Experimental Study on Absorption Behavior and Efficiency of Brine in Hazardous Gas Absorption Treatment
Abstract
1. Introduction
2. Research Purpose and Methodology
2.1. Experiment on Hazardous Gas Absorption Using Brine as an Absorbent
2.1.1. Experimental Device
2.1.2. Experimental Conditions and Methods
2.2. Verification and Expanded Application by Using ASPEN PLUS
2.2.1. Verification of Brine Gas Absorption Test Results
2.2.2. Expanded Application to Major Hazardous Gases by Using ASPEN PLUS
3. Research Results
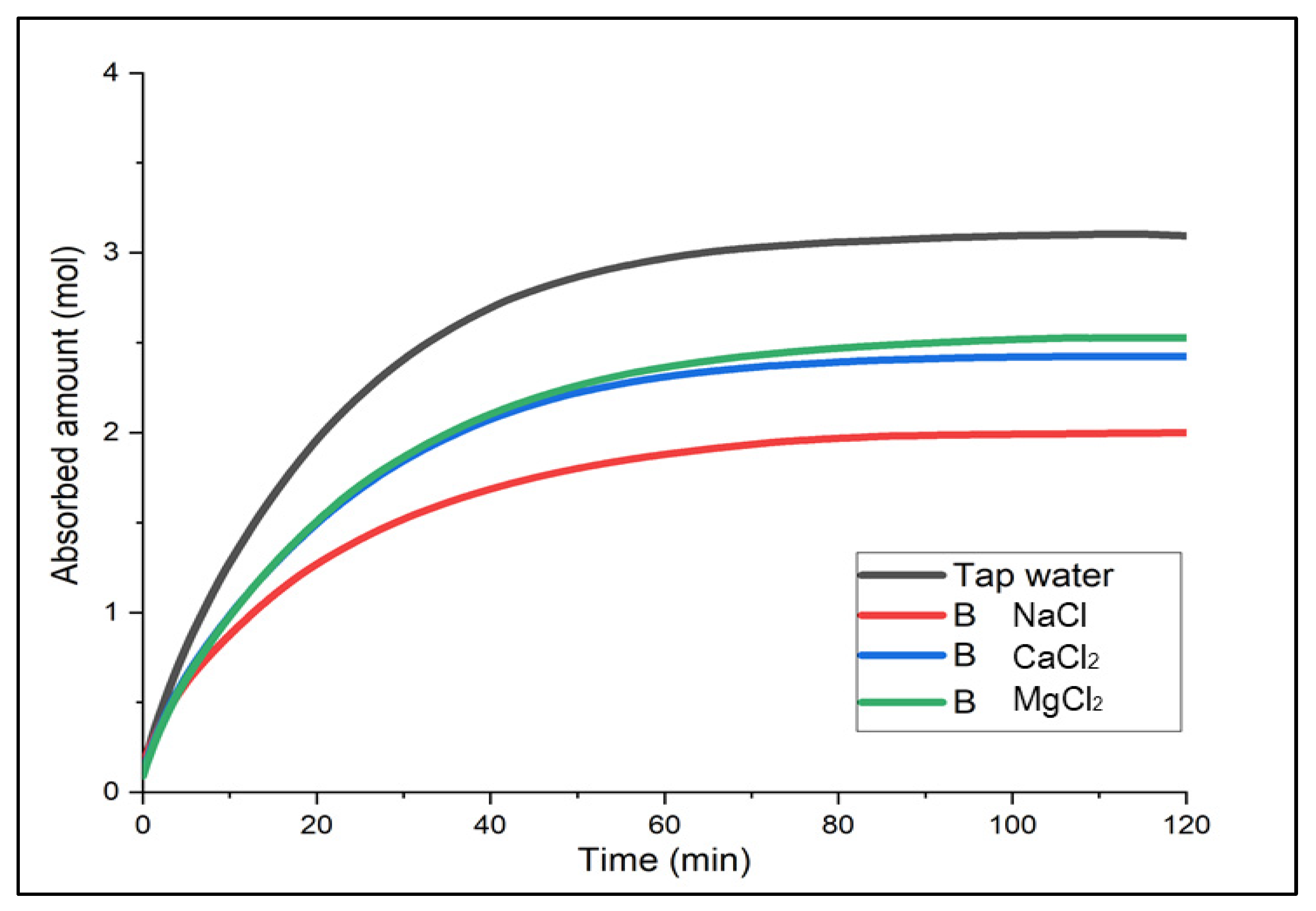
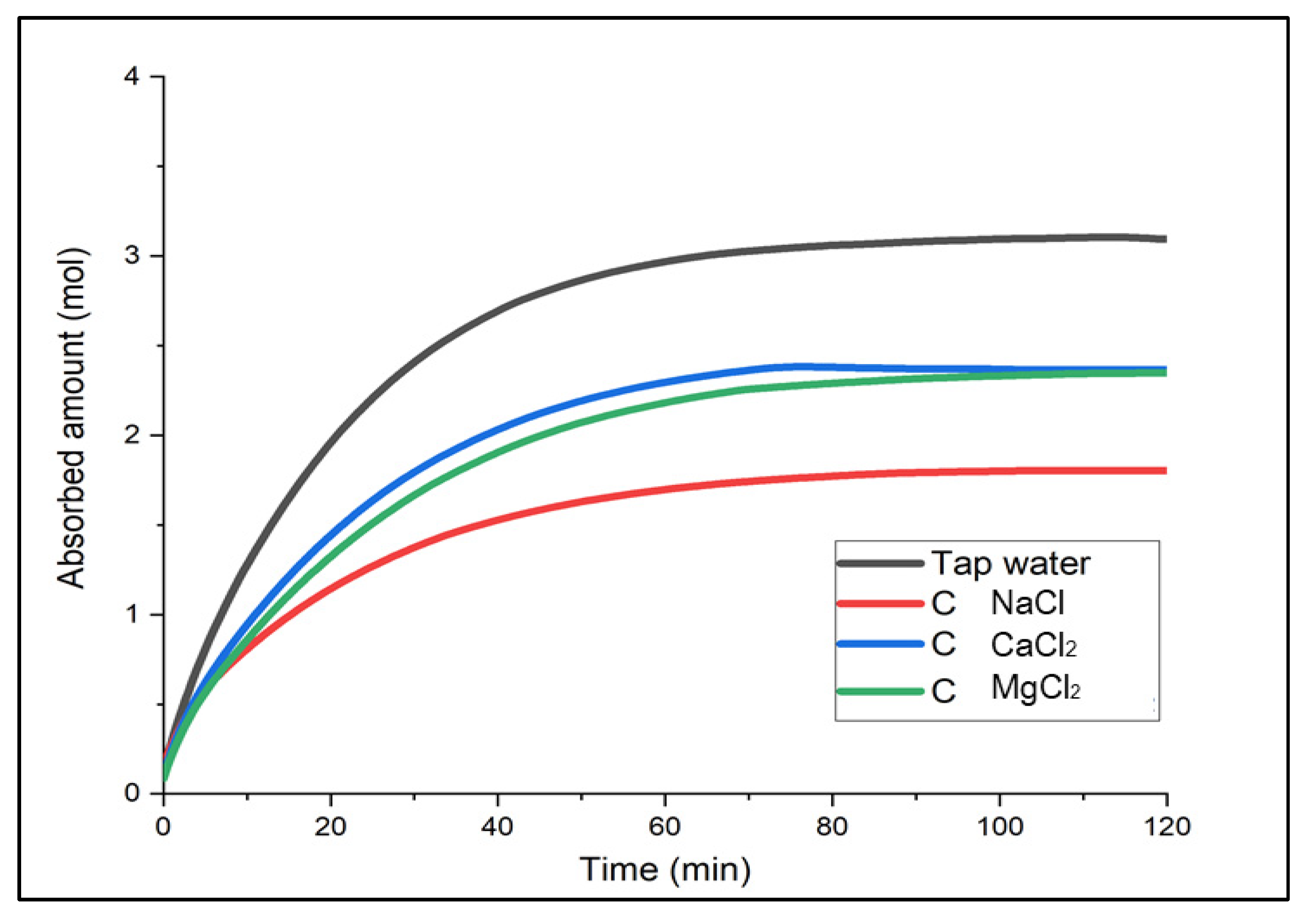
3.1. Experiment on Hazardous Gas Absorption Using Brine as an Absorbent
Determination of the Optimal Brine
3.2. Verification and Expanded Application by Using ASPEN PLUS
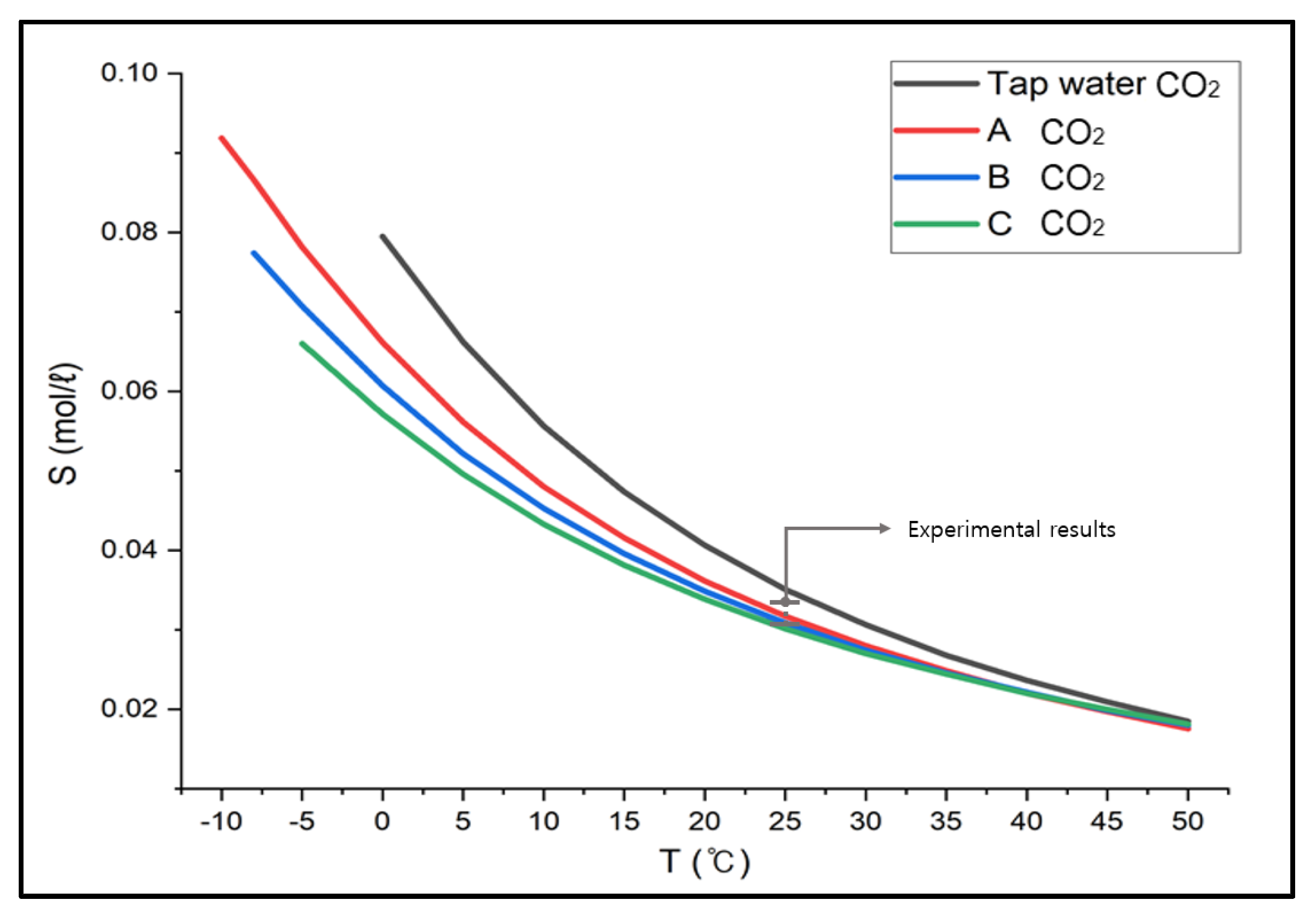
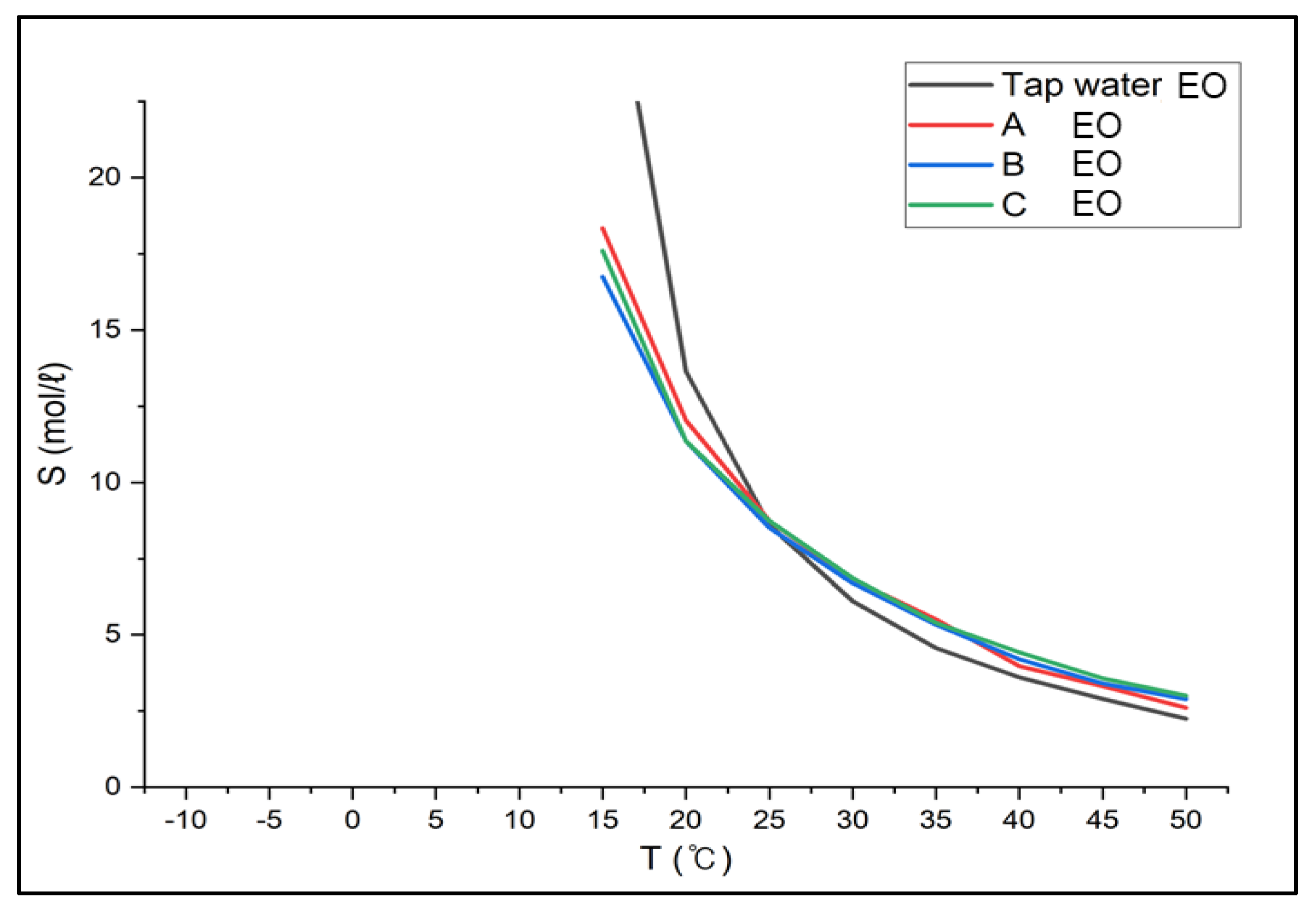
3.2.1. Verification of Brine Gas Absorption Test Results
3.2.2. Expanded Application to Major Hazardous Gases by Using ASPEN PLUS
4. Conclusions and Discussion
- When comparing the three different brines after varying the concentration required at different temperatures (−5 °C, −8 °C and −10 °C), CaCl2 brine was considered the most practical in terms of performance, affordability, and accessibility.
- When CaCl2 brine was used as an absorbent, the solubility of carbon dioxide decreased by about 25%, ammonia by about 20%, ethylene oxide by about 1%, and methylamine by about 33% compared to the solubility in tap water. However, since the gas solubility indicated higher values at low temperatures, no effort would be needed to increase the solubility.
- If the absorbent for bubble columns were substituted with brine (for carbon dioxide between 10 and 15 °C, for ammonia between 13 and 15 °C, for methylamine between 16 and 19 °C, and not required for ethylene oxide) at the temperatures about 15 °C, the bubble columns could operate stably and effectively without deterioration of the absorption efficiency.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lim, D.H.; Yoo, D.J.; Kang, Y. Characteristics of Gas-liquid Mass Transfer and Interfacial Area in a Bubble Column. Korean Chem. Eng. Res. 2015, 53, 315–320. [Google Scholar] [CrossRef][Green Version]
- Sastaravet, P.; Chuenchaem, C.; Thaphet, N.; Chawaloesphonsiya, N.; Painmanakul, P. Comparative Study of Mass Transfer and Bubble Hydrodynamic Parameters in Bubble Column Reactor: Physical Configurations and Operating Conditions. Environ. Eng. Res. 2014, 19, 345–354. [Google Scholar] [CrossRef]
- Lim, K. A Study on Absorption Treatment Method of Hazardous Gas Using Bubble Column. Master’s Thesis, Chonnam National University, Gwangju, Korea, 2021. [Google Scholar]
- Chen, P.-C. Absorption of Carbon Dioxide in a Bubble-Column Scrubber. In Greenhouse Gases—Capturing, Utilization and Reduction; Li, G., Ed.; Intech: Rijeka, Croatia, 2012; pp. 95–116. [Google Scholar] [CrossRef][Green Version]
- Onda, K.; Sada, E.; Kobayashi, T.; Kito, S.; Ito, K. Salting-out parameters of gas solubility in aqueous salt solutions. J. Chem. Eng. Jpn. 1970, 3, 18–24. [Google Scholar] [CrossRef]
- Yasunishi, A.; Yoshida, F. Solubility of carbon dioxide in aqueous electrolyte solutions. J. Chem. Eng. Data 1979, 24, 11–14. [Google Scholar] [CrossRef]
- Steel, L.; Liu, Q.; Mackay, E.; Maroto-Valer, M.M. CO2 solubility measurements in brine under reservoir conditions: A comparison of experimental and geochemical modeling methods. Greenh. Gases Sci. Technol. 2016, 6, 197–217. [Google Scholar] [CrossRef]
- Jung, G.Y. A Study on the Absorption Behavior and Efficiency in the Use of Brine for Absorption Treatment of Hazardous Gas. Master’s Thesis, Chonnam National University, Gwangju, Korea, 2021. [Google Scholar]
- Hefter, G.T.; Tomkins, R.P.T. The Experimental Determination of Solubilities; John Wiley Sons Inc.: Hoboken, NJ, USA, 2016; pp. 32–33. [Google Scholar]
- Liu, Y.; Hou, M.; Yang, G.; Han, B. Solubility of CO2 in aqueous solutions of NaCl, KCl, CaCl2 and their mixed salts at different temperatures and pressures. J. Supercrit. Fluids 2011, 56, 125–129. [Google Scholar] [CrossRef]
- Wilkinson, P.M.; Spek, A.P.; van Dierendonck, L.L. Design parameters estimation for scale-up of high-pressure bubble columns. AIChE J. 1992, 38, 544–554. [Google Scholar] [CrossRef]
- Besagni, G.; Gallazzini, L.; Inzoli, F. On the scale-up criteria for bubble columns. Petroleum 2017, 5, 114–122. [Google Scholar] [CrossRef]
- Jung, G.Y.; Lim, K.M.; Ma, B.C. Optimal design standard and application of low cost, high performance scrubber for absorbing hazardous gas. J. Korean Inst. Gas 2021, 25, 39–45. [Google Scholar] [CrossRef]
- Chang, R.; Goldsby, K.A. Chemistry, 11th ed.; McGraw-Hill: New York, NY, USA, 2014; pp. 500–515. [Google Scholar]
- Atkins, P.; de Paula, J. Elements of Physical Chemistry, 5th ed.; W.H.Freeman: New York, NY, USA, 2009; pp. 216–218. [Google Scholar]
- Gavrilescu, M.; Cozma, P.; Wukovits, W.; Mamaliga, I.; Friedl, A. Analysis and modelling of the solubility of biogas components in water for physical absorption processes. Environ. Eng. Manag. J. 2013, 12, 147–162. [Google Scholar] [CrossRef]
- Ministry of Environment, National Institute of Chemical Safety (NICS). Key Info Guide for Accident Preparedness Substances; NICS-GP2016-2; National Institute of Chemical Safety (NICS): Cheongju, Korea, 2017.
- Battino, R. The Ostwald coefficient of gas solubility. Fluid Phase Equilibria 1984, 15, 231–240. [Google Scholar] [CrossRef]
- Huerta-Diaz, M.A.; Rodriguez, S. Solubility measurements and determination of Setschenow constants for the pesticide carbaryl in seawater and other electrolyte solutions. Can. J. Chem. 1992, 70, 2864–2868. [Google Scholar] [CrossRef]
- Bastami, A.; Allahgholi, M.; Pourafshary, P. Experimental and modelling study of the solubility of CO2 in various CaCl2 solutions at different temperatures and pressures. Pet. Sci. 2014, 11, 569–577. [Google Scholar] [CrossRef]
- Liu, L.; Yan, H.; Zhao, G.; Zhuang, J. Experimental studies on the terminal velocity of air bubbles in water and glycerol aqueous solution. Exp. Therm. Fluid Sci. 2016, 78, 254–265. [Google Scholar] [CrossRef]
- McCabe, W.L.; Smith, J.C.; Harriott, P. Unit Operations of Chemical Engineering, 7th ed.; Mc Graw-Hill: New York, NY, USA, 2015; pp. 441–512. [Google Scholar]




| Substance | Solution | Temperature (°C) | Molarity (M) |
|---|---|---|---|
| NaCl | A | −5 | 1.30 |
| B | −8 | 2.06 | |
| C | −10 | 2.55 | |
| CaCl2 | A | −5 | 0.87 |
| B | −8 | 1.38 | |
| C | −10 | 1.71 | |
| MgCl2 | A | −5 | 0.88 |
| B | −8 | 1.38 | |
| C | −10 | 1.71 |
| Experiment No. | Condition (25 °C, 1 atm) | ||
|---|---|---|---|
| Experiment set (10 set) | Experiment 1 | Tap water | |
| Experiment 2 | NaCl | Solution A | |
| Experiment 3 | Solution B | ||
| Experiment 4 | Solution C | ||
| Experiment 5 | CaCl2 | Solution A | |
| Experiment 6 | Solution B | ||
| Experiment 7 | Solution C | ||
| Experiment 8 | MgCl2 | Solution A | |
| Experiment 9 | Solution B | ||
| Experiment 10 | Solution C | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, G.-y.; Lee, S.-g.; Lee, J.-s.; Ma, B.-c. Experimental Study on Absorption Behavior and Efficiency of Brine in Hazardous Gas Absorption Treatment. ChemEngineering 2022, 6, 4. https://doi.org/10.3390/chemengineering6010004
Jung G-y, Lee S-g, Lee J-s, Ma B-c. Experimental Study on Absorption Behavior and Efficiency of Brine in Hazardous Gas Absorption Treatment. ChemEngineering. 2022; 6(1):4. https://doi.org/10.3390/chemengineering6010004
Chicago/Turabian StyleJung, Ga-young, Seul-gi Lee, Jun-seo Lee, and Byung-chol Ma. 2022. "Experimental Study on Absorption Behavior and Efficiency of Brine in Hazardous Gas Absorption Treatment" ChemEngineering 6, no. 1: 4. https://doi.org/10.3390/chemengineering6010004
APA StyleJung, G.-y., Lee, S.-g., Lee, J.-s., & Ma, B.-c. (2022). Experimental Study on Absorption Behavior and Efficiency of Brine in Hazardous Gas Absorption Treatment. ChemEngineering, 6(1), 4. https://doi.org/10.3390/chemengineering6010004






