ionic liquid extractants [A336] [CA-12] and [A336] [CA-100] to the lanthanum extraction and separation from rare earths in the chloride medium. Ind. Eng. Chem. Res. 2011, 50, 7534–7541. [Google Scholar] [CrossRef]
- Maria, L.; Cruz, A.; Carretas, J.M.; Monteiro, B.; Galinha, C.; Gomes, S.S.; Araújo, M.F.; Paiva, I.; Marçalo, J.; Leal, J.P. Improving the selective extraction of lanthanides by using functionalised ionic liquids. Sep. Purif. Technol. 2020, 237, 116354. [Google Scholar] [CrossRef]
- de los Ríos, A.P.D.L.; Hernández-Fernández, F.; Alguacil, F.; Lozano, L.; Ginesta, A.; García-Díaz, I.; Sanchez-Segado, S.; López, F.; Godínez, C. On the use of imidazolium and ammonium-based ionic liquids as green solvents for the selective recovery of Zn (II), Cd (II), Cu (II) and Fe (III) from hydrochloride aqueous solutions. Sep. Purif. Technol. 2012, 97, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Corbett, P.J.; McIntosh, A.J.S.; Geeb, M.; Hallett, J.P. Use of ionic liquids to remove harmful M 2+ contaminants from hydrocarbon streams. Mol. Syst. Des. Eng. 2018, 3, 408–417. [Google Scholar] [CrossRef]
- Turgis, R.; Arrachart, G.; Michel, S.; Legeai, S.; Lejeune, M.; Draye, M.; Pellet-Rostaing, S. Ketone functionalized task specific ionic liquids for selective tantalum extraction. Sep. Purif. Technol. 2018, 196, 174–182. [Google Scholar] [CrossRef]
- Chauhan, G.; de Klerk, A. Acidified Ionic Liquid Assisted Recovery of Vanadium and Nickel from Oilsands Bitumen. Energy Fuels 2021, 35, 5963–5974. [Google Scholar] [CrossRef]
- Papaiconomou, N.; Génand-Pinaz, S.; Levequea, J.-M.; Guittonneau, S. Selective extraction of gold and platinum in water using ionic liquids. A simple two-step extraction process. Dalton Trans. 2013, 42, 1979–1982. [Google Scholar] [CrossRef]
- Tong, Y.; Hongxiao, Y.; Jing, L.; Yanzhao, Y. Extraction of Au (III) by ionic liquid from hydrochloric acid medium. Sep. Purif. Technol. 2013, 120, 367–372. [Google Scholar] [CrossRef]
- Yang, X.; Miao, C.; Sun, Y.; Lei, T.; Xie, Q.; Wang, S. Efficient extraction of gold (I) from alkaline aurocyanide solution using green ionic liquid-based aqueous biphasic systems. J. Taiwan Inst. Chem. Eng. 2018, 91, 176–185. [Google Scholar] [CrossRef]
- Wu, H.; Kim, S.-Y.; Takahashi, T.; Oosugi, H.; Ito, T.; Kanie, K. Extraction behaviors of platinum group metals in simulated high-level liquid waste by a hydrophobic ionic liquid bearing an amino moiety. Nucl. Eng. Technol. 2021, 53, 1218–1223. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Wieszczycka, K.; Wojciechowska, I.; Olszanowski, A. Lead (II) extraction from aqueous solutions by pyridine extractants. Sep. Purif. Technol. 2017, 177, 239–248. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Wieszczycka, K.; Wojciechowska, I. Efficient recovery of copper from aqueous solutions with pyridine extractants (oxime, ketone) and their quaternary pyridinium salts. Sep. Purif. Technol. 2017, 185, 103–111. [Google Scholar] [CrossRef]
- Wejman-Gibas, K.; Wieszczycka, K.; Wojciechowska, A.; Ochromowicz, K.; Pohl, P. Extraction of molybdenum (VI) from sulfate media by 3-pyridineketoxime and its quaternary salts. Sep. Purif. Technol. 2016, 158, 71–79. [Google Scholar] [CrossRef]
- Lee, J.M. Extraction of noble metal ions from aqueous solution by ionic liquids. Fluid Phase Equilibria 2012, 319, 30–36. [Google Scholar] [CrossRef]
- Lv, P.; Liu, R.; Wang, Y.; Liu, X.; Wu, Y.; Xue, W.; Yang, Y. Extraction of gold (III) using novel gemini-type benzimidazole ionic liquid from hydrochloric acid medium. Sep. Purif. Technol. 2021, 276, 119312. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Q.; Lu, W.; Ru, M.; Yang, Y. Extraction and stripping of platinum (IV) from acidic chloride media using guanidinium ionic liquid. J. Mol. Liq. 2019, 293, 111040. [Google Scholar] [CrossRef]
- Zarrougui, R.; Mdimagh, R.; Raouafi, N. Highly efficient and eco-friendly extraction of neodymium using, undiluted and non-fluorinated ionic liquids. Direct electrochemical metal separation. Sep. Purif. Technol. 2017, 175, 87–98. [Google Scholar] [CrossRef]
- Zarrougui, R.; Mdimagh, R.; Raouafi, N. Highly efficient extraction and selective separation of uranium (VI) from transition metals using new class of undiluted ionic liquids based on H-phosphonate anions. J. Hazard. Mater. 2018, 342, 464–476. [Google Scholar] [CrossRef]
- Mori, T.; Takao, K.; Sasaki, K.; Suzuki, T.; Arai, T.; Ikeda, Y. Homogeneous liquid-liquid extraction of U(VI) from HNO3 aqueous solution to betainium bis(trifluoromethylsulfonyl)imide ionic liquid and recovery of extracted U(VI). Sep. Purif. Technol. 2015, 155, 133–138. [Google Scholar] [CrossRef]
- Onghena, B.; Binnemans, K. Recovery of Scandium (III) from Aqueous Solutions by Solvent Extraction with the Functionalized Ionic Liquid Betanium Bis(trifluoromethylsulfonyl)imide. Ind. Eng. Chem. Res. 2015, 54, 1887–1898. [Google Scholar] [CrossRef] [Green Version]
- Volia, M.; Tereshatov, E.; Boltoeva, M.; Folden, C., III. Indium and thallium extraction into betainium bis(trifluoromethylsulfonyl)imide ionic liquid from aqueous hydrochloric acid media. New J. Chem. 2020, 44, 2527–2537. [Google Scholar] [CrossRef]
- Micheau, C.; Arrachart, G.; Turgis, R.; Lejeune, M.; Draye, M.; Michel, S.; Legeai, S.; Pellet-Rostaing, S. Ionic liquids as extraction media in a two-step eco-friendly process for selective tantalum recovery. ACS Sustain. Chem. Eng. 2020, 8, 1954–1963. [Google Scholar] [CrossRef]
- Zhou, C.; Yong, L.; Xue, X. Extraction mechanism of vanadium from vanadium slag leaching solution by N-Octylpyridine ionic liquids. Chin. J. Nonferrous Met. 2020, 30, 172–179. [Google Scholar] [CrossRef]
- Cholico-Gonzalez, D.; Chagnes, A.; Cote, G.; Avila-Rodriguez, M. Separation of Co (II) and Ni (II) from aqueous solutions by bis (2, 4, 4-trimethylpentyl) phosphinic acid (Cyanex 272) using trihexyl (tetradecyl) phosphonium chloride (Cyphos IL 101) as solvent. J. Mol. Liq. 2015, 209, 203–208. [Google Scholar] [CrossRef]
- Song, Y.; Tsuchida, Y.; Matsumiya, M.; Uchino, Y.; Yanagi, I. Separation of tungsten and cobalt from WC-Co hard metal wastes using ion-exchange and solvent extraction with ionic liquid. Miner. Eng. 2018, 128, 224–229. [Google Scholar] [CrossRef]
- Matsumiya, M.; Song, Y.; Tsuchida, Y.; Ota, H.; Tsunashima, K. Recovery of platinum by solvent extraction and direct electrodeposition using ionic liquid. Sep. Purif. Technol. 2019, 214, 162–167. [Google Scholar] [CrossRef]
- Sepúlveda, R.; Castillo, J.; Plaza, A.; Sanchez, J.; Torres, A.; Romero, J. Improvement of recovery performance in the solvent extraction of Cu (II) using [bmim][Tf2N] and a β-diketone as extractant and its stripping with supercritical carbon dioxide. J. Supercrit. Fluids 2017, 128, 26–31. [Google Scholar] [CrossRef]
- Takahashi, T.; Ito, T.; Kim, S.Y. Extraction behavior of Sr (II) from high-level liquid waste using ionic liquid extraction system with DtBuCH18C6. Energy Procedia 2017, 131, 170–177. [Google Scholar] [CrossRef]
- Lertlapwasin, R.; Bhawawet, N.; Imyim, A.; Fuangswasdi, S. Ionic liquid extraction of heavy metal ions by 2-aminothiophenol in 1-butyl-3-methylimidazolium hexafluorophosphate and their association constants. Sep. Purif. Technol. 2010, 72, 70–76. [Google Scholar] [CrossRef]
- Quijada-Maldonado, E.; Allaina, A.; Péreza, B.; Merleta, G.; Cabezasa, R.; Tapiab, R.; Romero, J. Selective liquid-liquid extraction of molybdenum (VI) and rhenium (VII) from a synthetic pregnant leach solution: Comparison between extractants and diluents. Miner. Eng. 2020, 145, 106060. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Wei, Z.; Zhou, Y.; Zhang, M.; Hongguo, H.; Tian, G.; Hui, H. Extraction behavior and third phase formation of neodymium (III) from nitric acid medium in N, N′-dimethyl-N, N′-dioctyl-3-oxadiglcolamide. J. Radioanal. Nucl. Chem. 2018, 318, 2087–2096. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, C.; Hu, Y.; Liu, Y.; Zhou, Y.; Jiao, C.; Zhang, M.; Hou, H.; Gao, Y.; Tian, G. Extraction behavior of several lanthanides from nitric acid with DMDODGA in [C 4 mim] [NTf 2] ionic liquid. J. Radioanal. Nucl. Chem. 2021, 327, 565–573. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.; Yuan, J.; Pu, N.; Wei, P.; Sun, T.; Shi, W.; Chen, J.; Wang, J.; Xu, C. Performance and mechanism for the selective separation of trivalent americium from lanthanides by a tetradentate phenanthroline ligand in ionic liquid. Inorg. Chem. 2020, 59, 3905–3911. [Google Scholar] [CrossRef]
- Xiao, C.L.; Wang, C.-Z.; Yuan, L.-Y.; Li, B.; He, H.; Wang, S.; Zhao, Y.-L.; Chai, Z.-F.; Shi, W.-Q. Excellent Selectivity for Actinides with a Tetradentate 2,9-Diamide-1,10-Phenanthroline Ligand in Highly Acidic Solution: A Hard-Soft Donor Combined Strategy. Inorg. Chem. 2014, 53, 1712–1720. [Google Scholar] [CrossRef]
- Zhang, Y.; Kogelnig, D.; Morgenbesser, C.; Stojanovic, A.; Jirsa, F.; Lichtscheidl-Schultz, I.; Krachler, R.; Li, Y.; Keppler, B.K. Preparation and characterization of immobilized [A336] [MTBA] in PVA–alginate gel beads as novel solid-phase extractants for an efficient recovery of Hg (II) from aqueous solutions. J. Hazard. Mat. 2011, 196, 201–209. [Google Scholar] [CrossRef]
- Ciopec, M.; Gabor, A.; Davidescu, C.M.; Negrea, A.; Negrea, P.; Duteanu, N. Eu (III) removal by tetrabutylammonium di-hydrogen phosphate (TBAH2P) functionalized polymers. Arab. J. Chem. 2020, 13, 3534–3545. [Google Scholar] [CrossRef]
- Dautoo, U.K.; Yashwant, S.; Sunita, R.; Shivani, J.; Rohini, D.; Chauhan, G.S. New crosslinked poly (ionic liquids) networks as As (V) extractants. J. Environ. Chem. Eng. 2019, 7, 103154. [Google Scholar] [CrossRef]
- Mokhodoeva, O.; Shkinev, V.; Maksimova, V.; Dzhenloda, R.; Spivakov, B. Recovery of platinum group metals using magnetic nanoparticles modified with ionic liquids. Sep. Purif. Technol. 2020, 248, 117049. [Google Scholar] [CrossRef]
- Ansari, S.A.; Mohapatra, P.K. A review on solid phase extraction of actinides and lanthanides with amide based extractants. J. Chromatogr. A 2017, 1499, 1–20. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yudaev, P.A.; Chistyakov, E.M. Ionic Liquids as Components of Systems for Metal Extraction. ChemEngineering 2022, 6, 6. https://doi.org/10.3390/chemengineering6010006
Yudaev PA, Chistyakov EM. Ionic Liquids as Components of Systems for Metal Extraction. ChemEngineering. 2022; 6(1):6. https://doi.org/10.3390/chemengineering6010006
Chicago/Turabian StyleYudaev, Pavel A., and Evgeniy M. Chistyakov. 2022. "Ionic Liquids as Components of Systems for Metal Extraction" ChemEngineering 6, no. 1: 6. https://doi.org/10.3390/chemengineering6010006
APA StyleYudaev, P. A., & Chistyakov, E. M. (2022). Ionic Liquids as Components of Systems for Metal Extraction. ChemEngineering, 6(1), 6. https://doi.org/10.3390/chemengineering6010006