Amino Ethers of Ortho-Phosphoric Acid as Extragents for Ethanol Dehydration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Process
2.3. Preparation of Aqueous Solutions and Mixtures
2.4. Phase Equilibrium Experiments
2.5. Viscosity and Density Measurements
3. Results
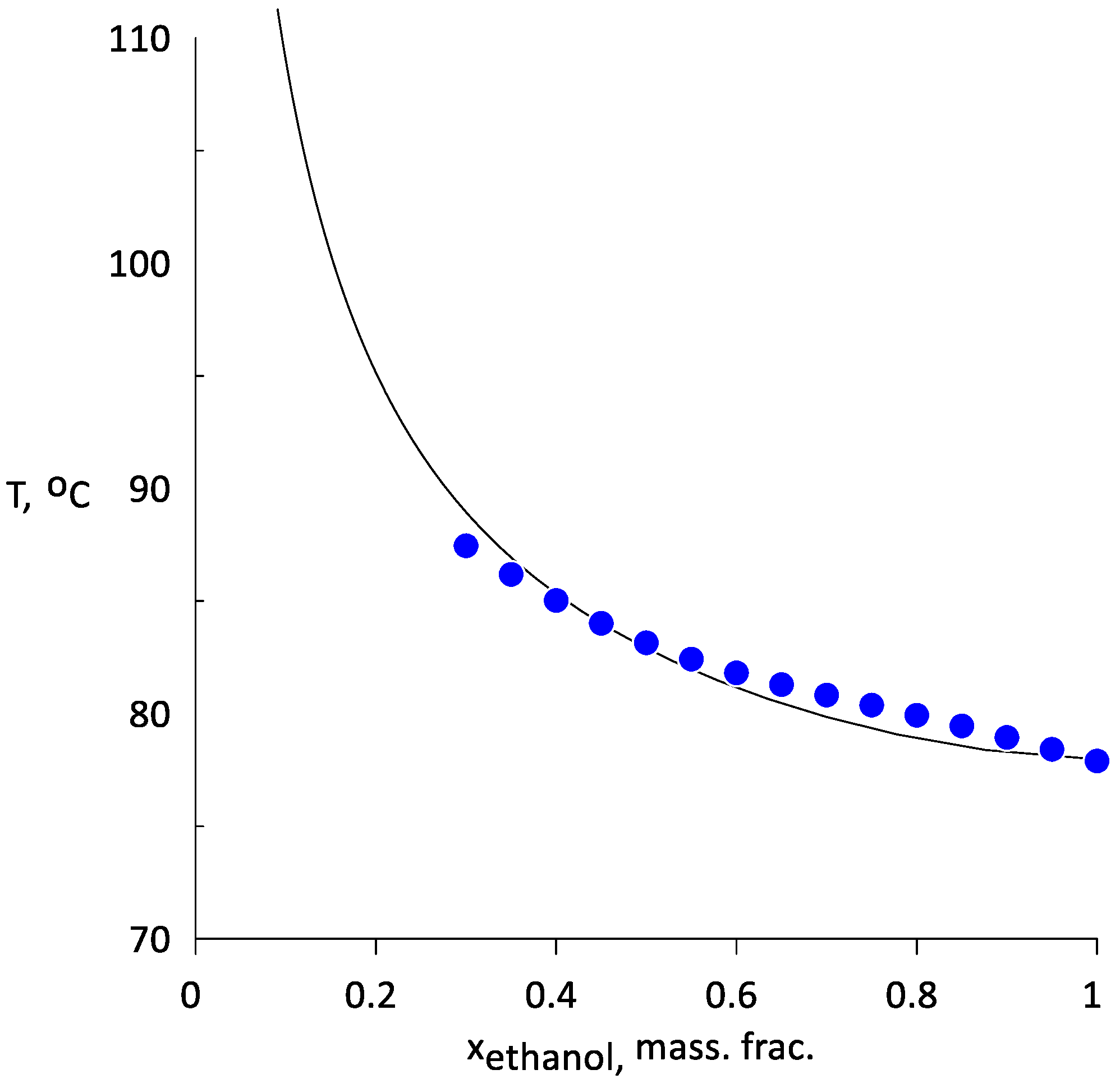
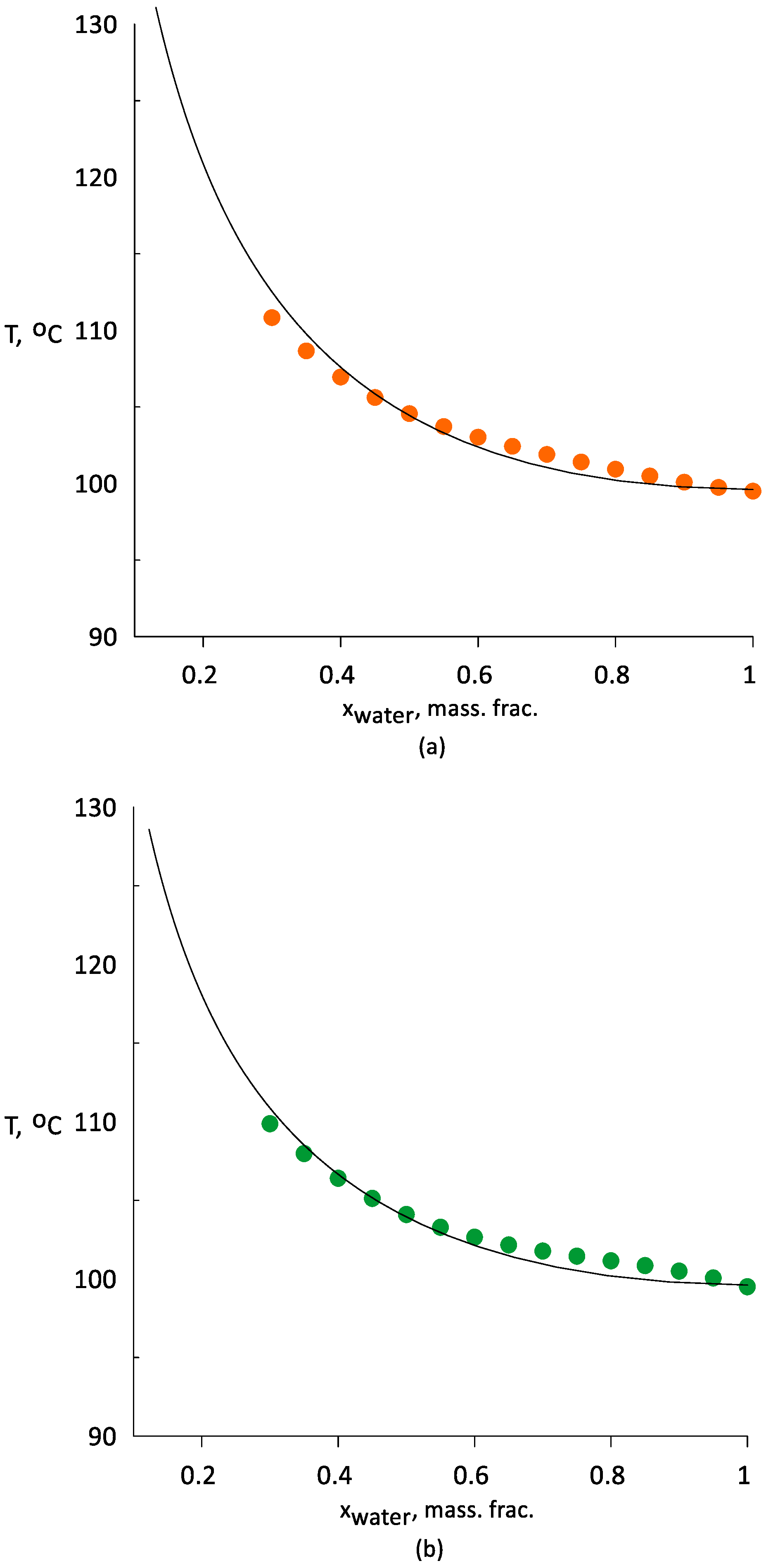
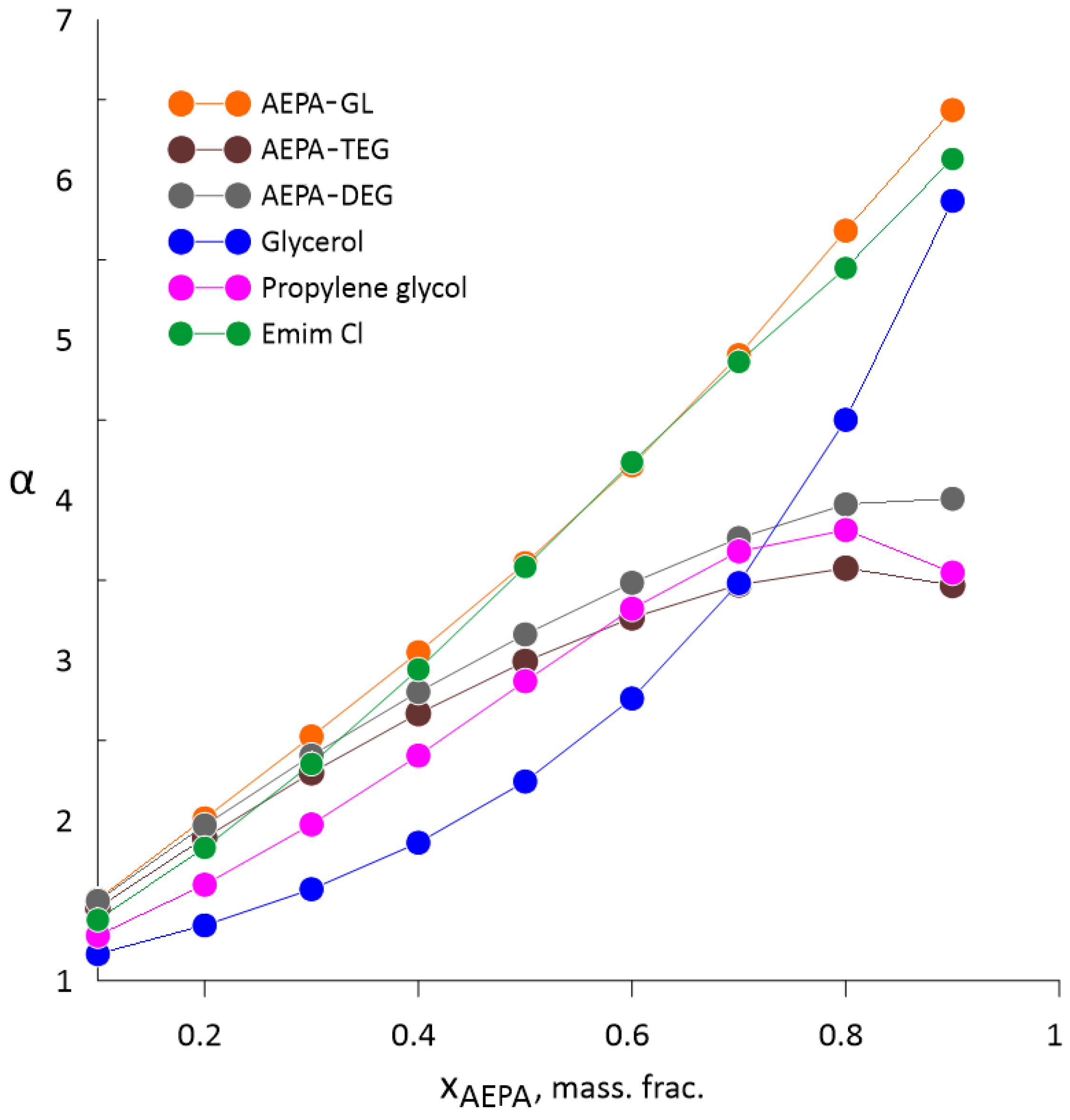
3.1. Vapor–Liquid Equilibrium of Water–Ethanol Mixtures with AEPA-(PEG/TEG/DEG/GI)
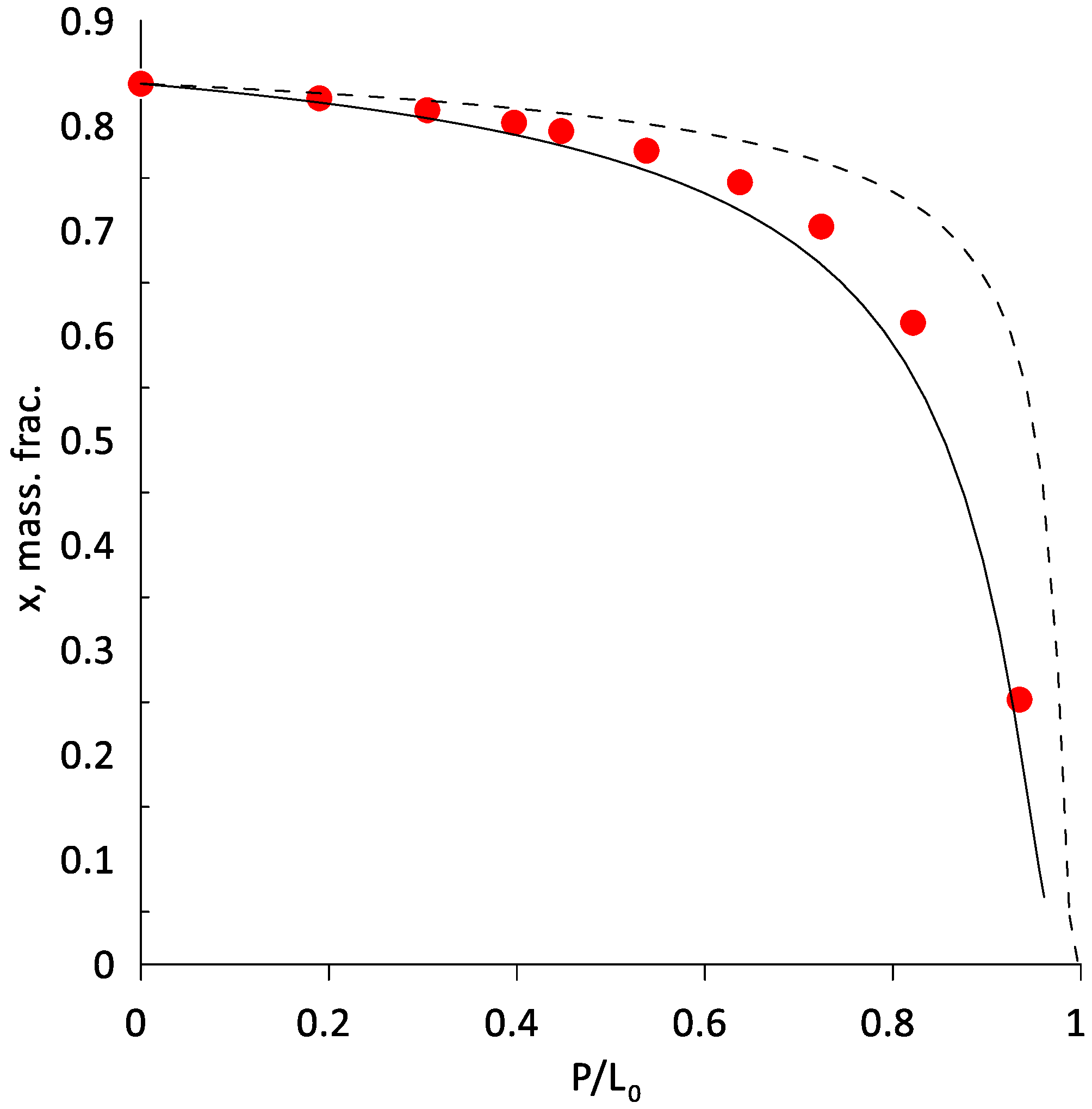
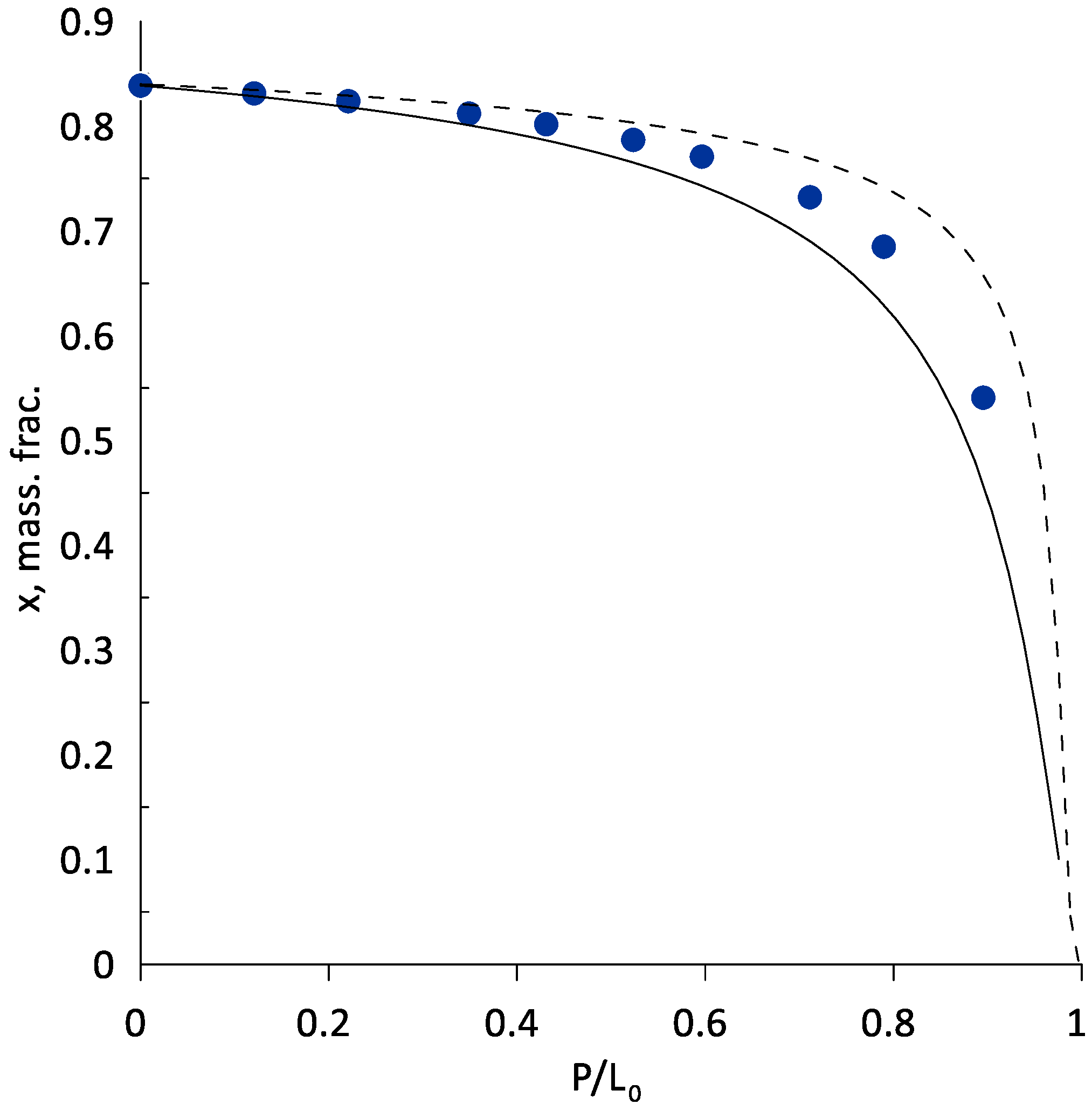
3.2. Extractive Distillation of the Ethanol–Water Mixture Using AEPA-Gl
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chianese, A.; Zinnamosca, F. Ethanol Dehydration by Azeotropic Distillation with Mixed Solvent Entrainer. Chem. Eng. J. 1990, 43, 59–65. [Google Scholar] [CrossRef]
- Gomis, V.; Pedraza, R.; Frances, O.; Font, A.; Asensi, J. Dehydration of Ethanol Using Azeotropic Distillation with Isooctane. Ind. Eng. Chem. Res. 2007, 46, 4572–4576. [Google Scholar] [CrossRef]
- Barba, D.; Brandani, V.; Giacomo, G.D. Hyperazeotropic Ethanol Salted-Out by Extractive Distillation Theorical Evaluation and Experimental Check. Chem. Eng. Sci. 1985, 40, 2287–2292. [Google Scholar] [CrossRef]
- Granjo, J.F.O.; Nunes, D.S.; Duarte, B.P.M.; Oliveira, N.M.C. A Comparison of Process Alternatives for Energy-Efficient Bioethanol Downstream Processing. Sep. Purif. Technol. 2020, 238, 116414. [Google Scholar] [CrossRef]
- Meirelles, A.; Weiss, S.; Herfurth, H. Ethanol Dehydration by Extractive Distillation. J. Chem. Tech. Biotechnol. 1992, 53, 181–188. [Google Scholar] [CrossRef]
- Fu, J. Simulation of Salt-Containing Extractive Distillation for the System of Ethanol/Water/Ethanediol/KAc. 2. Simulation of Salt-Containing Extractive Distillation. Ind. Eng. Chem. Res. 2004, 43, 1279–1283. [Google Scholar] [CrossRef]
- Furter, W.F. Extractive Distillation by Salt Effect. Chem. Eng. Commun. 1992, 116, 35–40. [Google Scholar] [CrossRef]
- Pinto, R.T.P.; Wolf-Maciel, M.R.; Lintomen, L. Saline Extractive Distillation Process for Ethanol Purification. Comput. Chem. Eng. 2000, 24, 1689–1694. [Google Scholar] [CrossRef]
- Pan, Q.; Shang, X.; Sun, L. Energy-Efficient Separation Process and Control Scheme for Extractive Distillation of Ethanol-Water Using Deep Eutectic Solvent. Sep. Purif. Technol. 2019, 219, 113–126. [Google Scholar] [CrossRef]
- Duan, C.; Li, C. Novel Energy-Saving Methods to Improve the Three-Column Extractive Distillation Process for Separating Ethyl Acetate and Ethanol Using Furfural. Sep. Purif. Technol. 2021, 272, 118887. [Google Scholar] [CrossRef]
- Delgado, J.A.; Agueda, V.I.; Uguina, M.A.; Sotelo, J.L.; Garcia-Sanz, A.; Garcia, A. Separation of Ethanol-Water Liquid Mixtures by Adsorption on BPL Activated Carbon with Air Regeneration. Sep. Purif. Technol. 2015, 149, 370–380. [Google Scholar] [CrossRef]
- Morales, J.Y.R.; Lopez, G.L.; Martinez, V.M.A.; Vazquez, F.J.S.; Mendoza, J.A.B.; Garcia, M.M. Parametric Study and Control of a Pressure Swing Adsorption Process to Separate the Water-Ethanol Mixture under Disturbances. Sep. Purif. Technol. 2019, 236, 116214. [Google Scholar] [CrossRef]
- Jacques, K.; Lyons, T.P.; Kelsall, D.R. Alcohol Textbook; Nottingham University Press: Nottingham, UK, 2003; p. 446. [Google Scholar]
- Liu, G.; Jin, W. Pervaporation Membrane Materials: Recent Trends and Perspectives. J. Membr. Sci. 2021, 636, 119557. [Google Scholar] [CrossRef]
- Ulrich, S.; Pavel, S. Design and Operation of a Pervaporation Plant for Ethanol Dehydration. J. Membr. Sci. 1988, 36, 463–475. [Google Scholar]
- Wu, Y.; Ding, L.; Lu, Z.; Deng, J.; Wei, Y. Two-Dimensional MXene Membrane for Ethanol Dehydration. J. Membr. Sci. 2019, 590, 117300. [Google Scholar] [CrossRef]
- Xu, C.; Zhou, C.; Wang, S.; Huang, A. Copper-Exchanged LTA Zeolite Membranes with Enhanced Water Flux for Ethanol Dehydration. Chin. Chem. Lett. 2019, 30, 1204–1206. [Google Scholar] [CrossRef]
- Jianga, H.; Shic, W.; Liua, Q.; Wang, H.; Lic, J.; Wua, C.; Li, Y.; Weia, Z. Intensification of Water/Ethanol Separation by PVA Hybrid Membrane with Different Functional Ligand UiO-66-X Nanochannels in Pervaporation Process. Sep. Purif. Technol. 2020, 256, 117802. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Tsuru, T. Pervaporation of Water/Ethanol Mixtures through Microporous Silica Membranes. Sep. Purif. Technol. 2009, 66, 479–485. [Google Scholar] [CrossRef]
- Hietaharju, J.; Kangas, J.; Tanskanen, J. Analysis of the Permeation Behavior of Ethanol/Water Mixtures through a Polydimethylsiloxane (PDMS) Membrane in Pervaporation and Vapor Permeation Conditions. Sep. Purif. Technol. 2019, 227, 115738. [Google Scholar] [CrossRef]
- Kiss, A.A.; Suszwalak, D.J.-P.C. Enhanced Bioethanol Dehydration by Extractive and Azeotropic Distillation in Dividing-Wall Columns. Sep. Purif. Technol. 2012, 86, 70–78. [Google Scholar] [CrossRef]
- Ma, S.; Hou, Y.; Sun, Y.; Li, J.; Li, Y.; Sun, L. Simulation and Experiment for Ethanol Dehydration Using Low Transition Temperature Mixtures (LTTMs) as Entrainers. Chem. Eng. Process. Process. Intensif. 2017, 121, 71–80. [Google Scholar] [CrossRef]
- Vazquez-Ojeda, M.; Segovia-Hernandez, J.G.; Hernandez, S.; Hernandez-Aguirre, A.; Kiss, A.A. Design and Optimization of an Ethanol Dehydration Process Using Stochastic Methods. Sep. Purif. Technol. 2013, 105, 90–97. [Google Scholar] [CrossRef]
- Ravagnania, M.A.S.S.; Reisa, M.H.M.; Filho, R.M.; Wolf-Maciel, M.R. Anhydrous Ethanol Production by Extractive Distillation: A Solvent Case Study. Process. Saf. Environ. Prot. 2010, 88, 67–73. [Google Scholar] [CrossRef]
- Gil, I.D.; Gomez, J.M.; Rodriguez, G. Control of an Extractive Distillation Process to Dehydrate Ethanol Using Glycerol as Entrainer. Comput. Chem. Eng. 2012, 39, 129–142. [Google Scholar] [CrossRef]
- Lee, F.M.; Pahl, R.H. Solvent Screening Study and Conceptual Extractive Distillation Process to Produce Anhydrous Ethanol from Fermentation Broth. Ind. Eng. Chem. Process. Des. Dev. 1985, 24, 168–172. [Google Scholar] [CrossRef]
- Gu, Y.; Jerome, F. Glycerol as a Sustainable Solvent for Green Chemistry. Green Chem. 2010, 12, 1127–1138. [Google Scholar] [CrossRef]
- Yeh, A.I.; Berg, L. The Dehydration of Ethanol by Extractive Distillation. Chem. Eng. Commun. 1992, 113, 147–153. [Google Scholar] [CrossRef]
- Soares, R.B.; Pessoa, F.; Marisa, M. Dehydration of Ethanol with Different Salts in a Packed Distillation Column. Process. Saf. Environ. Prot. 2014, 93, 147–153. [Google Scholar] [CrossRef]
- Lei, Z.; Dai, C.; Zhu, J.; Chen, B. Extractive Distillation with Ionic Liquids: A Review. AIChE J. 2014, 60, 3312–3329. [Google Scholar] [CrossRef]
- Zhua, Z.; Riab, Y.; Lia, M.; Jiaa, H.; Wanga, Y.; Wanga, Y. Extractive Distillation for Ethanol Dehydration Using Imidazolium-Based Ionic Liquids as Solvents. Chem. Eng. Process.-Process. Intensif. 2016, 109, 190–198. [Google Scholar] [CrossRef]
- Meindersma, G.W.; Quijada-Maldonado, E.; Aelmans, T.A.M.; Gutierrez, J.P.; Haan, A.B. Ionic Liquids in Extractive Distillation of Ethanol/Water: From Laboratory to Pilot Plant. ACS Symp. Ser. 2012, 1117, 239–257. [Google Scholar]
- Graczova, E.; Steltenpohl, P. Application of Ionic Liquids in Extractive Distillation of Ethanol-Water System. Chem. Eng. Trans. 2015, 45, 1957–1962. [Google Scholar]
- Quijada-Maldonadoa, E.; Meindersm, G.W.; Haanc, A.B. Ionic Liquid Effects on Mass Transfer Efficiency in Extractive Distillation of Water-Ethanol Mixtures. Comput. Chem. Eng. 2014, 71, 210–219. [Google Scholar] [CrossRef]
- Yao, C.; Hou, Y.; Ren, S.; Wu, W.; Liu, H. Selective Extraction of Aromatics from Aliphatics Using Dicationic Ionic Liquid-Solvent Composite Extractants. J. Mol. Liq. 2019, 291, 111267. [Google Scholar] [CrossRef]
- Gjineci, N.; Boli, E.; Tzani, A.; Detsi, A.; Voutsas, E. Separation of the Ethanol/Water Azeotropic Mixture Using Ionic Liquids and Deep Eutectic Solvents. Fluid Phase Equilibria 2016, 424, 1–7. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Klinov, A.V.; Khairullina, A.R.; Malygin, A.V.; Dulmaev, S.E.; Davletbaeva, A.R.; Mukhametzyanov, T.A. Organoboron Ionic Liquids as Extractants for Distillation Process of Binary Ethanol + Water Mixtures. Processes 2020, 8, 628. [Google Scholar] [CrossRef]
- Klinov, A.V.; Malygin, A.V.; Khairullina, A.R.; Dulmaev, S.E.; Davletbaeva, I.M. Alcohols Dehydration by Extractive Distillation with Use of Aminoethers of Boric Acid. Processes 2020, 8, 1466. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Sazonov, O.O.; Zakirov, I.N.; Gumerov, A.M.; Klinov, A.V.; Fazlyev, A.R.; Malygin, A.V. Organophosphorus Polyurethane Ionomers as Water Vapor Permeable and Pervaporation Membranes. Polymers 2021, 13, 1442. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Sazonov, O.O.; Fazlyev, A.R.; Davletbaev, R.S.; Efimov, S.V.; Klochkov, V.V. Polyurethane Ionomers Based on Amino Ethers of Orto-Phosphoric Acid. RSC Adv. 2019, 9, 18599–18608. [Google Scholar] [CrossRef] [Green Version]
- Davletbaeva, I.M.; Sazonov, O.O.; Fazlyev, A.R.; Zakirov, I.N.; Davletbaev, R.S.; Efimov, S.V.; Klochkov, V.V. Thermal Behavior of Polyurethane Ionomers Based on Amino Ethers of Ortho-Phosphoric Acid. Polym. Sci. Ser. A 2020, 62, 337–349. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Sazonov, O.O.; Nikitina, E.A.; Kapralova, V.M.; Nizamov, A.A.; Akhmetov, I.G.; Arkhipov, A.V.; Sudar, N.T. Dielectric Properties of Organophosphorus Polyurethane Ionomers. J. Appl. Polym. Sci. 2021, e51751. [Google Scholar] [CrossRef]
- Hala, E.; Pick, J.; Fried, V. Vapor-Liquid Equilibrium; Pergamon Press: Prague, Czech Republic, 1967; p. 599. [Google Scholar]
- Gmehling, J.; Kleiber, M.; Kolbe, B.; Rarey, J. Chemical Thermodynamics for Process Simulation; Wiley-VCH: Weinheim, Germany, 2019; p. 808. [Google Scholar]
- Reid, R.C.; Prausnitz, J.M.; Poling, B.E. The Properties of Gases and Liquids, 4th ed.; McGraw-Hill: New York, NY, USA, 1987; p. 753. [Google Scholar]
- Li, Z.; Chen, J.; Bao, T.; Shang, Y.; Li, Y. Prediction of Phase Equilibria in Tributyl Phosphate Extraction System Using the Unifac Group Contribution Method. Thermochim. Acta 1990, 169, 287–300. [Google Scholar] [CrossRef]
- Abrams, D.S.; Prausnitz, J.M. Statistical Thermodynamics of Liquid Mixtures: A New Expression for the Excess Gibbs Energy of Partly or Completely Miscible Systems. AIChE J. 1975, 21, 116–128. [Google Scholar] [CrossRef]
- Wittig, R.; Lohmann, J.; Gmehling, J. Vapor-Liquid Equilibria by UNIFAC Group Contribution. 6. Revision and Extension. Ind. Eng. Chem. Res. 2003, 42, 183–188. [Google Scholar] [CrossRef]
- Online UNIFAC Source. Available online: http://unifac.ddbst.de/unifac-matrix.html (accessed on 24 September 2021).
- Gmehling, J.; Mo1llmann, C. Synthesis of Distillation Processes Using Thermodynamic Models and the Dortmund Data Bank. Ind. Eng. Chem. Res. 1998, 37, 3112–3123. [Google Scholar] [CrossRef]
- Wlazlo, M.; Karpinska, M.; Domanska, U. Thermodynamics and Selectivity of Separation Based on Activity Coefficients at Infinite Dilution of Various Solutes in 1-Allyl-3-Methylimidazolium Bis{(Trifluoromethyl)Sulfonyl}imide Ionic Liquid. J. Chem. Thermodyn. 2016, 102, 39–47. [Google Scholar] [CrossRef]
- Dortmund Data Bank. Thermophysical Data for Process Design. Available online: http://ddbonline.ddbst.com/DDBSearch/onlineddboverview.exe (accessed on 24 September 2021).
- Li, J.-D.; Li, Y.-G.; Chen, J.; Lu, J.-F.; Teng, T. Activity Coefficient Data and Their Correlation for Tributyl Phosphate-Hydrocarbon and Uranyl Nitrate Tributyl Phosphate Complex-Hydrocarbon Solutions. Fluid Phase Equilibria 1990, 58, 307–318. [Google Scholar] [CrossRef]
- Reid, R.C.; Sherwood, T.K.; Street, R.E. The Properties of Gases and Liquids, 3rd ed.; McGraw-Hill: New York, NY, USA, 1977; p. 688. [Google Scholar]
- Zhang, L.; Yang, B.; Zhang, W. Vapor-Liquid Equilibrium of Water + Ethanol + Glycerol: Experimental Measurement and Modeling for Ethanol Dehydration by Extractive Distillation. J. Chem. Eng. Data 2015, 60, 1892–1899. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, J.; Li, Q.; Chen, B. UNIFAC Model for Ionic Liquids. Ind. Eng. Chem. Res. 2009, 48, 2697–2704. [Google Scholar] [CrossRef]
- Dong, Y.; Guo, Y.; Zhu, R.; Zhang, J.; Lei, Z. UNIFAC Model for Ionic Liquids. Revision and Extension. Ind. Eng. Chem. Res. 2020, 59, 10172–10184. [Google Scholar] [CrossRef]
- Column Equipment. Online Source. Available online: https://ingehim.ru/files/kolonnoe-oborudovanie.pdf (accessed on 24 September 2021).
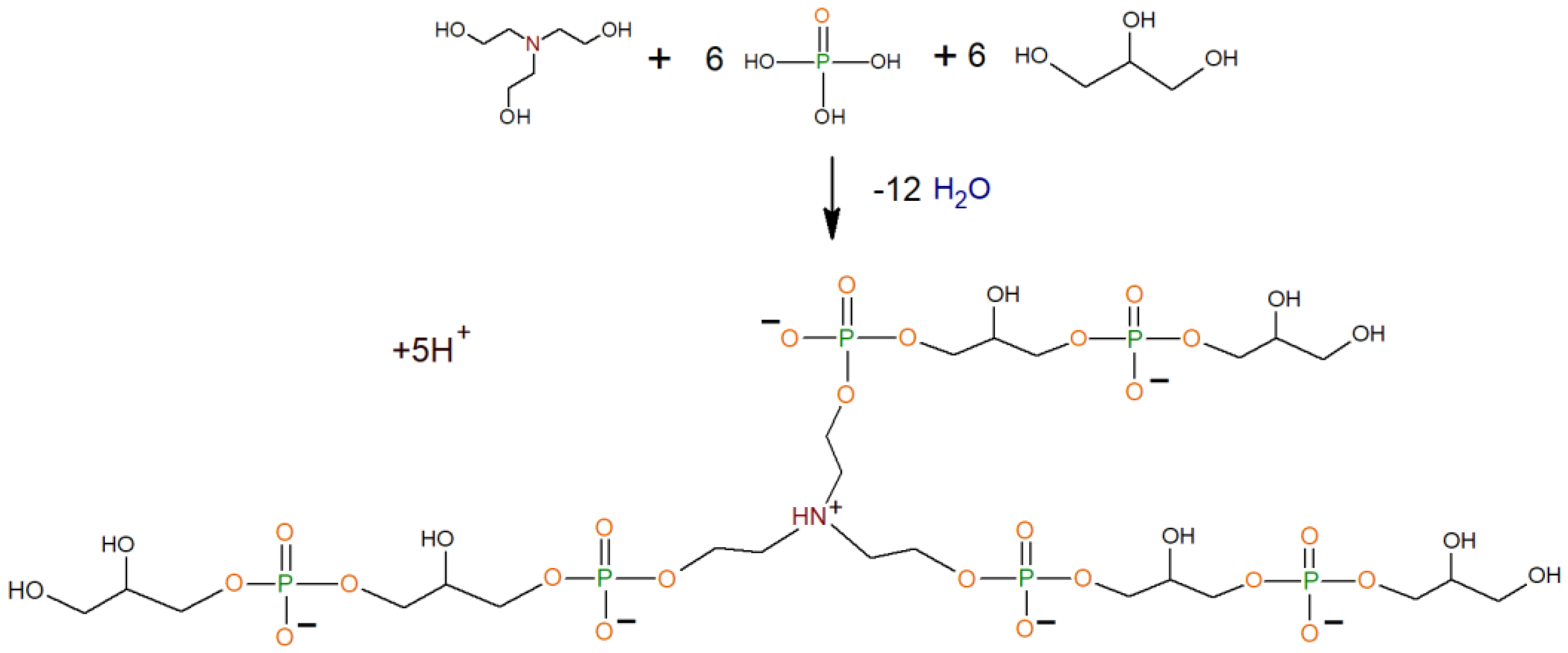



| Group | CH | CH2 | OCH2 | PO | OH | NHCH2 | Molar Mass, g/mol. | |
|---|---|---|---|---|---|---|---|---|
| Structure | ||||||||
| AEPA–Gl | 6 | 5 | 12 | 6 | 15 | 1 | 1074.54 | |
| AEPA–DEG | 0 | 11 | 18 | 6 | 9 | 1 | 1158.70 | |
| AEPA–TEG | 0 | 17 | 24 | 6 | 9 | 1 | 1423.01 | |
| Groups (i) | Parameters of Interaction from Equation (6) | |
|---|---|---|
| aij | aji | |
| CH2 | 557.93 | 797.94 |
| OCH2 | 396.85 | −185.04 |
| OH | −1897.4 | −591.94 |
| CNH | −1137.3 | 407.45 |
| H2O | 471.35 | −876.48 |
| Water Concentration, wt.% | ||||||
|---|---|---|---|---|---|---|
| T, °C | 1.027 | 5 | 20 | 40 | 60 | 80 |
| 20 | 7245.0 | 1548.0 | 51.708 | 25.682 | 4.0648 | 1.8555 |
| 30 | 2890.9 | 704.95 | 32.329 | 16.939 | 3.0468 | 1.4570 |
| 40 | 1291.4 | 354.96 | 21.253 | 11.774 | 2.3658 | 1.1801 |
| 50 | 638.22 | 193.99 | 14.787 | 8.5541 | 1.8888 | 0.9775 |
| 60 | 343.56 | 114.64 | 10.735 | 6.4500 | 1.5457 | 0.8269 |
| 70 | 198.83 | 72.123 | 8.0849 | 5.0088 | 1.2870 | |
| 80 | 122.37 | 47.792 | 6.2644 | 3.9877 | ||
| 90 | 79.389 | 33.144 | 4.9808 | |||
| 100 | 53.896 | 23.827 | 4.0429 | |||
| Water Concentration, wt.% | ||||||
|---|---|---|---|---|---|---|
| T, °C | 1.027 | 5 | 20 | 40 | 60 | 80 |
| 20 | 1.4380 | 1.4091 | 1.3180 | 1.2823 | 1.1542 | 1.0722 |
| 30 | 1.4322 | 1.4031 | 1.3122 | 1.2764 | 1.1490 | 1.0680 |
| 40 | 1.4265 | 1.3969 | 1.3065 | 1.2704 | 1.1440 | 1.0637 |
| 50 | 1.4205 | 1.3909 | 1.3004 | 1.2643 | 1.1384 | 1.0588 |
| 60 | 1.4142 | 1.385 | 1.2941 | 1.2579 | 1.1325 | 1.0534 |
| 70 | 1.4083 | 1.3791 | 1.2876 | 1.2514 | 1.1253 | |
| 80 | 1.4022 | 1.373 | 1.2810 | 1.2446 | ||
| 90 | 1.3963 | 1.3666 | 1.2740 | |||
| 100 | 1.3905 | 1.3603 | 1.2670 | |||
| Heating power, kW | 1.5 |
| Water content in AEPA-Gl, wt.% | 0.61 |
| Reflux consumption, g/s | 0.94 |
| Extractant consumption, g/s | 0.55 |
| Water content in the cube before the extractant supply, wt.% | 89.01 |
| Water content in the distillate before the extractant feed, wt.% | 6.75 |
| Time from the Beginning of the Extractant Supply, min | Distillate Water Content, wt.% | ||
|---|---|---|---|
| Experimental Data | Simulation Results | ||
| 6 Plates | 5 Plates | ||
| 15 | 1.5824 | 0.790917 | 1.89509 |
| 20 | 1.3593 | 0.776604 | 1.93154 |
| 25 | 1.3078 | 0.787001 | 1.97293 |
| 30 | 1.2963 | 0.800911 | 2.01389 |
| 35 | 1.2784 | 0.815832 | 2.05232 |
| 40 | 1.2574 | 0.829021 | 2.08367 |
| 45 | 1.2762 | 0.837874 | 2.11209 |
| 50 | 1.2636 | 0.848029 | 2.13466 |
| 55 | 1.2734 | 0.855868 | 2.15442 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klinov, A.V.; Malygin, A.V.; Khairullina, A.R.; Davletbaeva, A.R.; Sazonov, O.O.; Anashkin, I.P.; Davletbaeva, I.M. Amino Ethers of Ortho-Phosphoric Acid as Extragents for Ethanol Dehydration. ChemEngineering 2021, 5, 71. https://doi.org/10.3390/chemengineering5040071
Klinov AV, Malygin AV, Khairullina AR, Davletbaeva AR, Sazonov OO, Anashkin IP, Davletbaeva IM. Amino Ethers of Ortho-Phosphoric Acid as Extragents for Ethanol Dehydration. ChemEngineering. 2021; 5(4):71. https://doi.org/10.3390/chemengineering5040071
Chicago/Turabian StyleKlinov, Alexander V., Alexander V. Malygin, Alina R. Khairullina, Alisa R. Davletbaeva, Oleg O. Sazonov, Ivan P. Anashkin, and Ilsiya M. Davletbaeva. 2021. "Amino Ethers of Ortho-Phosphoric Acid as Extragents for Ethanol Dehydration" ChemEngineering 5, no. 4: 71. https://doi.org/10.3390/chemengineering5040071
APA StyleKlinov, A. V., Malygin, A. V., Khairullina, A. R., Davletbaeva, A. R., Sazonov, O. O., Anashkin, I. P., & Davletbaeva, I. M. (2021). Amino Ethers of Ortho-Phosphoric Acid as Extragents for Ethanol Dehydration. ChemEngineering, 5(4), 71. https://doi.org/10.3390/chemengineering5040071








