Comparison of the Fluidized State Stability from Radioactive Particle Tracking Results
Abstract
1. Introduction
2. Materials and Methods
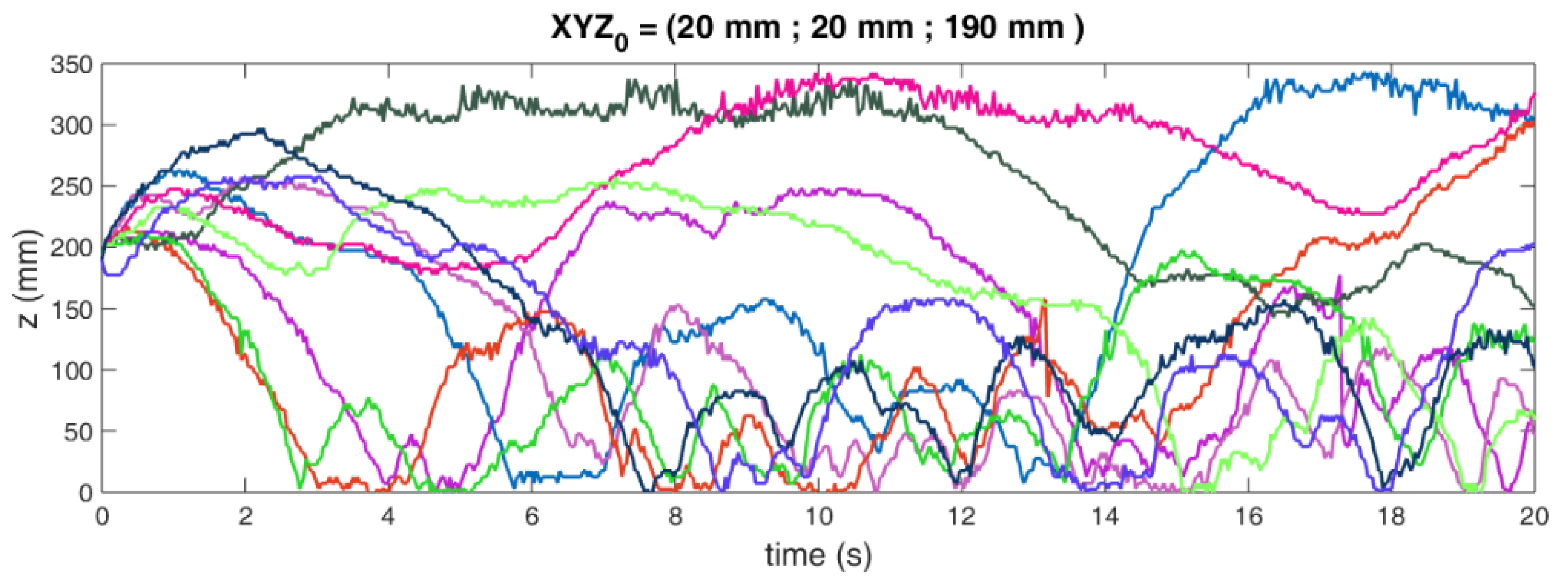
2.1. The Radioactive Particle Tracking Technique
2.2. Liquid–Solid Fluidized Bed (LSFB) Operational Parameters
2.3. Gas–Liquid–Solid Fluidized Bed (GLSFB) Operational Parameters
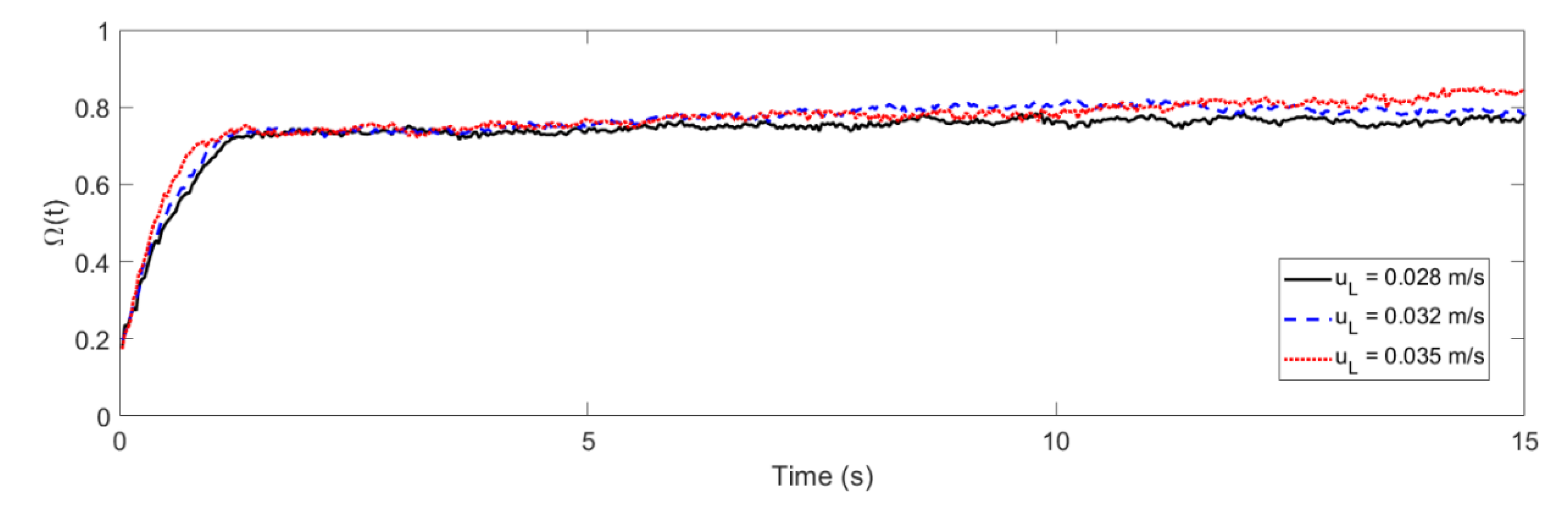
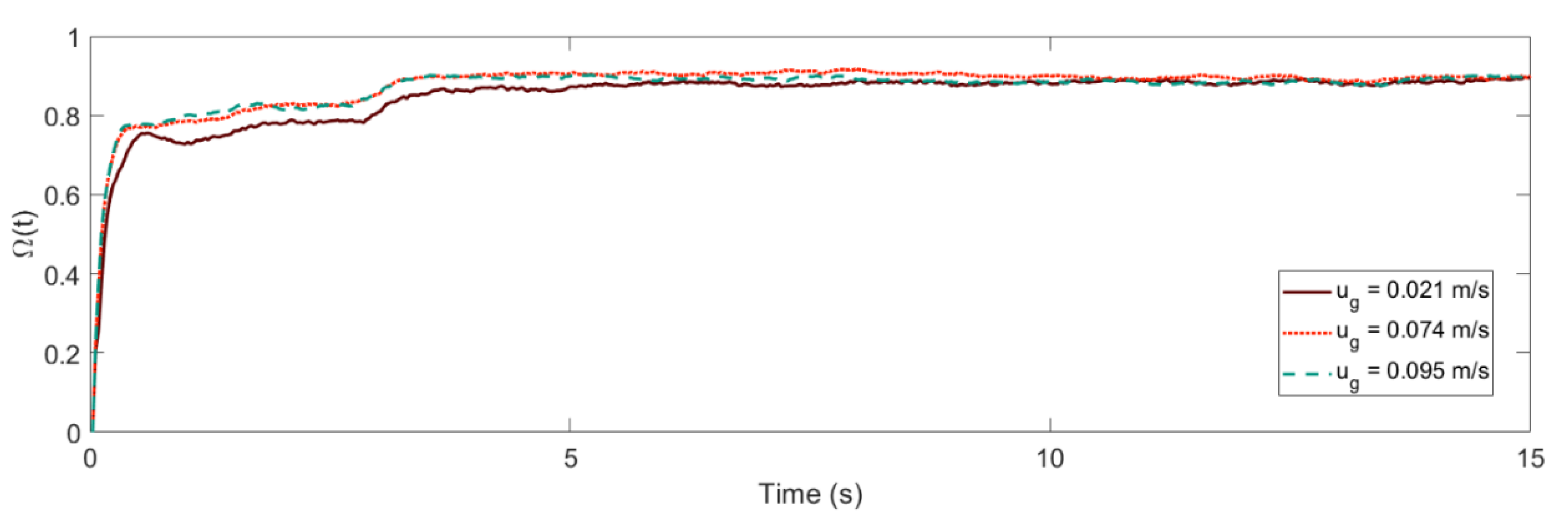
3. Shannon Entropy and Mixing Behavior
4. Mixing and Stability Assessment Based on Information Geometry
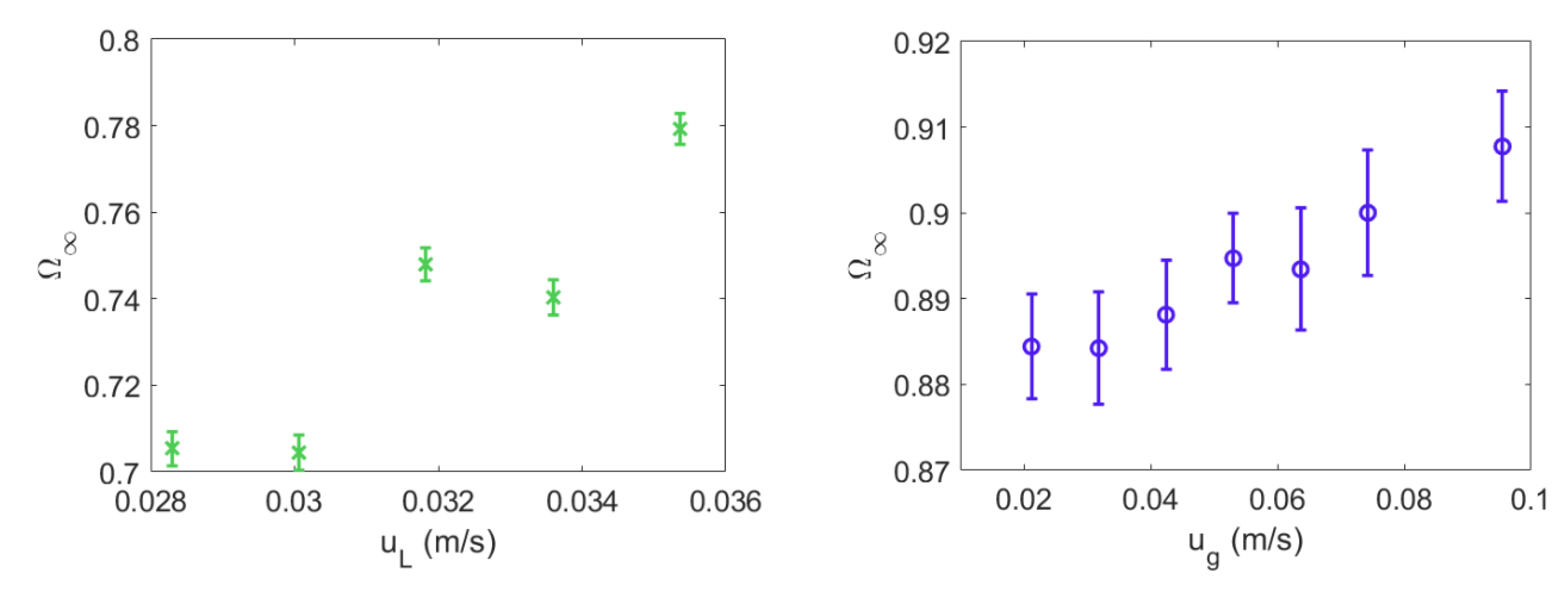
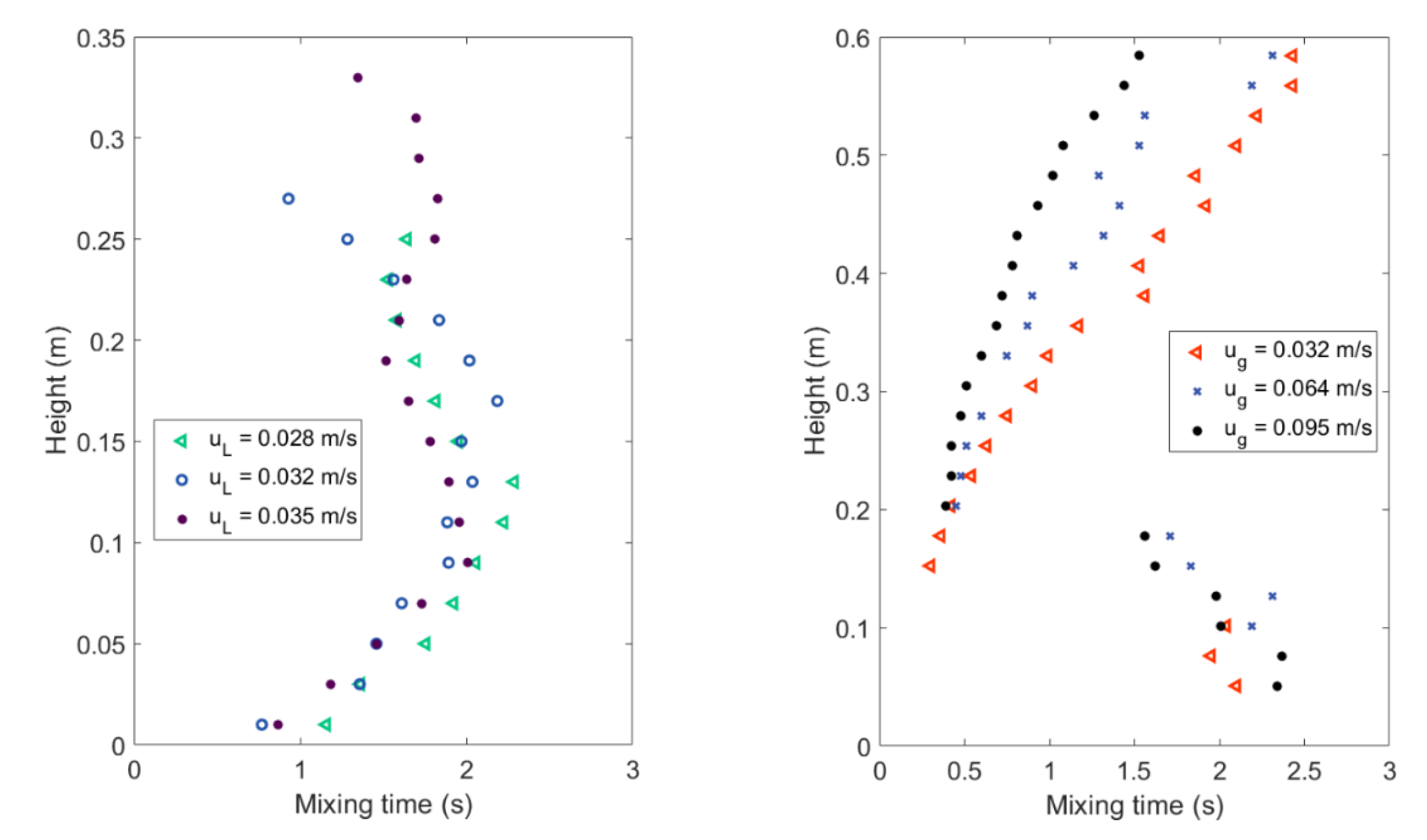
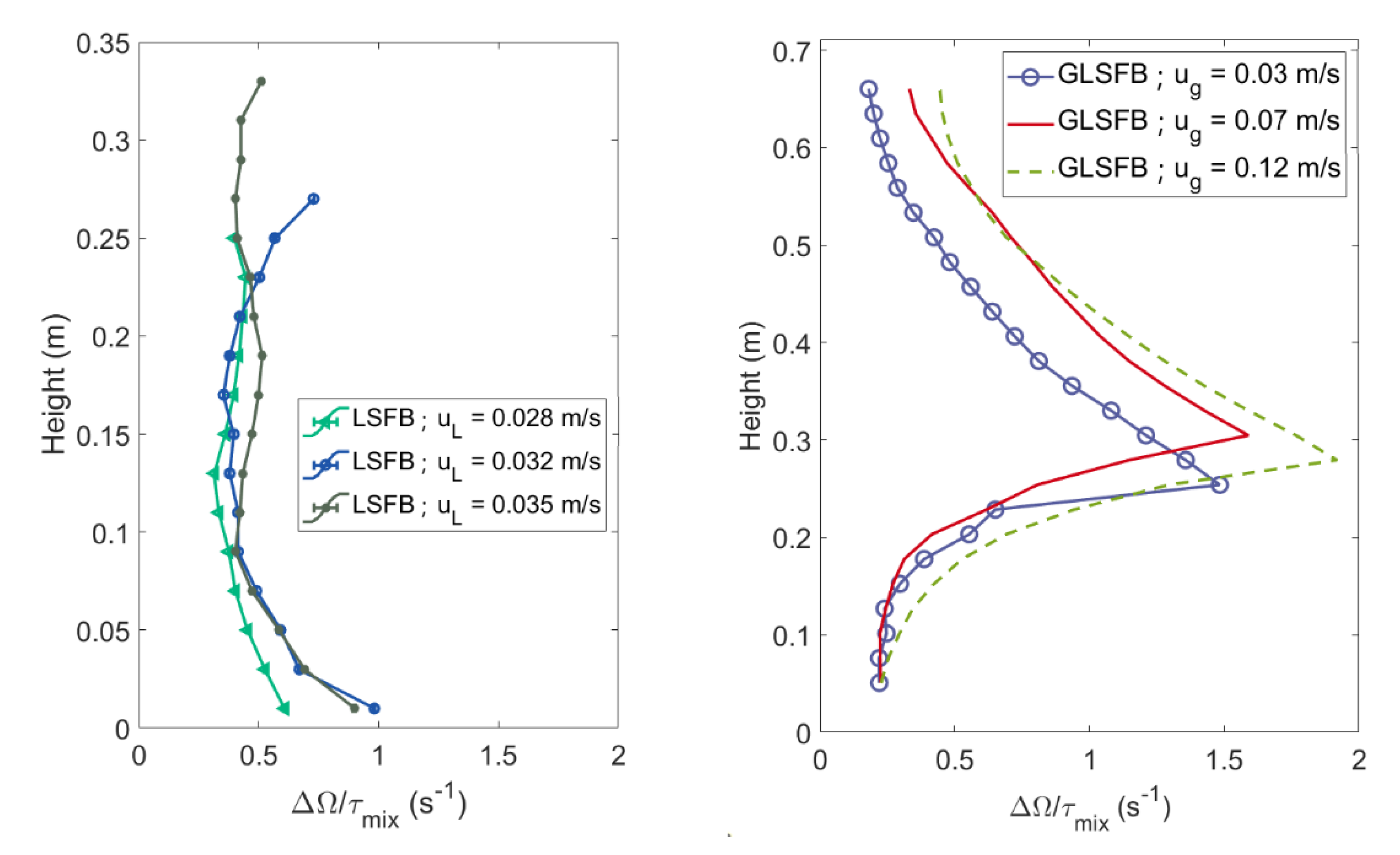
4.1. Mixing Times and Entropy Production
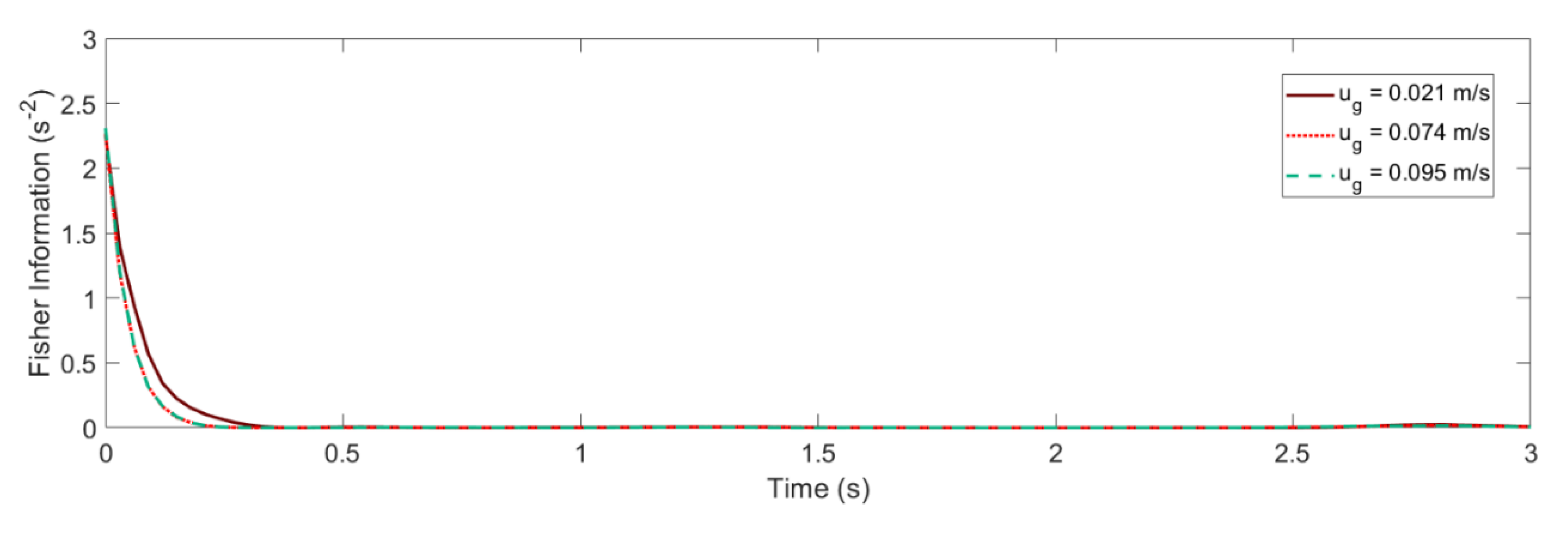
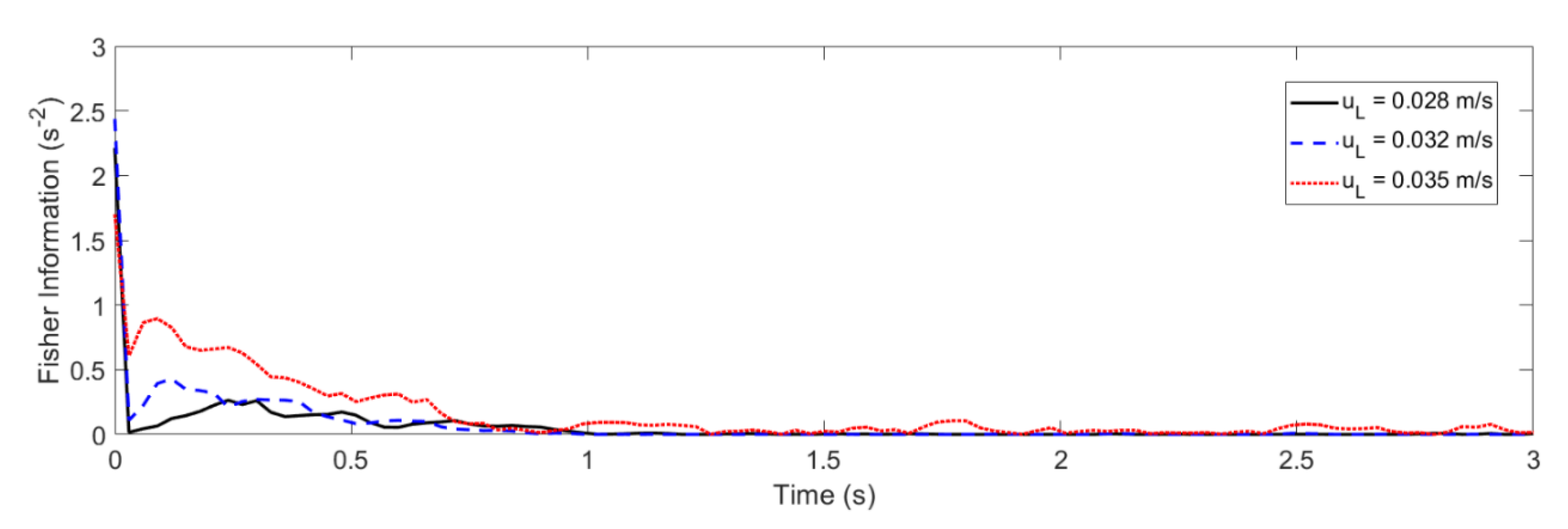
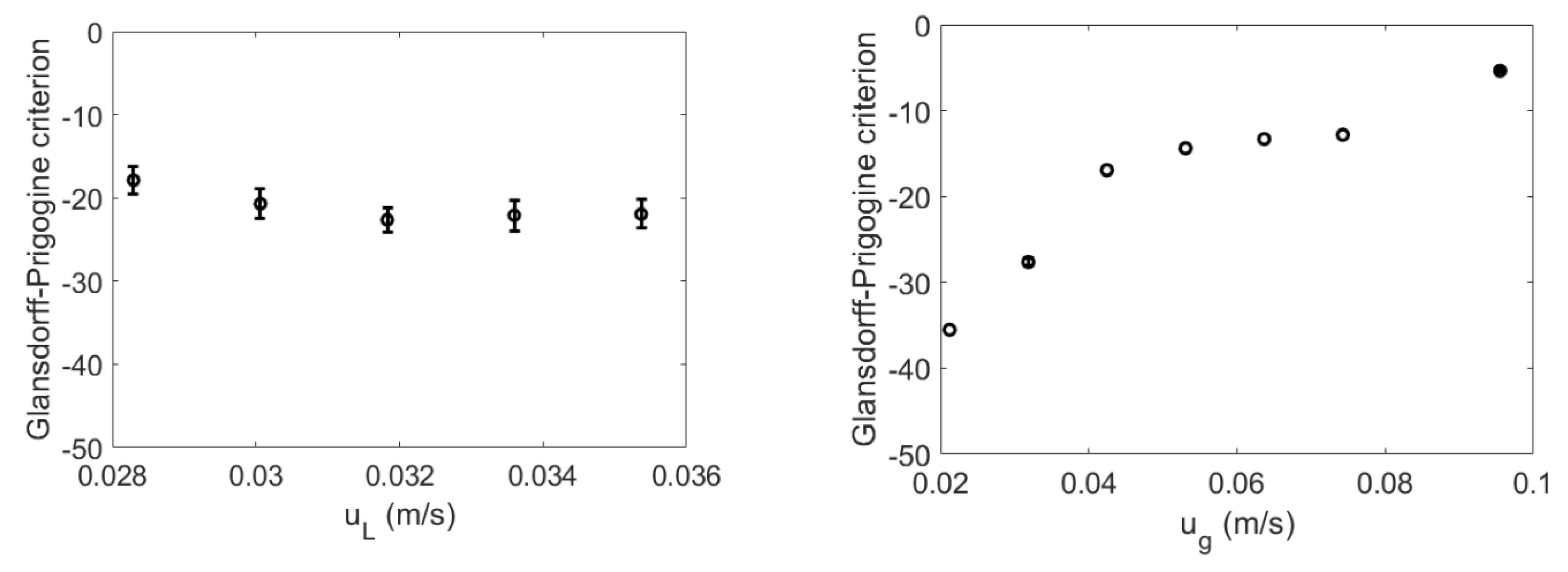
4.2. Glansdorff–Prigogine Criterion Based on the Fisher Information
- If the Glandsdorff–Prigogine stability measure sign is positive, the Fisher information diverges, so the entropy also diverges rapidly; thus, the system is unstable.
- If the Glandsdorff–Prigogine stability measure sign is negative, the Fisher information will decay to zero; thus, the Shannon entropy reaches a state where it does not change over time, making the system stable.
- A Glandsdorff–Prigogine stability measure tending to zero means that the system is either stable (e.g., fixed bed) or metastable, in which cases could be used to set operating window limits.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agricola, G. De Re Metallica; Dover Publications: Mineola, NY, USA, 2013; ISBN 978-1-306-32529-5. [Google Scholar]
- Piovano, S.; Salierno, G.L.; Montmany, E.; D’Agostino, M.; Maestri, M.; Cassanello, M. Bed Expansion and Particle Classification in Liquid Fluidized Beds with Structured Internals. Chem. Eng. Technol. 2015, 38, 423–430. [Google Scholar] [CrossRef]
- Salucci, E.; Russo, V.; Salmi, T.; Di Serio, M.; Tesser, R. Intraparticle Model for Non-Uniform Active Phase Distribution Catalysts in a Batch Reactor. Chem. Eng. 2021, 5, 38. [Google Scholar] [CrossRef]
- Loewert, M.; Pfeifer, P. Dynamically Operated Fischer-Tropsch Synthesis in PtL-Part 1: System Response on Intermittent Feed. Chem. Eng. 2020, 4, 21. [Google Scholar] [CrossRef]
- Chen, K.-H.; Wang, S.S.-S.; Show, P.-L.; Lin, G.-T.; Chang, Y.-K. A Rapid and Efficient Technique for Direct Extraction of C-Phycocyanin from Highly Turbid Spirulina Platensis Algae Using Hydrophobic Interaction Chromatography in Stirred Fluidized Bed. Biochem. Eng. J. 2018, 140, 47–56. [Google Scholar] [CrossRef]
- Maciel, K.S.; Santos, L.S.; Bonomo, R.C.F.; Verissimo, L.A.A.; Minim, V.P.R.; Minim, L.A. Purification of Lactoferrin from Sweet Whey Using Ultrafiltration Followed by Expanded Bed Chromatography. Sep. Purif. Technol. 2020, 251, 117324. [Google Scholar] [CrossRef]
- Pangarkar, V.G. Process Intensification in Multiphase Reactors: From Concept to Reality. Chem. Eng. Process. 2017, 120, 1–8. [Google Scholar] [CrossRef]
- Van Gerven, T.; Stankiewicz, A. Structure, Energy, Synergy, Time—The Fundamentals of Process Intensification. Ind. Eng. Chem. Res. 2009, 48, 2465–2474. [Google Scholar] [CrossRef]
- Reay, D.; Ramshaw, C.; Harvey, A. Process Intensification. In Process Intensification; Elsevier: Amsterdam, The Netherlands, 2013; pp. 27–55. ISBN 978-0-08-098304-2. [Google Scholar]
- Duduković, M.; Mills, P. Scale-up and Multiphase Reaction Engineering. Curr. Opin. Chem. 2015, 9, 49–58. [Google Scholar] [CrossRef]
- Ali, N.; Al-Juwaya, T.; Al-Dahhan, M. An Advanced Evaluation of the Mechanistic Scale-up Methodology of Gas–Solid Spouted Beds Using Radioactive Particle Tracking. Particuology 2017, 34, 48–60. [Google Scholar] [CrossRef]
- Goniva, C.; Kloss, C.; Deen, N.G.; Kuipers, J.A.M.; Pirker, S. Influence of Rolling Friction on Single Spout Fluidized Bed Simulation. Particuology 2012, 10, 582–591. [Google Scholar] [CrossRef]
- Hager, A.; Kloss, C.; Goniva, C. Combining Open Source and Easy Access in the field of DEM and coupled CFD-DEM: LIGGGHTS®, CFDEM®coupling and CFDEM®workbench. In Computer Aided Chemical Engineering; 28 European Symposium on Computer Aided Process Engineering; Friedl, A., Klemeš, J.J., Radl, S., Varbanov, P.S., Wallek, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 43, pp. 1699–1704. [Google Scholar]
- Roy, S. Radiotracer and Particle Tracking Methods, Modeling and Scale-Up. AIChE J. 2017, 63, 314–326. [Google Scholar] [CrossRef]
- Salierno, G.; Maestri, M.; Piovano, S.; Cassanello, M.; Cardona, M.A.; Hojman, D.; Somacal, H. Solid Motion in a Three-Phase Bubble Column Examined with Radioactive Particle Tracking. Flow Meas. Instrum. 2018, 62, 196–204. [Google Scholar] [CrossRef]
- Wang, M. Industrial Tomography: Systems and Applications; Elsevier: Boston, MA, USA, 2015; ISBN 978-1-78242-118-4. [Google Scholar]
- Chaouki, J.; Larachi, F.; Dudukovic, M.P. (Eds.) Non-Invasive Monitoring of Multiphase Flows; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 1997; ISBN 978-0-444-82521-6. [Google Scholar]
- Bhusarapu, S.; Cassanello, M.; Al-Dahhan, M.H.; Dudukovic, M.P.; Trujillo, S.; O’Hern, T.J. Dynamical Features of the Solid Motion in Gas–Solid Risers. Int. J. Multiph. Flow 2007, 33, 164–181. [Google Scholar] [CrossRef]
- Salierno, G.L.; Maestri, M.; Piovano, S.; Cassanello, M.; Cardona, M.A.; Hojman, D.; Somacal, H. Discrete Axial Motion of a Radioactive Tracer Reconstructed from the Response of Axially Aligned Detectors: Application to the Analysis of a Bubble Column Dynamics. Chem. Eng. Sci. 2013, 100, 402–412. [Google Scholar] [CrossRef]
- Shiraishi, N.; Sagawa, T. Fluctuation Theorem for Partially Masked Nonequilibrium Dynamics. Phys. Rev. E 2015, 91, 012130. [Google Scholar] [CrossRef]
- Cover, T.M.; Thomas, J.A. Elements of Information Theory; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; ISBN 978-0-471-20061-1. [Google Scholar]
- Salierno, G.; Maestri, M.; Piovano, S.; Cassanello, M.; Cardona, M.A.; Hojman, D.; Somacal, H. Features of the Motion of Gel Particles in a Three-Phase Bubble Column under Foaming and Non-Foaming Conditions. Chin. J. Chem. Eng. 2018, 26, 1370–1382. [Google Scholar] [CrossRef]
- Liu, M.; Liu, L.; Zhang, H.; Yi, B.; Everaert, N. Alginate Oligosaccharides Preparation, Biological Activities and Their Application in Livestock and Poultry. J. Integr. Agric. 2021, 20, 24–34. [Google Scholar] [CrossRef]
- Sutirman, Z.A.; Sanagi, M.M.; Wan Aini, W.I. Alginate-Based Adsorbents for Removal of Metal Ions and Radionuclides from Aqueous Solutions: A Review. Int. J. Biol. Macromol. 2021, 174, 216–228. [Google Scholar] [CrossRef]
- Borgiallo, A.; Rojas, R. Reactivity and Heavy Metal Removal Capacity of Calcium Alginate Beads Loaded with Ca–Al Layered Double Hydroxides. Chem. Eng. 2019, 3, 22. [Google Scholar] [CrossRef]
- Zhan, T.; Lu, S.; Liu, X.; Teng, H.; Hou, W. Alginate Derived Co3O4/Co Nanoparticles Decorated in N-Doped Porous Carbon as an Efficient Bifunctional Catalyst for Oxygen Evolution and Reduction Reactions. Electrochim. Acta 2018, 265, 681–689. [Google Scholar] [CrossRef]
- Ghorbani-Vaghei, R.; Veisi, H.; Aliani, M.H.; Mohammadi, P.; Karmakar, B. Alginate Modified Magnetic Nanoparticles to Immobilization of Gold Nanoparticles as an Efficient Magnetic Nanocatalyst for Reduction of 4-Nitrophenol in Water. J. Mol. Liq. 2021, 327, 114868. [Google Scholar] [CrossRef]
- Lorenzoni, A.S.G.; Aydos, L.F.; Klein, M.P.; Ayub, M.A.Z.; Rodrigues, R.C.; Hertz, P.F. Continuous Production of Fructooligosaccharides and Invert Sugar by Chitosan Immobilized Enzymes: Comparison between in Fluidized and Packed Bed Reactors. J. Mol. Catal. B Enzym. 2015, 111, 51–55. [Google Scholar] [CrossRef]
- Mehrotra, T.; Dev, S.; Banerjee, A.; Chatterjee, A.; Singh, R.; Aggarwal, S. Use of Immobilized Bacteria for Environmental Bioremediation: A Review. J. Environ. Chem. Eng. 2021, 9, 105920. [Google Scholar] [CrossRef]
- Ren, S.; Chen, R.; Wu, Z.; Su, S.; Hou, J.; Yuan, Y. Enzymatic Characteristics of Immobilized Carbonic Anhydrase and Its Applications in CO2 Conversion. Colloids Surf. B Biointerfaces 2021, 204, 111779. [Google Scholar] [CrossRef]
- Derksen, J.J. Simulations of Solid–Liquid Mass Transfer in Fixed and Fluidized Beds. Chem. Eng. J. 2014, 255, 233–244. [Google Scholar] [CrossRef]
- Cassanello, M.; Larachi, F.; Guy, C.; Chaouki, J. Solids Mixing in Gas-Liquid-Solid Fluidized Beds: Experiments and Modelling. Chem. Eng. Sci. 1996, 51, 2011–2020. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; Wiley: New York, NY, USA, 1999; ISBN 978-0-471-25424-9. [Google Scholar]
- Wu, D.; Gu, Z.; Li, Y. Attrition of Catalyst Particles in a Laboratory-Scale Fluidized-Bed Reactor. Chem. Eng. Sci. 2015, 135, 431–440. [Google Scholar] [CrossRef]
- Martin, C.; Olmos, É.; Collignon, M.-L.; De Isla, N.; Blanchard, F.; Chevalot, I.; Marc, A.; Guedon, E. Revisiting MSC Expansion from Critical Quality Attributes to Critical Culture Process Parameters. Process. Biochem. 2017, 59, 231–243. [Google Scholar] [CrossRef]
- Paidoussis, M.P. Fluid-Structure Interactions: Slender Structures and Axial Flow, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2014; ISBN 978-0-12-397312-2. [Google Scholar]
- Salierno, G.; Maestri, M.; Piovano, S.; Cassanello, M.; Cardona, M.A.; Hojman, D.; Somacal, H. Calcium Alginate Beads Motion in a Foaming Three-Phase Bubble Column. Chem. Eng. J. 2017, 324, 358–369. [Google Scholar] [CrossRef]
- Maestri, M.; Salierno, G.; Piovano, S.; Cassanello, M.; Cardona, M.A.; Hojman, D.; Somacal, H. CFD-DEM Modeling of Solid Motion in a Water-Calcium Alginate Fluidized Column and Its Comparison with Results from Radioactive Particle Tracking. Chem. Eng. J. 2019, 377, 120339. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Abel, M.; Biferale, L.; Cencini, M.; Falcioni, M.; Vergni, D.; Vulpiani, A. Exit-Times and ϵ-Entropy for Dynamical Systems, Stochastic Processes, and Turbulence. Phys. D Nonlinear Phenom. 2000, 147, 12–35. [Google Scholar] [CrossRef][Green Version]
- Guida, A.; Nienow, A.W.; Barigou, M. Shannon Entropy for Local and Global Description of Mixing by Lagrangian Particle Tracking. Chem. Eng. Sci. 2010, 65, 2865–2883. [Google Scholar] [CrossRef]
- Prigogine, I.; Nicolis, G. Self-Organisation in Nonequilibrium Systems: Towards A Dynamics of Complexity. In Bifurcation Analysis; Hazewinkel, M., Jurkovich, R., Paelinck, J.H.P., Eds.; Springer: Dordrecht, The Netherlands, 1985; pp. 3–12. ISBN 978-94-009-6241-5. [Google Scholar]
- Ito, S. Thermodynamics of Information Geometry as a Generalization of the Glansdorff-Prigogine Criterion for Stability. arXiv 2019, arXiv:1908.09446. [Google Scholar]
- Ito, S.; Dechant, A. Stochastic Time-Evolution, Information Geometry and the Cramer-Rao Bound. arXiv 2019, arXiv:1810.06832. [Google Scholar] [CrossRef]
- Yoshimura, K.; Ito, S. Information Geometric Inequalities of Chemical Thermodynamics. arXiv 2020, arXiv:2005.08444. [Google Scholar]
- Ebrahimi-Mamaghani, A.; Sotudeh-Gharebagh, R.; Zarghami, R.; Mostoufi, N. Dynamics of Two-Phase Flow in Vertical Pipes. J. Fluids Struct. 2019, 87, 150–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, P.; Li, W.; Yu, K. Turbulent Mass Transfer Model for the Simulation of Liquid-Solid CFB Risers and Its Verification. Powder Technol. 2021, 377, 847–856. [Google Scholar] [CrossRef]
- Salierno, G.; Maestri, M.; Picabea, J.; Cassanello, M.; De Blasio, C.; Cardona, M.A.; Hojman, D.; Somacal, H. Industrially Relevant Radioactive Particle Tracking Study on the Motion of Adsorbent Granules Suspended in a Pilot-Scale Water–Air Three-Phase Fluidized Bed. Chem. Eng. Res. Des. 2021, 173, 305–316. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salierno, G.; Gradišek, A.; Maestri, M.; Picabea, J.; Cassanello, M.; De Blasio, C.; Cardona, M.A.; Hojman, D.; Somacal, H. Comparison of the Fluidized State Stability from Radioactive Particle Tracking Results. ChemEngineering 2021, 5, 65. https://doi.org/10.3390/chemengineering5040065
Salierno G, Gradišek A, Maestri M, Picabea J, Cassanello M, De Blasio C, Cardona MA, Hojman D, Somacal H. Comparison of the Fluidized State Stability from Radioactive Particle Tracking Results. ChemEngineering. 2021; 5(4):65. https://doi.org/10.3390/chemengineering5040065
Chicago/Turabian StyleSalierno, Gabriel, Anton Gradišek, Mauricio Maestri, Julia Picabea, Miryan Cassanello, Cataldo De Blasio, María Angélica Cardona, Daniel Hojman, and Héctor Somacal. 2021. "Comparison of the Fluidized State Stability from Radioactive Particle Tracking Results" ChemEngineering 5, no. 4: 65. https://doi.org/10.3390/chemengineering5040065
APA StyleSalierno, G., Gradišek, A., Maestri, M., Picabea, J., Cassanello, M., De Blasio, C., Cardona, M. A., Hojman, D., & Somacal, H. (2021). Comparison of the Fluidized State Stability from Radioactive Particle Tracking Results. ChemEngineering, 5(4), 65. https://doi.org/10.3390/chemengineering5040065









