Application of Gold Nanoparticle-Based Materials in Cancer Therapy and Diagnostics
Abstract
1. Introduction
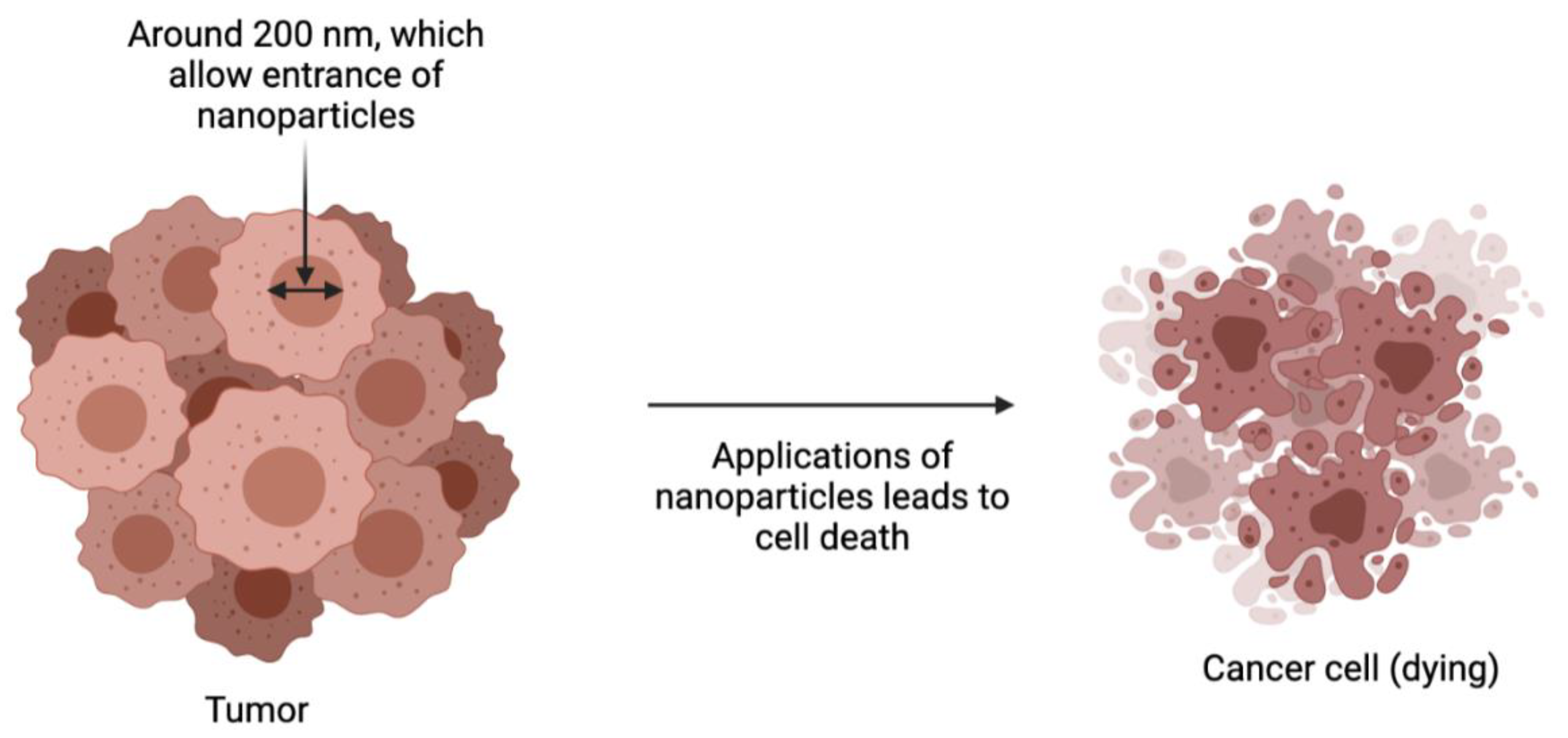
2. Form and Size Dependence on the Biological Properties of Gold Nanoparticles
- (a)
- The size and shape strongly affect the kinetics of accumulation and excretion of AuNPs in filtering organs;
- (b)
- Spherical and star-shaped AuNPs showed the same percentage of accumulation, but others localize in the liver;
- (c)
- Only stellate (or star-shaped) AuNPs can accumulate in the lungs;
- (d)
- Changes in geometry did not improve the passage of the blood–brain barrier. Overall, the study is a reliable starting point for the synthesis and functionalization of potential candidates for theranostic purposes in many research areas [54].
3. Drug Delivery
- Conjugation (attachment to the surface of a nanoparticle through linker molecules);
- Sorption (fixation on the surface due to non-covalent bonds and the developed surface of nanoparticles).
- Passive transfer (delivery is carried out in areas of increased permeability, which cancer cells often have);
- Active transfer (accumulation in the tumor due to the binding of a specific ligand and a damage marker).
3.1. Peptide Delivery
3.2. Nucleic Acid Delivery
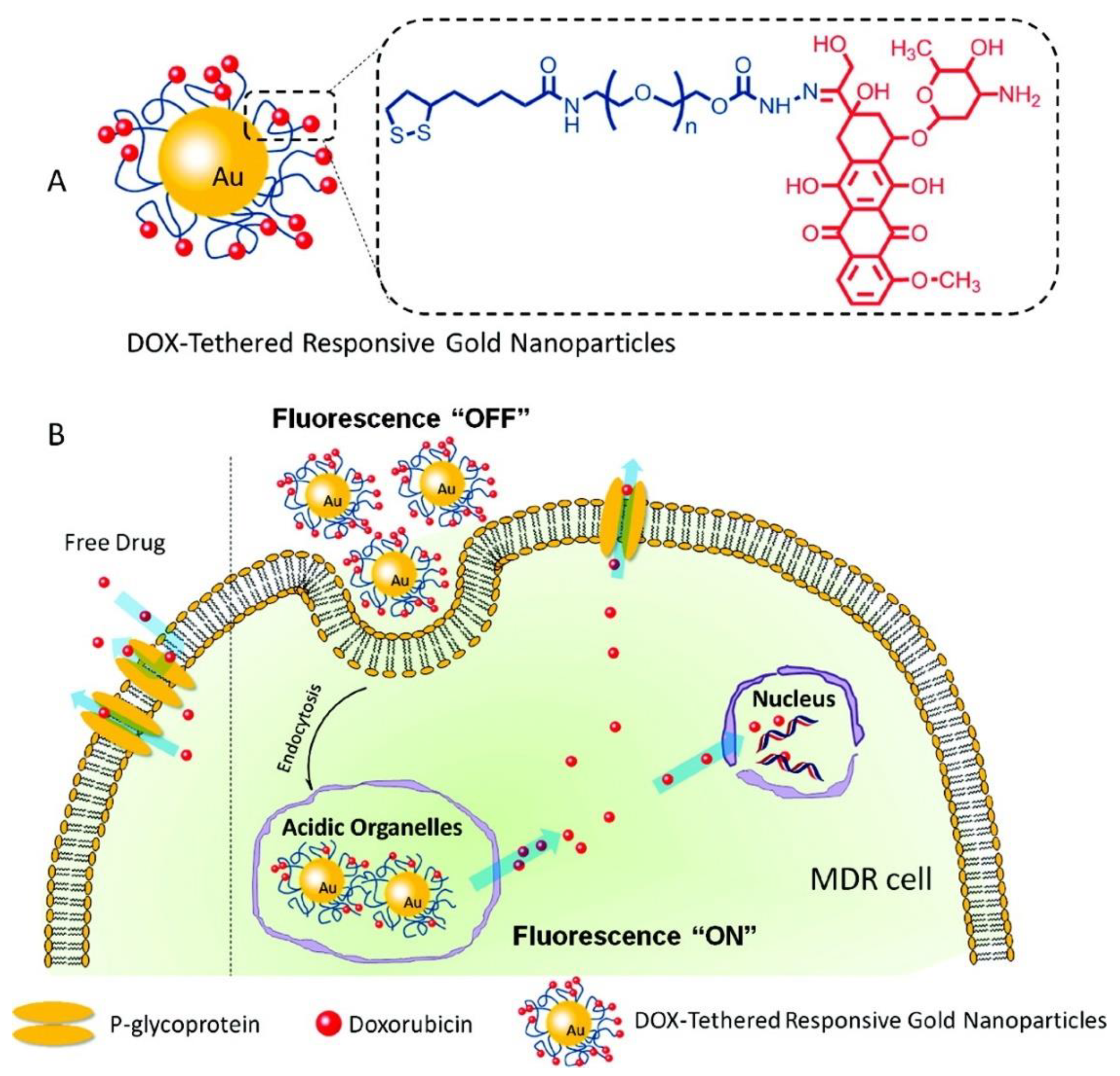
3.3. Hybrid Gold-Based Materials for Drug Delivery
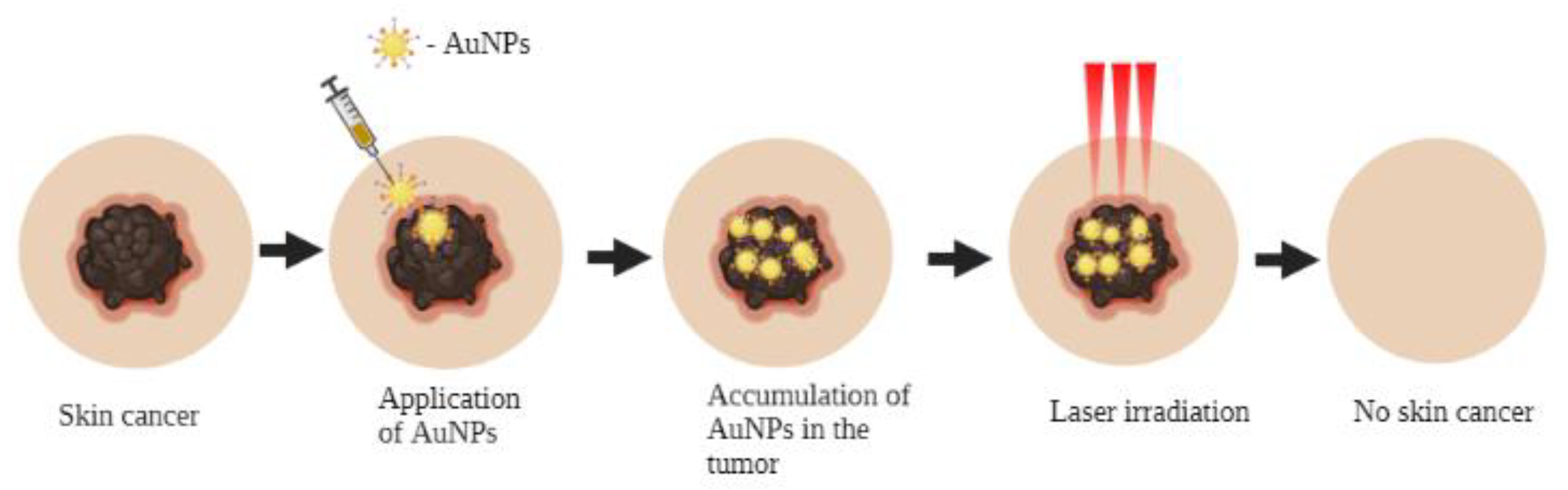
4. Photothermal Therapy
- (i)
- nanoparticles of suitable size and uniform shape;
- (ii)
- possessing a good dispersibility in aqueous solutions;
- (iii)
- respond to near-infrared light in range (650–950 nm) to prevent damage to surrounding healthy tissue, to ensure sufficient photothermal efficiency, and to ensure enough penetration depth;
- (iv)
- sufficiently photostable to allow adequate diffusion time to reach tumors before losing their light sensitivity;
- (v)
- exhibit low or no cytotoxicity in living systems.
- (1)
- The ability to focus on the local region of the tumor while minimizing non-specific distribution;
- (2)
- They can be activated through near-infrared (NIR) laser light, creating the ability to penetrate deep into biological tissues;
- (3)
- They can be modulated to create multifaceted drug delivery systems and cancer photothermal therapy.
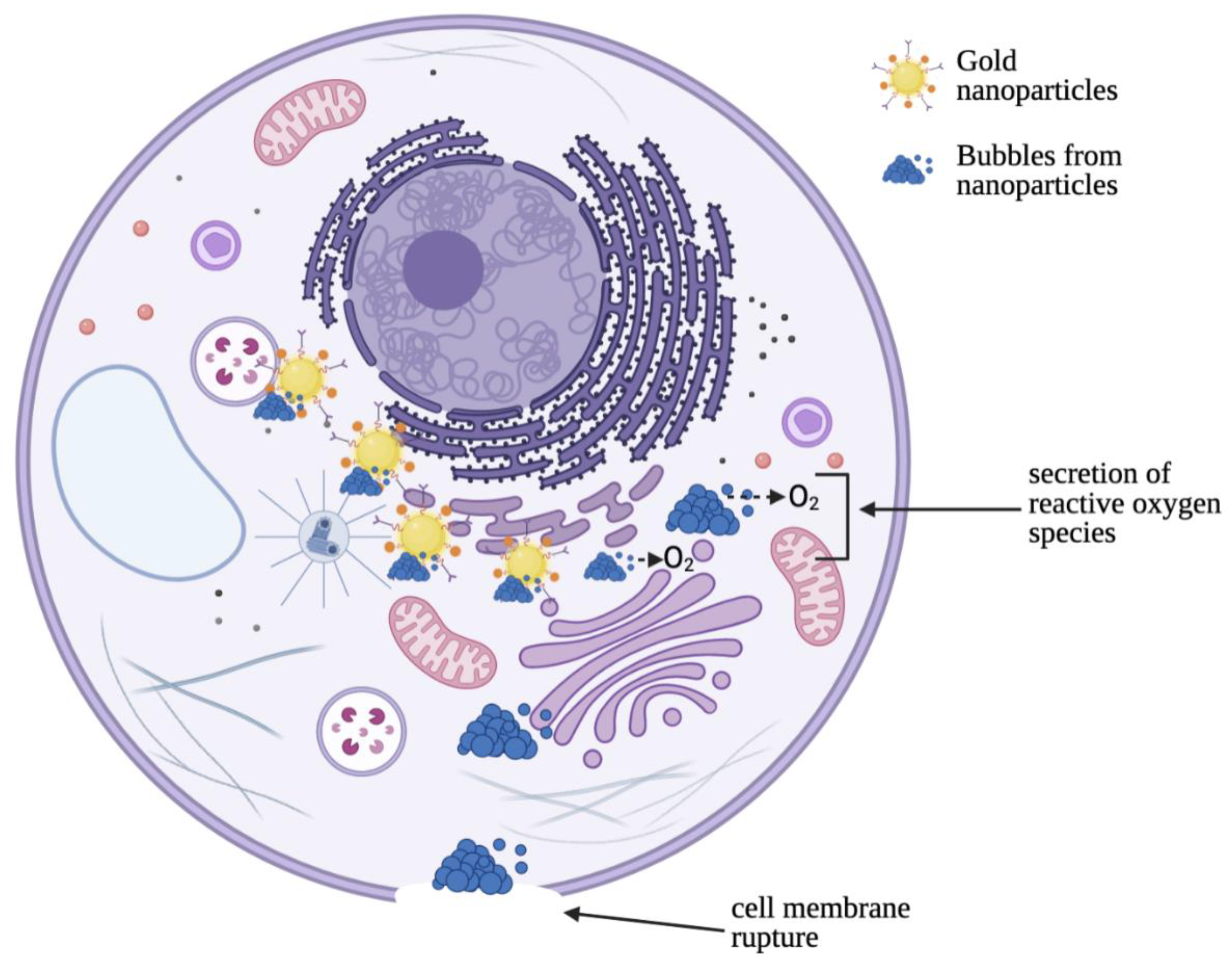
5. Sonochemical Therapy
6. Gold Nanoparticles as a Diagnostic Material
7. Current Major Restrictions on the Use of Gold Nanoparticles for Medical Purposes
7.1. Toxicity: Safety Test
7.2. Adsorption from Physiological Media
7.3. Pharmacodynamics: Pharmacokinetics
7.4. Low Efficiency
7.5. Lack of Clinical Trials
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; World Health Organization: Geneva, Switzerland, 2020; ISBN 9789240001299. [Google Scholar]
- Abdel-Qadir, H.; Austin, P.C.; Lee, D.S.; Amir, E.; Tu, J.V.; Thavendiranathan, P.; Fung, K.; Anderson, G.M. A Population-Based Study of Cardiovascular Mortality Following Early-Stage Breast Cancer. JAMA Cardiol. 2017, 2, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Sud, A.; Kinnersley, B.; Houlston, R.S. Genome-Wide Association Studies of Cancer: Current Insights and Future Perspectives. Nat. Rev. Cancer 2017, 17, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Cancer Collaboration Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018, 4, 1553–1568. [CrossRef]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie-Meder, C.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients with Cervical Cancer. Virchows Arch. 2018, 472, 919–936. [Google Scholar] [CrossRef]
- Tu, H.; Wen, C.P.; Tsai, S.P.; Chow, W.-H.; Wen, C.; Ye, Y.; Zhao, H.; Tsai, M.K.; Huang, M.; Dinney, C.P.; et al. Cancer Risk Associated with Chronic Diseases and Disease Markers: Prospective Cohort Study. BMJ 2018, 360, k134. [Google Scholar] [CrossRef]
- Palesh, O.; Scheiber, C.; Kesler, S.; Mustian, K.; Koopman, C.; Schapira, L. Management of Side Effects during and Post-Treatment in Breast Cancer Survivors. Breast J. 2018, 24, 167–175. [Google Scholar] [CrossRef]
- Zaorsky, N.G.; Churilla, T.M.; Egleston, B.L.; Fisher, S.G.; Ridge, J.A.; Horwitz, E.M.; Meyer, J.E. Causes of Death among Cancer Patients. Ann. Oncol. 2017, 28, 400–407. [Google Scholar] [CrossRef]
- Yarchoan, R.; Uldrick, T.S. HIV-Associated Cancers and Related Diseases. N. Engl. J. Med. 2018, 378, 1029–1041. [Google Scholar] [CrossRef]
- Tocut, M.; Brenner, R.; Zandman-Goddard, G. Autoimmune Phenomena and Disease in Cancer Patients Treated with Immune Checkpoint Inhibitors. Autoimmun. Rev. 2018, 17, 610–616. [Google Scholar] [CrossRef]
- Delaunay, M.; Cadranel, J.; Lusque, A.; Meyer, N.; Gounant, V.; Moro-Sibilot, D.; Michot, J.-M.; Raimbourg, J.; Girard, N.; Guisier, F.; et al. Immune-Checkpoint Inhibitors Associated with Interstitial Lung Disease in Cancer Patients. Eur. Respir. J. 2017, 50, 1700050. [Google Scholar] [CrossRef]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of Cardiac Disease in Cancer Patients throughout Oncological Treatment: ESMO Consensus Recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Pearman, T.P.; Beaumont, J.L.; Mroczek, D.; O’Connor, M.; Cella, D. Validity and Usefulness of a Single-Item Measure of Patient-Reported Bother from Side Effects of Cancer Therapy. Cancer 2018, 124, 991–997. [Google Scholar] [CrossRef]
- Pearce, A.; Haas, M.; Viney, R.; Pearson, S.-A.; Haywood, P.; Brown, C.; Ward, R. Incidence and Severity of Self-Reported Chemotherapy Side Effects in Routine Care: A Prospective Cohort Study. PLoS ONE 2017, 12, e0184360. [Google Scholar] [CrossRef]
- Demaria, M.; O’Leary, M.N.; Chang, J.; Shao, L.; Liu, S.; Alimirah, F.; Koenig, K.; Le, C.; Mitin, N.; Deal, A.M.; et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 2017, 7, 165–176. [Google Scholar] [CrossRef]
- Ariza-Garcia, A.; Lozano-Lozano, M.; Galiano-Castillo, N.; Postigo-Martin, P.; Arroyo-Morales, M.; Cantarero-Villanueva, I. A Web-Based Exercise System (e-CuidateChemo) to Counter the Side Effects of Chemotherapy in Patients with Breast Cancer: Randomized Controlled Trial. J. Med. Internet Res. 2019, 21, e14418. [Google Scholar] [CrossRef]
- Carlson, L.E.; Subnis, U.B.; Piedalue, K.-A.L.; Vallerand, J.; Speca, M.; Lupichuk, S.; Tang, P.; Faris, P.; Wolever, R.Q. The ONE-MIND Study: Rationale and Protocol for Assessing the Effects of ONlinE MINDfulness-Based Cancer Recovery for the Prevention of Fatigue and Other Common Side Effects during Chemotherapy. Eur. J. Cancer Care 2019, 28, e13074. [Google Scholar] [CrossRef]
- Gegechkori, N.; Haines, L.; Lin, J.J. Long-Term and Latent Side Effects of Specific Cancer Types. Med. Clin. N. Am. 2017, 101, 1053–1073. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef]
- Mostafavi, E.; Soltantabar, P.; Webster, T.J. Nanotechnology and picotechnology. In Biomaterials in Translational Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 191–212. ISBN 9780128134771. [Google Scholar]
- Chen, S.; Li, R.; Li, X.; Xie, J. Electrospinning: An Enabling Nanotechnology Platform for Drug Delivery and Regenerative Medicine. Adv. Drug Deliv. Rev. 2018, 132, 188–213. [Google Scholar] [CrossRef] [PubMed]
- Abadeer, N.S.; Murphy, C.J. Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles. J. Phys. Chem. C Nanomater. Interfaces 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Song, W.; Anselmo, A.C.; Huang, L. Nanotechnology Intervention of the Microbiome for Cancer Therapy. Nat. Nanotechnol. 2019, 14, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Deng, X.; Ding, J.; Zhou, W.; Zheng, X.; Tang, G. Mechanisms of Drug Release in pH-Sensitive Micelles for Tumour Targeted Drug Delivery System: A Review. Int. J. Pharm. 2018, 535, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Ji, X.; Xu, X.; Islam, M.A.; Li, Z.; Chen, S.; Saw, P.E.; Zhang, H.; Bharwani, Z.; Guo, Z.; et al. Antimonene Quantum Dots: Synthesis and Application as near-Infrared Photothermal Agents for Effective Cancer Therapy. Angew. Chem. Weinh. Bergstr. Ger. 2017, 129, 12058–12062. [Google Scholar] [CrossRef]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef]
- Jia, Q.; Ge, J.; Liu, W.; Zheng, X.; Wang, M.; Zhang, H.; Wang, P. Biocompatible Iron Phthalocyanine-Albumin Assemblies as Photoacoustic and Thermal Theranostics in Living Mice. ACS Appl. Mater. Interfaces 2017, 9, 21124–21132. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, L.; Zheng, W.; Cong, L.; Guo, Z.; Xie, Y.; Wang, L.; Tang, R.; Feng, Q.; Hamada, Y.; et al. Thermo-Triggered Release of CRISPR-Cas9 System by Lipid-Encapsulated Gold Nanoparticles for Tumor Therapy. Angew. Chem. Int. Ed. Engl. 2018, 57, 1491–1496. [Google Scholar] [CrossRef]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A. Application of Gold Nanoparticles in Biomedical and Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 410–422. [Google Scholar] [CrossRef]
- Cai, Y.; Liang, P.; Tang, Q.; Yang, X.; Si, W.; Huang, W.; Zhang, Q.; Dong, X. Diketopyrrolopyrrole-Triphenylamine Organic Nanoparticles as Multifunctional Reagents for Photoacoustic Imaging-Guided Photodynamic/Photothermal Synergistic Tumor Therapy. ACS Nano 2017, 11, 1054–1063. [Google Scholar] [CrossRef]
- Aghebati-Maleki, A.; Dolati, S.; Ahmadi, M.; Baghbanzhadeh, A.; Asadi, M.; Fotouhi, A.; Yousefi, M.; Aghebati-Maleki, L. Nanoparticles and Cancer Therapy: Perspectives for Application of Nanoparticles in the Treatment of Cancers. J. Cell. Physiol. 2020, 235, 1962–1972. [Google Scholar] [CrossRef]
- Xuan, M.; Shao, J.; Zhao, J.; Li, Q.; Dai, L.; Li, J. Magnetic Mesoporous Silica Nanoparticles Cloaked by Red Blood Cell Membranes: Applications in Cancer Therapy. Angew. Chem. Int. Ed. Engl. 2018, 57, 6049–6053. [Google Scholar] [CrossRef] [PubMed]
- Mishra, H.; Mishra, P.K.; Ekielski, A.; Jaggi, M.; Iqbal, Z.; Talegaonkar, S. Melanoma Treatment: From Conventional to Nanotechnology. J. Cancer Res. Clin. Oncol. 2018, 144, 2283–2302. [Google Scholar] [CrossRef] [PubMed]
- Candido, N.M.; de Melo, M.T.; Franchi, L.P.; Primo, F.L.; Tedesco, A.C.; Rahal, P.; Calmon, M.F. Combining Photodynamic Therapy and Chemotherapy: Improving Breast Cancer Treatment with Nanotechnology. J. Biomed. Nanotechnol. 2018, 14, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Janicka, M.; Gubernator, J. Use of Nanotechnology for Improved Pharmacokinetics and Activity of Immunogenic Cell Death Inducers Used in Cancer Chemotherapy. Expert Opin. Drug Deliv. 2017, 14, 1059–1075. [Google Scholar] [CrossRef]
- Zhao, C.-Y.; Cheng, R.; Yang, Z.; Tian, Z.-M. Nanotechnology for Cancer Therapy Based on Chemotherapy. Molecules 2018, 23, 826. [Google Scholar] [CrossRef]
- Falagan-Lotsch, P.; Grzincic, E.M.; Murphy, C.J. New Advances in Nanotechnology-Based Diagnosis and Therapeutics for Breast Cancer: An Assessment of Active-Targeting Inorganic Nanoplatforms. Bioconjug. Chem. 2017, 28, 135–152. [Google Scholar] [CrossRef]
- Matos, A.I.; Carreira, B.; Peres, C.; Moura, L.I.F.; Conniot, J.; Fourniols, T.; Scomparin, A.; Martínez-Barriocanal, Á.; Arango, D.; Conde, J.P.; et al. Nanotechnology Is an Important Strategy for Combinational Innovative Chemo-Immunotherapies against Colorectal Cancer. J. Control. Release 2019, 307, 108–138. [Google Scholar] [CrossRef]
- Pillai, G. Nanotechnology toward treating cancer. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 221–256. ISBN 9780128140291. [Google Scholar]
- Sielaff, C.M.; Mousa, S.A. Status and Future Directions in the Management of Pancreatic Cancer: Potential Impact of Nanotechnology. J. Cancer Res. Clin. Oncol. 2018, 144, 1205–1217. [Google Scholar] [CrossRef]
- Song, G.; Cheng, L.; Chao, Y.; Yang, K.; Liu, Z. Emerging Nanotechnology and Advanced Materials for Cancer Radiation Therapy. Adv. Mater. 2017, 29, 1604894. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, Z. The Application of Nanotechnology in Immune Checkpoint Blockade for Cancer Treatment. J. Control. Release 2018, 290, 28–45. [Google Scholar] [CrossRef]
- Cryer, A.M.; Thorley, A.J. Nanotechnology in the Diagnosis and Treatment of Lung Cancer. Pharmacol. Ther. 2019, 198, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, M.; Pang, B.; Vara, M.; Xia, Y. Gold Nanomaterials at Work in Biomedicine. Chem. Rev. 2015, 115, 10410–10488. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The Golden Age: Gold Nanoparticles for Biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Beik, J.; Khateri, M.; Khosravi, Z.; Kamrava, S.K.; Kooranifar, S.; Ghaznavi, H.; Shakeri-Zadeh, A. Gold Nanoparticles in Combinatorial Cancer Therapy Strategies. Coord. Chem. Rev. 2019, 387, 299–324. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor Vascular Permeability and the EPR Effect in Macromolecular Therapeutics: A Review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Wei, Y.; Quan, L.; Zhou, C.; Zhan, Q. Factors Relating to the Biodistribution & Clearance of Nanoparticles & Their Effects on in Vivo Application. Nanomedicine 2018, 13, 1495–1512. [Google Scholar] [CrossRef]
- Angelov, B.; Angelova, A.; Filippov, S.K.; Drechsler, M.; Štěpánek, P.; Lesieur, S. Multicompartment Lipid Cubic Nanoparticles with High Protein Upload: Millisecond Dynamics of Formation. ACS Nano 2014, 8, 5216–5226. [Google Scholar] [CrossRef]
- Dou, Y.; Yang, X. Novel High-Sensitive Fluorescent Detection of Deoxyribonuclease I Based on DNA-Templated Gold/silver Nanoclusters. Anal. Chim. Acta 2013, 784, 53–58. [Google Scholar] [CrossRef]
- Weadick, D.S.; Liu, J. Phosphorothioate DNA Stabilized Fluorescent Gold and Silver Nanoclusters. Nanomaterials 2015, 5, 804–813. [Google Scholar] [CrossRef]
- Lee, Y.J.; Ahn, E.-Y.; Park, Y. Shape-Dependent Cytotoxicity and Cellular Uptake of Gold Nanoparticles Synthesized Using Green Tea Extract. Nanoscale Res. Lett. 2019, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The Effect of Shape on Cellular Uptake of Gold Nanoparticles in the Forms of Stars, Rods, and Triangles. Sci. Rep. 2017, 7, 3827. [Google Scholar] [CrossRef] [PubMed]
- Scarabelli, L.; Coronado-Puchau, M.; Giner-Casares, J.J.; Langer, J.; Liz-Marzán, L.M. Monodisperse Gold Nanotriangles: Size Control, Large-Scale Self-Assembly, and Performance in Surface-Enhanced Raman Scattering. ACS Nano 2014, 8, 5833–5842. [Google Scholar] [CrossRef] [PubMed]
- Sironi, L.; Freddi, S.; Caccia, M.; Pozzi, P.; Rossetti, L.; Pallavicini, P.; Donà, A.; Cabrini, E.; Gualtieri, M.; Rivolta, I.; et al. Gold Branched Nanoparticles for Cellular Treatments. J. Phys. Chem. C Nanomater. Interfaces 2012, 116, 18407–18418. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Barcinska, E.; Malankowska, A.; Zauszkiewicz-Pawlak, A.; Nowaczyk, G.; Zaleska-Medynska, A.; Inkielewicz-Stepniak, I. Impact of Gold Nanoparticles Shape on Their Cytotoxicity against Human Osteoblast and Osteosarcoma in in Vitro Model. Evaluation of the Safety of Use and Anti-Cancer Potential. J. Mater. Sci. Mater. Med. 2019, 30, 22. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, P.; Kvitek, L.; Prucek, R.; Panacek, A.; Halder, A.; Vajda, S.; Zboril, R. Simple Size-Controlled Synthesis of Au Nanoparticles and Their Size-Dependent Catalytic Activity. Sci. Rep. 2018, 8, 4589. [Google Scholar] [CrossRef]
- Xu, M.; Soliman, M.G.; Sun, X.; Pelaz, B.; Feliu, N.; Parak, W.J.; Liu, S. How Entanglement of Different Physicochemical Properties Complicates the Prediction of in Vitro and in Vivo Interactions of Gold Nanoparticles. ACS Nano 2018, 12, 10104–10113. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Rosfa, S.; Wlodarski, A.; Kuharev, J.; Rekik, A.; Knauer, S.K.; Bantz, C.; Nawroth, T.; Bier, C.; et al. Nanoparticle Size Is a Critical Physicochemical Determinant of the Human Blood Plasma Corona: A Comprehensive Quantitative Proteomic Analysis. ACS Nano 2011, 5, 7155–7167. [Google Scholar] [CrossRef]
- Walkey, C.D.; Olsen, J.B.; Song, F.; Liu, R.; Guo, H.; Olsen, D.W.H.; Cohen, Y.; Emili, A.; Chan, W.C.W. Protein Corona Fingerprinting Predicts the Cellular Interaction of Gold and Silver Nanoparticles. ACS Nano 2014, 8, 2439–2455. [Google Scholar] [CrossRef]
- Rozengurt, E.; Heppel, L.A. A Specific Effect of External ATP on the Permeability of Transformed 3T3 Cells. Biochem. Biophys. Res. Commun. 1975, 67, 1581–1588. [Google Scholar] [CrossRef]
- Liu, H.; Pierre-Pierre, N.; Huo, Q. Dynamic Light Scattering for Gold Nanorod Size Characterization and Study of Nanorod–protein Interactions. Gold Bull. 2012, 45, 187–195. [Google Scholar] [CrossRef]
- Duncan, B.; Kim, C.; Rotello, V.M. Gold Nanoparticle Platforms as Drug and Biomacromolecule Delivery Systems. J. Control. Release 2010, 148, 122–127. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef]
- Hostetler, M.J.; Wingate, J.E.; Zhong, C.-J.; Harris, J.E.; Vachet, R.W.; Clark, M.R.; Londono, J.D.; Green, S.J.; Stokes, J.J.; Wignall, G.D.; et al. Alkanethiolate Gold Cluster Molecules with Core Diameters from 1.5 to 5.2 Nm: Core and Monolayer Properties as a Function of Core Size. Langmuir 1998, 14, 17–30. [Google Scholar] [CrossRef]
- Rana, S.; Bajaj, A.; Mout, R.; Rotello, V.M. Monolayer Coated Gold Nanoparticles for Delivery Applications. Adv. Drug Deliv. Rev. 2012, 64, 200–216. [Google Scholar] [CrossRef]
- Perrault, S.D.; Walkey, C.; Jennings, T.; Fischer, H.C.; Chan, W.C.W. Mediating Tumor Targeting Efficiency of Nanoparticles through Design. Nano Lett. 2009, 9, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR Effect for Macromolecular Drug Delivery to Solid Tumors: Improvement of Tumor Uptake, Lowering of Systemic Toxicity, and Distinct Tumor Imaging in Vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef]
- Park, J.; Choi, Y.; Chang, H.; Um, W.; Ryu, J.H.; Kwon, I.C. Alliance with EPR Effect: Combined Strategies to Improve the EPR Effect in the Tumor Microenvironment. Theranostics 2019, 9, 8073–8090. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ortiz, M.; Zapata-Urzúa, C.; Acosta, G.A.; Álvarez-Lueje, A.; Albericio, F.; Kogan, M.J. Gold Nanoparticles as an Efficient Drug Delivery System for GLP-1 Peptides. Colloids Surf. B Biointerfaces 2017, 158, 25–32. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Yang, D.; Qin, M.; Zhang, Y.; He, B.; Dai, W.; Wang, X.; Zhang, Q.; Zhang, H.; Yin, C. Increased Cellular Uptake of Peptide-Modified PEGylated Gold Nanoparticles. Biochem. Biophys. Res. Commun. 2017, 494, 339–345. [Google Scholar] [CrossRef]
- Halamoda-Kenzaoui, B.; Ceridono, M.; Urbán, P.; Bogni, A.; Ponti, J.; Gioria, S.; Kinsner-Ovaskainen, A. The Agglomeration State of Nanoparticles Can Influence the Mechanism of Their Cellular Internalisation. J. Nanobiotechnol. 2017, 15, 48. [Google Scholar] [CrossRef]
- Odhner, J.H.; Moore Tibbetts, K.; Tangeysh, B.; Wayland, B.B.; Levis, R.J. Mechanism of Improved Au Nanoparticle Size Distributions Using Simultaneous Spatial and Temporal Focusing for Femtosecond Laser Irradiation of Aqueous KAuCl4. J. Phys. Chem. C Nanomater. Interfaces 2014, 118, 23986–23995. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef]
- Nakase, I.; Niwa, M.; Takeuchi, T.; Sonomura, K.; Kawabata, N.; Koike, Y.; Takehashi, M.; Tanaka, S.; Ueda, K.; Simpson, J.C.; et al. Cellular Uptake of Arginine-Rich Peptides: Roles for Macropinocytosis and Actin Rearrangement. Mol. Ther. 2004, 10, 1011–1022. [Google Scholar] [CrossRef]
- Kapur, A.; Medina, S.H.; Wang, W.; Palui, G.; Schneider, J.P.; Mattoussi, H. Intracellular Delivery of Gold Nanocolloids Promoted by a Chemically Conjugated Anticancer Peptide. ACS Omega 2018, 3, 12754–12762. [Google Scholar] [CrossRef]
- Gaspar, D.; Veiga, A.S.; Sinthuvanich, C.; Schneider, J.P.; Castanho, M.A.R.B. Anticancer Peptide SVS-1: Efficacy Precedes Membrane Neutralization. Biochemistry 2012, 51, 6263–6265. [Google Scholar] [CrossRef][Green Version]
- Sinthuvanich, C.; Veiga, A.S.; Gupta, K.; Gaspar, D.; Blumenthal, R.; Schneider, J.P. Anticancer β-Hairpin Peptides: Membrane-Induced Folding Triggers Activity. J. Am. Chem. Soc. 2012, 134, 6210–6217. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold Nanoparticles for Biology and Medicine. Angew. Chem. Int. Ed. Engl. 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.C.; Giljohann, D.A.; Daniel, W.L.; Zheng, D.; Prigodich, A.E.; Mirkin, C.A. Scavenger Receptors Mediate Cellular Uptake of Polyvalent Oligonucleotide-Functionalized Gold Nanoparticles. Bioconjug. Chem. 2010, 21, 2250–2256. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, C.M.; Esposito, E.A., 3rd; Boal, A.K.; Simard, J.M.; Martin, C.T.; Rotello, V.M. Inhibition of DNA Transcription Using Cationic Mixed Monolayer Protected Gold Clusters. J. Am. Chem. Soc. 2001, 123, 7626–7629. [Google Scholar] [CrossRef]
- Sandhu, K.K.; McIntosh, C.M.; Simard, J.M.; Smith, S.W.; Rotello, V.M. Gold Nanoparticle-Mediated Transfection of Mammalian Cells. Bioconjug. Chem. 2002, 13, 3–6. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C.S.; Kim, S.T.; Landis, R.F.; Rotello, V.M. Gold Nanoparticles for Nucleic Acid Delivery. Mol. Ther. 2014, 22, 1075–1083. [Google Scholar] [CrossRef]
- Kunoh, T.; Takeda, M.; Matsumoto, S.; Suzuki, I.; Takano, M.; Kunoh, H.; Takada, J. Green Synthesis of Gold Nanoparticles Coupled with Nucleic Acid Oxidation. ACS Sustain. Chem. Eng. 2018, 6, 364–373. [Google Scholar] [CrossRef]
- Labala, S.; Mandapalli, P.K.; Kurumaddali, A.; Venuganti, V.V.K. Layer-by-Layer Polymer Coated Gold Nanoparticles for Topical Delivery of Imatinib Mesylate to Treat Melanoma. Mol. Pharm. 2015, 12, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Labala, S.; Jose, A.; Chawla, S.R.; Khan, M.S.; Bhatnagar, S.; Kulkarni, O.P.; Venuganti, V.V.K. Effective Melanoma Cancer Suppression by Iontophoretic Co-Delivery of STAT3 siRNA and Imatinib Using Gold Nanoparticles. Int. J. Pharm. 2017, 525, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Kwong, L.N.; Davies, M.A. Targeted Therapy for Melanoma: Rational Combinatorial Approaches. Oncogene 2014, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Ji, B.; Yu, H.; Bao, W.; Yang, Z.; Yu, Y.; Cui, Y.; Du, Y.; Song, M.; Liu, S.; et al. Multifunctional Gold Nanoparticles Overcome MicroRNA Regulatory Network Mediated-Multidrug Resistant Leukemia. Sci. Rep. 2019, 9, 5348. [Google Scholar] [CrossRef]
- Lynn, R.C.; Poussin, M.; Kalota, A.; Feng, Y.; Low, P.S.; Dimitrov, D.S.; Powell, D.J., Jr. Targeting of Folate Receptor β on Acute Myeloid Leukemia Blasts with Chimeric Antigen Receptor-Expressing T Cells. Blood 2015, 125, 3466–3476. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, Z.; Liu, T.; Li, X.; Hu, X.; Wei, X.; Zhang, X.; Tan, W. Smart Human-Serum-Albumin-As O Nanodrug with Self-Amplified Folate Receptor-Targeting Ability for Chronic Myeloid Leukemia Treatment. Angew. Chem. Int. Ed. Engl. 2017, 56, 10845–10849. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, Z.; Liu, Z.; Feng, B.; Ren, G.; Li, K.; Zhou, L.; Sun, Y.; Li, M.; Zhou, J.; et al. miR-508-5p Regulates Multidrug Resistance of Gastric Cancer by Targeting ABCB1 and ZNRD1. Oncogene 2014, 33, 3267–3276. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, L.; Hu, J.; Ruan, J. miR-138 Might Reverse Multidrug Resistance of Leukemia Cells. Leuk. Res. 2010, 34, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, K.-J.; Moon, S.U.; Kim, S. Theragnosis-Based Combined Cancer Therapy Using Doxorubicin-Conjugated microRNA-221 Molecular Beacon. Biomaterials 2016, 74, 109–118. [Google Scholar] [CrossRef]
- Shiao, Y.-S.; Chiu, H.-H.; Wu, P.-H.; Huang, Y.-F. Aptamer-Functionalized Gold Nanoparticles as Photoresponsive Nanoplatform for Co-Drug Delivery. ACS Appl. Mater. Interfaces 2014, 6, 21832–21841. [Google Scholar] [CrossRef]
- Xiang, D.; Shigdar, S.; Qiao, G.; Wang, T.; Kouzani, A.Z.; Zhou, S.-F.; Kong, L.; Li, Y.; Pu, C.; Duan, W. Nucleic Acid Aptamer-Guided Cancer Therapeutics and Diagnostics: The next Generation of Cancer Medicine. Theranostics 2015, 5, 23–42. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Hu, X.; Venkatachalam, K.; Wang, M.-H. Nucleolin Targeted Delivery of Aptamer Tagged Trichoderma Derived Crude Protein Coated Gold Nanoparticles for Improved Cytotoxicity in Cancer Cells. Process. Biochem. 2021, 102, 325–332. [Google Scholar] [CrossRef]
- Shaabani, E.; Sharifiaghdam, M.; de Keersmaecker, H.; de Rycke, R.; de Smedt, S.; Faridi-Majidi, R.; Braeckmans, K.; Fraire, J.C. Layer by Layer Assembled Chitosan-Coated Gold Nanoparticles for Enhanced siRNA Delivery and Silencing. Int. J. Mol. Sci. 2021, 22, 831. [Google Scholar] [CrossRef] [PubMed]
- Sreelakshmi, C.; Goel, N.; Datta, K.K.R.; Addlagatta, A.; Ummanni, R.; Reddy, B.V.S. Green Synthesis of Curcumin Capped Gold Nanoparticles and Evaluation of Their Cytotoxicity. Nanosci. Nanotechnol. Lett. 2013, 5, 1258–1265. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Lazzara, G.; Merli, M.; Milioto, S.; Parisi, F.; Sciascia, L. Effect of the Biopolymer Charge and the Nanoclay Morphology on Nanocomposite Materials. Ind. Eng. Chem. Res. 2016, 55, 7373–7380. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Biopolymer-Targeted Adsorption onto Halloysite Nanotubes in Aqueous Media. Langmuir 2017, 33, 3317–3323. [Google Scholar] [CrossRef]
- Deljoo, S.; Rabiee, N.; Rabiee, M. Curcumin-Hybrid Nanoparticles in Drug Delivery System (Review). Asian J. Nanosci. Mater. 2019, 2, 66–91. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.-C.; Dou, S.; Xiong, M.-H.; Sun, T.-M.; Wang, J. Doxorubicin-Tethered Responsive Gold Nanoparticles Facilitate Intracellular Drug Delivery for Overcoming Multidrug Resistance in Cancer Cells. ACS Nano 2011, 5, 3679–3692. [Google Scholar] [CrossRef]
- Ruan, S.; Hu, C.; Tang, X.; Cun, X.; Xiao, W.; Shi, K.; He, Q.; Gao, H. Increased Gold Nanoparticle Retention in Brain Tumors by in Situ Enzyme-Induced Aggregation. ACS Nano 2016, 10, 10086–10098. [Google Scholar] [CrossRef]
- Mats, L.; Logue, F.; Oleschuk, R.D. “Particle-Free” Magnetic Actuation of Droplets on Superhydrophobic Surfaces Using Dissolved Paramagnetic Salts. Anal. Chem. 2016, 88, 9486–9494. [Google Scholar] [CrossRef]
- Suarasan, S.; Focsan, M.; Potara, M.; Soritau, O.; Florea, A.; Maniu, D.; Astilean, S. Doxorubicin-Incorporated Nanotherapeutic Delivery System Based on Gelatin-Coated Gold Nanoparticles: Formulation, Drug Release, and Multimodal Imaging of Cellular Internalization. ACS Appl. Mater. Interfaces 2016, 8, 22900–22913. [Google Scholar] [CrossRef]
- Ou, Y.-C.; Webb, J.A.; Faley, S.; Shae, D.; Talbert, E.M.; Lin, S.; Cutright, C.C.; Wilson, J.T.; Bellan, L.M.; Bardhan, R. Gold Nanoantenna-Mediated Photothermal Drug Delivery from Thermosensitive Liposomes in Breast Cancer. ACS Omega 2016, 1, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, G.; Alves, C.S.; Tomás, H.; Xiong, Z.; Shen, M.; Rodrigues, J.; Shi, X. Multifunctional Dendrimer-Entrapped Gold Nanoparticles Conjugated with Doxorubicin for pH-Responsive Drug Delivery and Targeted Computed Tomography Imaging. Langmuir 2018, 34, 12428–12435. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.-Q.; Liu, L.-N.; Du, Y.-Z.; Yuan, H. Synthesis and Antitumor Activity of Doxorubicin Conjugated Stearic Acid-G-Chitosan Oligosaccharide Polymeric Micelles. Biomaterials 2009, 30, 6955–6963. [Google Scholar] [CrossRef]
- Shen, W.C.; Ryser, H.J. Cis-Aconityl Spacer between Daunomycin and Macromolecular Carriers: A Model of pH-Sensitive Linkage Releasing Drug from a Lysosomotropic Conjugate. Biochem. Biophys. Res. Commun. 1981, 102, 1048–1054. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Xu, Y.; Shen, M.; Zhao, J.; Zhang, G.; Shi, X. Synthesis of PEGylated Low Generation Dendrimer-Entrapped Gold Nanoparticles for CT Imaging Applications. Nanoscale 2014, 6, 4521–4526. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zheng, L.; Chen, Q.; Shen, M.; Guo, R.; Wang, H.; Cao, X.; Zhang, G.; Shi, X. PEGylated Dendrimer-Entrapped Gold Nanoparticles for in Vivo Blood Pool and Tumor Imaging by Computed Tomography. Biomaterials 2012, 33, 1107–1119. [Google Scholar] [CrossRef]
- Khutale, G.V.; Casey, A. Synthesis and Characterization of a Multifunctional Gold-Doxorubicin Nanoparticle System for pH Triggered Intracellular Anticancer Drug Release. Eur. J. Pharm. Biopharm. 2017, 119, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Mallick, A.; More, P.; Ghosh, S.; Chippalkatti, R.; Chopade, B.A.; Lahiri, M.; Basu, S. Dual Drug Conjugated Nanoparticle for Simultaneous Targeting of Mitochondria and Nucleus in Cancer Cells. ACS Appl. Mater. Interfaces 2015, 7, 7584–7598. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, R.; Pichaimani, A.; Hari, K.; Balasubramanian, P.K.; Kulandaivel, J.; Premkumar, K. Doxorubicin Conjugated Gold Nanorods: A Sustained Drug Delivery Carrier for Improved Anticancer Therapy. J. Mater. Chem. B Mater. Biol. Med. 2013, 1, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Ahmadi, S.; Arab, Z.; Bagherzadeh, M.; Safarkhani, M.; Nasseri, B.; Rabiee, M.; Tahriri, M.; Webster, T.J.; Tayebi, L. Aptamer Hybrid Nanocomplexes as Targeting Components for Antibiotic/Gene Delivery Systems and Diagnostics: A Review. Int. J. Nanomed. 2020, 15, 4237–4256. [Google Scholar] [CrossRef]
- Ahmadi Nasab, N.; Hassani Kumleh, H.; Beygzadeh, M.; Teimourian, S.; Kazemzad, M. Delivery of Curcumin by a pH-Responsive Chitosan Mesoporous Silica Nanoparticles for Cancer Treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Fan, Z.; Li, Y.; Zhang, Y.; Yu, F.; Su, G.; Xie, L.; Hou, Z. Design of pH-Sensitive Methotrexate Prodrug-Targeted Curcumin Nanoparticles for Efficient Dual-Drug Delivery and Combination Cancer Therapy. Int. J. Nanomed. 2018, 13, 1381–1398. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Y.; Zhang, X.; Liu, S.; Tian, C.; Wang, H. Targeted Nanomedicine for Prostate Cancer Therapy: Docetaxel and Curcumin Co-Encapsulated Lipid-Polymer Hybrid Nanoparticles for the Enhanced Anti-Tumor Activity in Vitro and in Vivo. Drug Deliv. 2016, 23, 1757–1762. [Google Scholar] [CrossRef]
- Rao, K.M.; Kumar, A.; Suneetha, M.; Han, S.S. pH and near-Infrared Active; Chitosan-Coated Halloysite Nanotubes Loaded with Curcumin-Au Hybrid Nanoparticles for Cancer Drug Delivery. Int. J. Biol. Macromol. 2018, 112, 119–125. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, J.; Xiao, Y.; Li, W.; Tan, L.; Xu, X.; Qian, Z. Porous Au@Pt Nanoparticles: Therapeutic Platform for Tumor Chemo-Photothermal Co-Therapy and Alleviating Doxorubicin-Induced Oxidative Damage. ACS Appl. Mater. Interfaces 2018, 10, 150–164. [Google Scholar] [CrossRef]
- Norouzi, H.; Khoshgard, K.; Akbarzadeh, F. In Vitro Outlook of Gold Nanoparticles in Photo-Thermal Therapy: A Literature Review. Lasers Med. Sci. 2018, 33, 917–926. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, T.; Qin, X.; Qiao, Q.; Shang, L.; Song, Q.; Yang, C.; Zhang, Z. Intracellularly Generated Immunological Gold Nanoparticles for Combinatorial Photothermal Therapy and Immunotherapy against Tumor. Nano Lett. 2019, 19, 6635–6646. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold Nanoparticle-Mediated Photothermal Therapy: Applications and Opportunities for Multimodal Cancer Treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef]
- Cheng, X.; Sun, R.; Yin, L.; Chai, Z.; Shi, H.; Gao, M. Light-Triggered Assembly of Gold Nanoparticles for Photothermal Therapy and Photoacoustic Imaging of Tumors In Vivo. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.K.; Wu, Y.; El-Sayed, M.A. Gold-Nanoparticle-Assisted Plasmonic Photothermal Therapy Advances toward Clinical Application. J. Phys. Chem. C Nanomater. Interfaces 2019, 123, 15375–15393. [Google Scholar] [CrossRef]
- Liu, Y.; Crawford, B.M.; Vo-Dinh, T. Gold Nanoparticles-Mediated Photothermal Therapy and Immunotherapy. Immunotherapy 2018, 10, 1175–1188. [Google Scholar] [CrossRef]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold Nanoparticle Based Photothermal Therapy: Development and Application for Effective Cancer Treatment. Sustain. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- Sun, M.; Peng, D.; Hao, H.; Hu, J.; Wang, D.; Wang, K.; Liu, J.; Guo, X.; Wei, Y.; Gao, W. Thermally Triggered in Situ Assembly of Gold Nanoparticles for Cancer Multimodal Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 10453–10460. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, D.Y. Photothermal Therapy with Gold Nanoparticles as an Anticancer Medication. J. Pharm. Investig. 2017, 47, 19–26. [Google Scholar] [CrossRef]
- Beik, J.; Asadi, M.; Khoei, S.; Laurent, S.; Abed, Z.; Mirrahimi, M.; Farashahi, A.; Hashemian, R.; Ghaznavi, H.; Shakeri-Zadeh, A. Simulation-Guided Photothermal Therapy Using MRI-Traceable Iron Oxide-Gold Nanoparticle. J. Photochem. Photobiol. B 2019, 199, 111599. [Google Scholar] [CrossRef] [PubMed]
- Neshastehriz, A.; Tabei, M.; Maleki, S.; Eynali, S.; Shakeri-Zadeh, A. Photothermal Therapy Using Folate Conjugated Gold Nanoparticles Enhances the Effects of 6MV X-Ray on Mouth Epidermal Carcinoma Cells. J. Photochem. Photobiol. B 2017, 172, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Du, B.; Huang, Y.; Yu, M.; Zheng, J. Cancer Photothermal Therapy with ICG-Conjugated Gold Nanoclusters. Bioconjug. Chem. 2020, 31, 1522–1528. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, D.Y. Near-Infrared-Responsive Cancer Photothermal and Photodynamic Therapy Using Gold Nanoparticles. Polymers 2018, 10, 961. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, Y.; Yu, H.; Yan, C.; Liu, Y.; Hong, S.; Tao, H.; Robertson, A.W.; Wang, Z.; Pádua, A.A.H. New Solvent-Stabilized Few-Layer Black Phosphorus for Antibacterial Applications. Nanoscale 2018, 10, 12543–12553. [Google Scholar] [CrossRef]
- Wang, X.; Shao, J.; Abd El Raouf, M.; Xie, H.; Huang, H.; Wang, H.; Chu, P.K.; Yu, X.-F.; Yang, Y.; AbdEl-Aal, A.M.; et al. Near-Infrared Light-Triggered Drug Delivery System Based on Black Phosphorus for in Vivo Bone Regeneration. Biomaterials 2018, 179, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Liu, Z.; Li, Y.; Hou, Y.; Fei, X.; Su, C.; Wang, S.; Zhuang, Z.; Guo, Z. Facile Synthesis of Black Phosphorus-Au Nanocomposites for Enhanced Photothermal Cancer Therapy and Surface-Enhanced Raman Scattering Analysis. Biomater. Sci. 2017, 5, 2048–2055. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Mokhtari-Dizaji, M.; Ghassemi, F.; Sheibani, S.; Amoli, F.A. The Effect of Ultrasound Hyperthermia with Gold Nanoparticles on Retinoblastoma Y79 Cells. Gold Bull. 2020, 53, 111–120. [Google Scholar] [CrossRef]
- Katifelis, H.; Mukha, I.; Lyberopoulou, A.; Vityuk, N.; Grammatikaki, M.; Pylypchuk, I.; Lazaris, F.; Storozhuk, L.; Kouloulias, V.; Gazouli, M. In Vitro Effect of Hyperthermic Ag and Au Fe3O4 Nanoparticles in Cancer Cells. Beilstein Arch. 2019, 2019, 101. [Google Scholar]
- Shanei, A.; Sazgarnia, A. An Overview of Therapeutic Applications of Ultrasound Based on Synergetic Effects with Gold Nanoparticles and Laser Excitation. Iran. J. Basic Med. Sci. 2019, 22, 848–855. [Google Scholar] [CrossRef]
- Canavese, G.; Ancona, A.; Racca, L.; Canta, M.; Dumontel, B.; Barbaresco, F.; Limongi, T.; Cauda, V. Nanoparticle-Assisted Ultrasound: A Special Focus on Sonodynamic Therapy against Cancer. Chem. Eng. J. 2018, 340, 155–172. [Google Scholar] [CrossRef]
- Beik, J.; Khademi, S.; Attaran, N.; Sarkar, S.; Shakeri-Zadeh, A.; Ghaznavi, H.; Ghadiri, H. A Nanotechnology-Based Strategy to Increase the Efficiency of Cancer Diagnosis and Therapy: Folate-Conjugated Gold Nanoparticles. Curr. Med. Chem. 2017, 24, 4399–4416. [Google Scholar] [CrossRef]
- Izadifar, Z.; Izadifar, Z.; Chapman, D.; Babyn, P. An Introduction to High Intensity Focused Ultrasound: Systematic Review on Principles, Devices, and Clinical Applications. J. Clin. Med. Res. 2020, 9, 460. [Google Scholar] [CrossRef]
- Kennedy, J.E. High-Intensity Focused Ultrasound in the Treatment of Solid Tumours. Nat. Rev. Cancer 2005, 5, 321–327. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Z.; Jiang, S. Potentiation of Cytotoxicity of Adriamycin on Human Ovarian Carcinoma Cell Line 3AO by Low-Level Ultrasound. Ultrasonics 2001, 39, 307–309. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Z.; Mason, T.J. A Review of Research into the Uses of Low Level Ultrasound in Cancer Therapy. Ultrason. Sonochem. 2004, 11, 95–103. [Google Scholar] [CrossRef]
- Marmottant, P.; Hilgenfeldt, S. Controlled Vesicle Deformation and Lysis by Single Oscillating Bubbles. Nature 2003, 423, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Wang, C.C.J.; Blankschtein, D.; Langer, R. An Investigation of the Role of Cavitation in Low-Frequency Ultrasound-Mediated Transdermal Drug Transport. Pharm. Res. 2002, 19, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Barnett, S.B.; ter Haar, G.R.; Ziskin, M.C.; Nyborg, W.L.; Maeda, K.; Bang, J. Current Status of Research on Biophysical Effects of Ultrasound. Ultrasound Med. Biol. 1994, 20, 205–218. [Google Scholar] [CrossRef]
- Barnett, S.B. Conclusions and Recommendations on Thermal and Non-Thermal Mechanisms for Biological Effects of Ultrasound; CSIRO Research Publications Repository: Melbourne, Australia, 1996. [Google Scholar]
- Tuziuti, T.; Yasui, K.; Sivakumar, M.; Iida, Y.; Miyoshi, N. Correlation between Acoustic Cavitation Noise and Yield Enhancement of Sonochemical Reaction by Particle Addition. J. Phys. Chem. A 2005, 109, 4869–4872. [Google Scholar] [CrossRef]
- Farny, C.H.; Wu, T.; Holt, R.G.; Murray, T.W.; Roy, R.A. Nucleating Cavitation from Laser-Illuminated Nano-Particles. Acoust. Res. Lett. Online 2005, 6, 138–143. [Google Scholar] [CrossRef]
- Victor, E.G.; Silveira, P.C.L.; Possato, J.C.; da Rosa, G.L.; Munari, U.B.; de Souza, C.T.; Pinho, R.A.; da Silva, L.; Streck, E.L.; Paula, M.M.S. Pulsed ultrasound Associated with Gold Nanoparticle Gel Reduces Oxidative Stress Parameters and Expression of pro-Inflammatory Molecules in an Animal Model of Muscle Injury. J. Nanobiotechnol. 2012, 10, 11. [Google Scholar] [CrossRef]
- Serrano, A.L.; Baeza-Raja, B.; Perdiguero, E.; Jardí, M.; Muñoz-Cánoves, P. Interleukin-6 Is an Essential Regulator of Satellite Cell-Mediated Skeletal Muscle Hypertrophy. Cell Metab. 2008, 7, 33–44. [Google Scholar] [CrossRef]
- Li, Y.-P. TNF-Alpha Is a Mitogen in Skeletal Muscle. Am. J. Physiol. Cell Physiol. 2003, 285, C370–C376. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Shiau, A.-L.; Chen, S.-Y.; Chen, Y.-H.; Cheng, P.-C.; Chang, M.-Y.; Chen, D.-H.; Chou, C.-H.; Wang, C.-R.; Wu, C.-L. Amelioration of Collagen-Induced Arthritis in Rats by Nanogold. Arthritis Rheum. 2007, 56, 544–554. [Google Scholar] [CrossRef]
- Beik, J.; Abed, Z.; Shakeri-Zadeh, A.; Nourbakhsh, M.; Shiran, M.B. Evaluation of the Sonosensitizing Properties of Nano-Graphene Oxide in Comparison with Iron Oxide and Gold Nanoparticles. Phys. E Low Dimens. Syst. Nanostruct. 2016, 81, 308–314. [Google Scholar] [CrossRef]
- Aminabad, N.S.; Farshbaf, M.; Akbarzadeh, A. Recent Advances of Gold Nanoparticles in Biomedical Applications: State of the Art. Cell Biochem. Biophys. 2019, 77, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Mieszawska, A.J.; Mulder, W.J.M.; Fayad, Z.A.; Cormode, D.P. Multifunctional Gold Nanoparticles for Diagnosis and Therapy of Disease. Mol. Pharm. 2013, 10, 831–847. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.; Ferreira Carlos, F.; Pedrosa, P.; Lopez, A.; Baptista, P.V. Gold Nanoparticles for Diagnostics: Advances towards Points of Care. Diagnostics 2016, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; So, P.T.C.; Dasari, R.R.; Lim, D.-K. High Resolution Live Cell Raman Imaging Using Subcellular Organelle-Targeting SERS-Sensitive Gold Nanoparticles with Highly Narrow Intra-Nanogap. Nano Lett. 2015, 15, 1766–1772. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Tiwari, S.; Augustine, S.; Srivastava, S.; Yadav, B.K.; Malhotra, B.D. Highly Sensitive Protein Functionalized Nanostructured Hafnium Oxide Based Biosensing Platform for Non-Invasive Oral Cancer Detection. Sens. Actuators B Chem. 2016, 235, 1–10. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman Spectra of Pyridine Adsorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Feng, S.; Chen, R.; Lin, J.; Pan, J.; Wu, Y.; Li, Y.; Chen, J.; Zeng, H. Gastric Cancer Detection Based on Blood Plasma Surface-Enhanced Raman Spectroscopy Excited by Polarized Laser Light. Biosens. Bioelectron. 2011, 26, 3167–3174. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Li, B.; Wen, Z.; Luo, X.; Xue, L.; Li, L. Label-Free Blood Serum Detection by Using Surface-Enhanced Raman Spectroscopy and Support Vector Machine for the Preoperative Diagnosis of Parotid Gland Tumors. BMC Cancer 2015, 15, 650. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Yan, B.; Xue, L.; Li, Y.; Luo, X.; Ji, P. Surface-Enhanced Raman Spectroscopy of Blood Serum Based on Gold Nanoparticles for the Diagnosis of the Oral Squamous Cell Carcinoma. Lipids Health Dis. 2017, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Qu, Q.; Zhao, Y.; Luo, Z.; Zhao, Y.; Ng, K.W.; Zhao, Y. Graphene Oxide Wrapped Gold Nanoparticles for Intracellular Raman Imaging and Drug Delivery. J. Mater. Chem. B Mater. Biol. Med. 2013, 1, 6495–6500. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, X. Gold Nanoparticles for Photoacoustic Imaging. Nanomedicine 2015, 10, 299–320. [Google Scholar] [CrossRef]
- Song, J.; Kim, J.; Hwang, S.; Jeon, M.; Jeong, S.; Kim, C.; Kim, S. “Smart” Gold Nanoparticles for Photoacoustic Imaging: An Imaging Contrast Agent Responsive to the Cancer Microenvironment and Signal Amplification via pH-Induced Aggregation. Chem. Commun. 2016, 52, 8287–8290. [Google Scholar] [CrossRef]
- Sun, I.-C.; Dumani, D.; Emelianov, S.Y. Ultrasound-Guided Photoacoustic Imaging of Lymph Nodes with Biocompatible Gold Nanoparticles as a Novel Contrast Agent (Conference Presentation). In Proceedings of the Colloidal Nanoparticles for Biomedical Applications XII, San Francisco, CA, USA, 28 January–2 February 2017; Liang, X.-J., Parak, W.J., Osiński, M., Eds.; SPIE: Bellingham, WA, USA, 2017. [Google Scholar]
- Jin, H.-Y.; Li, D.-W.; Zhang, N.; Gu, Z.; Long, Y.-T. Analyzing Carbohydrate-Protein Interaction Based on Single Plasmonic Nanoparticle by Conventional Dark Field Microscopy. ACS Appl. Mater. Interfaces 2015, 7, 12249–12253. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Huang, X.; Kang, B.; El-Sayed, M.A. Dark-Field Light Scattering Imaging of Living Cancer Cell Component from Birth through Division Using Bioconjugated Gold Nanoprobes. J. Biomed. Opt. 2010, 15, 046025. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Y.; Gao, P.F.; Zou, H.Y.; Huang, C.Z. Precision Improvement in Dark-Field Microscopy Imaging by Using Gold Nanoparticles as an Internal Reference: A Combined Theoretical and Experimental Study. Nanoscale 2016, 8, 8729–8736. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; He, Y.; Liu, H.; Luo, Y.; Shen, M.; Xia, J.; Shi, X. Targeted CT Imaging of Human Hepatocellular Carcinoma Using Low-Generation Dendrimer-Entrapped Gold Nanoparticles Modified with Lactobionic Acid. J. Mater. Chem. B Mater. Biol. Med. 2015, 3, 286–295. [Google Scholar] [CrossRef]
- Meir, R.; Shamalov, K.; Betzer, O.; Motiei, M.; Horovitz-Fried, M.; Yehuda, R.; Popovtzer, A.; Popovtzer, R.; Cohen, C.J. Nanomedicine for Cancer Immunotherapy: Tracking Cancer-Specific T-Cells in Vivo with Gold Nanoparticles and CT Imaging. ACS Nano 2015, 9, 6363–6372. [Google Scholar] [CrossRef]
- Kim, J.; Lee, N.; Hyeon, T. Recent Development of Nanoparticles for Molecular Imaging. Philos. Trans. A Math. Phys. Eng. Sci. 2017, 375. [Google Scholar] [CrossRef]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic Nanoparticles for MRI Contrast Agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Lin, H.-T.; Chen, T.-H.; Chen, C.-A.; Chang, H.-T.; Chen, C.-F. Signal Amplified Gold Nanoparticles for Cancer Diagnosis on Paper-Based Analytical Devices. ACS Sens. 2018, 3, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, Y.; Jiang, Y.; Ju, H. Polyadenine-Modulated DNA Conformation Monitored by Surface-Enhanced Raman Scattering (SERS) on Multibranched Gold Nanoparticles and Its Sensing Application. Chemistry 2017, 23, 9332–9337. [Google Scholar] [CrossRef] [PubMed]
- Cheung-Lau, J.C.; Liu, D.; Pulsipher, K.W.; Liu, W.; Dmochowski, I.J. Engineering a Well-Ordered, Functional Protein-Gold Nanoparticle Assembly. J. Inorg. Biochem. 2014, 130, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.K.; Vaze, A.; Shen, M.; Rusling, J.F. High-Throughput Electrochemical Microfluidic Immunoarray for Multiplexed Detection of Cancer Biomarker Proteins. ACS Sens. 2016, 1, 1036–1043. [Google Scholar] [CrossRef]
- Saeed, A.A.; Sánchez, J.L.A.; O’Sullivan, C.K.; Abbas, M.N. DNA Biosensors Based on Gold Nanoparticles-Modified Graphene Oxide for the Detection of Breast Cancer Biomarkers for Eary Diagnosis. Bioelectrochemistry 2017, 118, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Zeng, S.; Yong, K.-T.; Roy, I.; Dinh, X.-Q.; Yu, X.; Luan, F. A Review on Functionalized Gold Nanoparticles for Biosensing Applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Pingarrón, J.M.; Yáñez-Sedeño, P.; González-Cortés, A. Gold Nanoparticle-Based Electrochemical Biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Meola, A.; Rao, J.; Chaudhary, N.; Sharma, M.; Chang, S.D. Gold Nanoparticles for Brain Tumor Imaging: A Systematic Review. Front. Neurol. 2018, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2016, 20, 1–11. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Kim, M.; Shinde, S.; Sung, J.-S.; Ghodake, G. Cytotoxicity and Antibacterial Assessment of Gallic Acid Capped Gold Nanoparticles. Colloids Surf. B Biointerfaces 2017, 149, 162–167. [Google Scholar] [CrossRef]
- Bhamidipati, M.; Fabris, L. Multiparametric Assessment of Gold Nanoparticle Cytotoxicity in Cancerous and Healthy Cells: The Role of Size, Shape, and Surface Chemistry. Bioconjug. Chem. 2017, 28, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Walkey, C.D.; Olsen, J.B.; Guo, H.; Emili, A.; Chan, W.C.W. Secreted Biomolecules Alter the Biological Identity and Cellular Interactions of Nanoparticles. ACS Nano 2014, 8, 5515–5526. [Google Scholar] [CrossRef] [PubMed]
- Grainger, D.W.; Castner, D.G. Nanobiomaterials and Nanoanalysis: Opportunities for Improving the Science to Benefit Biomedical Technologies. Adv. Mater. 2008, 20, 867–877. [Google Scholar] [CrossRef]
- Walkey, C.D.; Olsen, J.B.; Guo, H.; Emili, A.; Chan, W.C.W. Nanoparticle Size and Surface Chemistry Determine Serum Protein Adsorption and Macrophage Uptake. J. Am. Chem. Soc. 2012, 134, 2139–2147. [Google Scholar] [CrossRef]
- Bailly, A.-L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.-A. In Vivo Evaluation of Safety, Biodistribution and Pharmacokinetics of Laser-Synthesized Gold Nanoparticles. Sci. Rep. 2019, 9, 12890. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Dai, Q.; Bertleff-Zieschang, N.; Braunger, J.A.; Björnmalm, M.; Cortez-Jugo, C.; Caruso, F. Particle Targeting in Complex Biological Media. Adv. Healthc. Mater. 2018, 7, 1700575. [Google Scholar] [CrossRef] [PubMed]


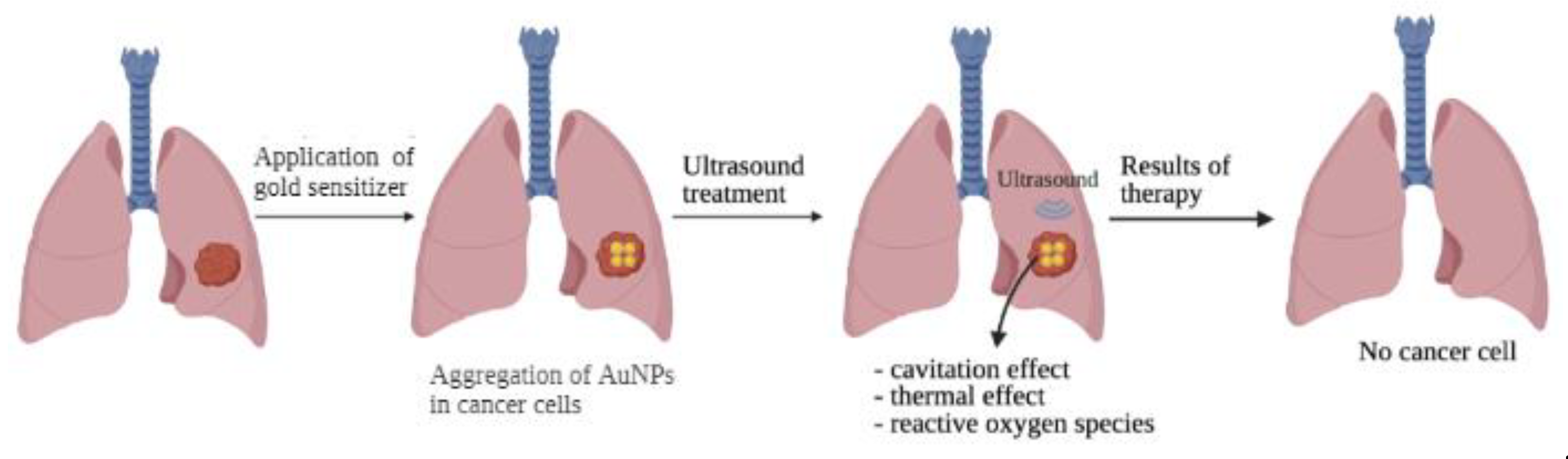

| Ultrasound Therapy | ||||
|---|---|---|---|---|
| Type of Therapy | Intensity, W/cm2 | Frequency | Effect | Features |
| High intensity focused ultrasound (HIFU) | 100–20,000 | 0.25–10 MHz | heat to destroy cells | local overheating of the tissue with a temperature from 60 to 85 °C |
| Low-intensity ultrasound (LIU) | 0.1–5 | 1 Hz–100 kHz | slight heating, improved permeability | increased the activity of chemotherapeutic molecules in cancer therapy; used for direct action on cells and their components (sonoporation); it has been used for the delivery or transfection of genes and for accelerating tissue heating, as well as for its anti-vascular effect on the neovascular network of the tumor |
| Sonodynamic therapy (SDT) | 1–10 | 0.5–2 MHz | free radical nature associated with the cavitation effects of ultrasound | the combined effect on the tumor of ultrasound and chemical non-medicinal compounds that enhance the therapeutic effect |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vodyashkin, A.A.; Rizk, M.G.H.; Kezimana, P.; Kirichuk, A.A.; Stanishevskiy, Y.M. Application of Gold Nanoparticle-Based Materials in Cancer Therapy and Diagnostics. ChemEngineering 2021, 5, 69. https://doi.org/10.3390/chemengineering5040069
Vodyashkin AA, Rizk MGH, Kezimana P, Kirichuk AA, Stanishevskiy YM. Application of Gold Nanoparticle-Based Materials in Cancer Therapy and Diagnostics. ChemEngineering. 2021; 5(4):69. https://doi.org/10.3390/chemengineering5040069
Chicago/Turabian StyleVodyashkin, Andrey A., Marko George Halim Rizk, Parfait Kezimana, Anatoly A. Kirichuk, and Yaroslav M. Stanishevskiy. 2021. "Application of Gold Nanoparticle-Based Materials in Cancer Therapy and Diagnostics" ChemEngineering 5, no. 4: 69. https://doi.org/10.3390/chemengineering5040069
APA StyleVodyashkin, A. A., Rizk, M. G. H., Kezimana, P., Kirichuk, A. A., & Stanishevskiy, Y. M. (2021). Application of Gold Nanoparticle-Based Materials in Cancer Therapy and Diagnostics. ChemEngineering, 5(4), 69. https://doi.org/10.3390/chemengineering5040069







