Water Purification of Classical and Emerging Organic Pollutants: An Extensive Review
Abstract
:1. Introduction
- Classical pollutants: “water purification”, “organic pollutants”
- Emerging pollutants: “water purification”, “emerging pollutants”.
Description of the Main Removal Techniques of Organic Pollutants from Water: Strengths and Weaknesses
2. Removal of Classical Organic Pollutants from Water
2.1. Halogenated Hydrocarbons
2.1.1. Adsorption
2.1.2. Catalysis
2.1.3. Reductive and Oxidative Processes
2.1.4. Phytoremediation
2.1.5. Biodegradation
2.1.6. Membranes
2.2. Aromatic Hydrocarbons
2.2.1. Adsorption
2.2.2. Photocatalysis
2.2.3. Reductive and Oxidative Processes
2.2.4. Liquid–Liquid Extraction (LLE)
2.2.5. Combined Methods
2.3. Phenols
2.3.1. Adsorption
2.3.2. Photocatalysis
2.3.3. Reductive and Oxidative Processes
2.3.4. Combined Methods
2.4. Aldehydes
2.4.1. Adsorption
2.4.2. Combined Methods
3. Removal of Emerging Organic Pollutants from Water
- dyes.
- endocrine disrupters and personal care products (PCP).
- pharmaceuticals (PhACs).
3.1. Dyes
3.1.1. Adsorption
3.1.2. Catalysis
3.1.3. Phytoremediation
3.1.4. Membranes
3.1.5. Combined Methods
3.2. Endocrine Disrupters (EDs) and Personal Care Products (PCPs)
3.2.1. Adsorption
3.2.2. Reductive and Oxidative Processes
3.2.3. Membranes
3.2.4. Combined Methods
3.3. Pharmaceuticals (PhACs)
3.3.1. Adsorption
3.3.2. Catalysis
3.3.3. Oxidative Processes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Water Use in Europe. Available online: https://www.eea.europa.eu/signals/signals-2018-content-list/infographic/water-use-in-europe/view (accessed on 16 March 2020).
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Alrozi, R. Removal of malachite green dye from aqueous solution using rambutan peel-based activated carbon: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2011, 171, 510–516. [Google Scholar] [CrossRef]
- Sweetman, M.; May, S.; Mebberson, N.; Pendleton, P.; Vasilev, K.; Plush, S.; Hayball, J. Activated carbon, carbon nanotubes and graphene: Materials and composites for advanced water purification. C J. Carbon Res. 2017, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Qu, F.; Zhu, L.; Yang, K. Adsorption behaviors of volatile organic compounds (VOCs) on porous clay heterostructures (PCH). J. Hazard. Mater. 2009, 170, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liu, G.; Wang, Q.; Zhu, A.; Liu, P.; Wu, Q. Synthesis of a novel mesoporous Fe3O4@SiO2/CTAB-SiO2 composite material and its application in the efficient removal of bisphenol A from water. Colloid Polym. Sci. 2021, 299, 807–822. [Google Scholar] [CrossRef]
- Liu, F.; Chen, C.; Qian, J. Film-like bacterial cellulose/cyclodextrin oligomer composites with controllable structure for the removal of various persistent organic pollutants from water. J. Hazard. Mater. 2021, 405, 124122. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Fan, B.M.; Wen, B.Y.; Jiang, C. Experimental and theoretical studies on the removal mechanism of formaldehyde from water by mesoporous calcium silicate. Sci. China Technol. Sci. 2020, 63, 2098–2112. [Google Scholar] [CrossRef]
- Azam, K.; Raza, R.; Shezad, N.; Shabir, M.; Yang, W.; Ahmad, N.; Shafiq, I.; Akhter, P.; Razzaq, A.; Hussain, M. Development of recoverable magnetic mesoporous carbon adsorbent for removal of methyl blue and methyl orange from wastewater. J. Environ. Chem. Eng. 2020, 8, 104220. [Google Scholar] [CrossRef]
- Rego, R.M.; Sriram, G.; Ajeya, K.V.; Jung, H.Y.; Kurkuri, M.D.; Kigga, M. Cerium based UiO-66 MOF as a multipollutant adsorbent for universal water purification. J. Hazard. Mater. 2021, 416, 125941. [Google Scholar] [CrossRef]
- Zhai, L.; Bai, Z.; Zhu, Y.; Wang, B.; Luo, W. Fabrication of chitosan microspheres for efficient adsorption of methyl orange. Chin. J. Chem. Eng. 2018, 26, 657–666. [Google Scholar] [CrossRef]
- Peng, L.; Qin, P.; Lei, M.; Zeng, Q.; Song, H.; Yang, J.; Shao, J.; Liao, B.; Gu, J. Modifying Fe3O4 nanoparticles with humic acid for removal of Rhodamine B in water. J. Hazard. Mater. 2012, 209–210, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Markandeya; Dhiman, N.; Shukla, S.P.; Mohan, D.; Kisku, G.C.; Patnaik, S. Comprehensive remediation study of disperse dyes in wastewater using cenospheres nanosyntactic foam. J. Clean. Prod. 2018, 182, 206–216. [Google Scholar] [CrossRef]
- Viotti, P.V.; Moreira, W.M.; dos Santos, O.A.A.; Bergamasco, R.; Vieira, A.M.S.; Vieira, M.F. Diclofenac removal from water by adsorption on Moringa oleifera pods and activated carbon: Mechanism, kinetic and equilibrium study. J. Clean. Prod. 2019, 219, 809–817. [Google Scholar] [CrossRef]
- Ali, I.; Al-Othman, Z.A.; Alwarthan, A. Synthesis of composite iron nano adsorbent and removal of ibuprofen drug residue from water. J. Mol. Liq. 2016, 219, 858–864. [Google Scholar] [CrossRef]
- Alhooshani, K.R. Adsorption of chlorinated organic compounds from water with cerium oxide-activated carbon composite. Arab. J. Chem. 2019, 12, 2585–2596. [Google Scholar] [CrossRef] [Green Version]
- Dastjerdi, M.H.T.; Habibagahi, G.; Ghahramani, A.; Karimi-Jashni, A.; Zeinali, S. Removal of dissolved toluene in underground water with nanowires of manganese oxide. Adsorpt. Sci. Technol. 2018, 36, 393–407. [Google Scholar] [CrossRef] [Green Version]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Bartosiewicz, I.; Kulisa, K. Advanced oxidation/reduction processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS)—A review of recent advances. Chem. Eng. J. 2018, 336, 170–199. [Google Scholar] [CrossRef]
- Doty, S.L. Enhancing phytoremediation through the use of transgenics and endophytes. New Phytol. 2008, 179, 318–333. [Google Scholar] [CrossRef]
- Gerhardt, K.E.; Huang, X.D.; Glick, B.R.; Greenberg, B.M. Phytoremediation and rhizoremediation of organic soil contaminants: Potential and challenges. Plant Sci. 2009, 176, 20–30. [Google Scholar] [CrossRef]
- Moccia, E.; Intiso, A.; Cicatelli, A.; Proto, A.; Guarino, F.; Iannece, P.; Castiglione, S.; Rossi, F. Use of Zea mays L. in phytoremediation of trichloroethylene. Environ. Sci. Pollut. Res. 2017, 24, 11053–11060. [Google Scholar] [CrossRef]
- Zemb, O.; Lee, M.; Low, A.; Manefield, M. Reactive iron barriers: A niche enabling microbial dehalorespiration of 1,2-dichloroethane. Appl. Microbiol. Biotechnol. 2010, 88, 319–325. [Google Scholar] [CrossRef]
- Wen, L.L.; Zhang, Y.; Chen, J.X.; Zhang, Z.X.; Yi, Y.Y.; Tang, Y.; Rittmann, B.E.; Zhao, H.P. The dechlorination of TCE by a perchlorate reducing consortium. Chem. Eng. J. 2017, 313, 1215–1221. [Google Scholar] [CrossRef]
- Xiu, Z.M.; Jin, Z.H.; Li, T.L.; Mahendra, S.; Lowry, G.V.; Alvarez, P.J.J. Effects of nano-scale zero-valent iron particles on a mixed culture dechlorinating trichloroethylene. Bioresour. Technol. 2010, 101, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Mulder, M. The use of membrane processes in environmental problems. An introduction. In Membrane Processes in Separation and Purification, 1st ed.; Crespo, J.G., Böddeker, K.W., Eds.; Springer: Enschede, The Netherlands, 1994; pp. 229–262. [Google Scholar]
- Qu, X.; Brame, J.; Li, Q.; Alvarez, P.J.J. Nanotechnology for a safe and sustainable water supply: Enabling integrated water treatment and reuse. Acc. Chem. Res. 2013, 46, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U. The Preparation of Chitosan/PVA/zeolite Electrospun Composite Nanofibrous Membrane for Heavy Metal Removal Application. Master’s Thesis, Engineering Science, University Malaya, Kuala Lumpur, Malaysia, 2016. [Google Scholar]
- Zhang, W.; Tang, S.; Zhang, S.; Chen, Y. Purification of nitrogen-doped graphene quantum dots: Via the liquid-liquid extraction system of tetrahydrofuran-(NH4)2SO4-water and its application to sensitive iron(III) ions determination. Anal. Methods 2017, 9, 5691–5696. [Google Scholar] [CrossRef]
- Cazoir, D.; Fine, L.; Ferronato, C.; Chovelon, J.M. Hydrocarbon removal from bilgewater by a combination of air-stripping and photocatalysis. J. Hazard. Mater. 2012, 235–236, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Ghoreyshi, A.A.; Sadeghifar, H.; Entezarion, F. Efficiency assessment of air stripping packed towers for removal of VOCs (volatile organic compounds) from industrial and drinking waters. Energy 2014, 73, 838–843. [Google Scholar] [CrossRef]
- Siggins, A.; Abram, F.; Healy, M.G. Pyrolysed waste materials show potential for remediation of trichloroethylene-contaminated water. J. Hazard. Mater. 2020, 390, 121909. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Elmchaouri, A.; El Mouzdahir, Y.; Korili, S.A. Removal of tetrachloroethylene from aqueous solutions by adsorption on clay minerals. Adsorpt. Sci. Technol. 2015, 33, 355–367. [Google Scholar] [CrossRef]
- Ibrahim, A.O.; Adegoke, K.A.; Adegoke, R.O.; AbdulWahab, Y.A.; Oyelami, V.B.; Adesina, M.O. Adsorptive removal of different pollutants using metal-organic framework adsorbents. J. Mol. Liq. 2021, 333, 115593. [Google Scholar] [CrossRef]
- Aranzabal, A.; Pereda-Ayo, B.; González-Marcos, M.P.; González-Marcos, J.A.; López-Fonseca, R.; González-Velasco, J.R. State of the art in catalytic oxidation of chlorinated volatile organic compounds. Chem. Pap. 2014, 68, 1169–1186. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Suzuki, K. Catalytic oxidation of VOCs over Al- and Fe-pillared montmorillonite. Appl. Clay Sci. 2013, 77–78, 56–60. [Google Scholar] [CrossRef]
- Ma, H.; Huang, Y.; Shen, M.; Guo, R.; Cao, X.; Shi, X. Enhanced dechlorination of trichloroethylene using electrospun polymer nanofibrous mats immobilized with iron/palladium bimetallic nanoparticles. J. Hazard. Mater. 2012, 211–212, 349–356. [Google Scholar] [CrossRef]
- Huang, C.C.; Lo, S.L.; Lien, H.L. Zero-valent copper nanoparticles for effective dechlorination of dichloromethane using sodium borohydride as a reductant. Chem. Eng. J. 2012, 203, 95–100. [Google Scholar] [CrossRef]
- Mouhamad, R.; Ghanem, I.; AlOrfi, M.; Ibrahim, K.; Ali, N.; Al-Daoude, A. Phytoremediation of trichloroethylene and dichlorodiphenyltrichloroethane-polluted water using transgenic sesbania grandiflora and arabidopsis thaliana plants harboring rabbit cytochrome P450 2E1. Int. J. Phytoremediat. 2012, 14, 656–668. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.Y.; Wang, X.L.; Yang, J.; Gu, J.D.; Zhu, R.L.; Wang, P.; Lin, K.F.; Liu, Y. Di Aerobic biodegradation of trichloroethylene and phenol co-contaminants in groundwater by a bacterial community using hydrogen peroxide as the sole oxygen source. Environ. Technol. 2015, 36, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.-S.; Wang, F.; Zhang, X.-L.; Xu, J. Removal mechanism of benzene and chlorobenzene in water by modified biochar activates persulfate. China Environ. Sci. 2020, 40, 5280–5289. [Google Scholar]
- Ono, Y.; Sekiguchi, K.; Sankoda, K.; Nii, S.; Namiki, N. Improved ultrasonic degradation of hydrophilic and hydrophobic aldehydes in water by combined use of atomization and UV irradiation onto the mist surface. Ultrason. Sonochem. 2020, 60, 104766. [Google Scholar] [CrossRef] [PubMed]
- Talaiekhozani, A.; Salari, M.; Talaei, M.R.; Bagheri, M.; Eskandari, Z. Formaldehyde removal from wastewater and air by using UV, ferrate(VI) and UV/ferrate(VI). J. Environ. Manag. 2016, 184, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Ainscough, T.J.; Oatley-Radcliffe, D.L.; Barron, A.R. Groundwater remediation of volatile organic compounds using nanofiltration and reverse osmosis membranes—A field study. Membranes 2021, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lei, C.; Wei, C.; Zeng, G. Chlorinated volatile organic compounds (Cl-VOCs) in environment—Sources, potential human health impacts, and current remediation technologies. Environ. Int. 2014, 71, 118–138. [Google Scholar] [CrossRef]
- De Miranda, B.R.; Greenamyre, J.T. Trichloroethylene, a ubiquitous environmental contaminant in the risk for Parkinson’s disease. Environ. Sci. Process Impacts 2020, 22, 543–554. [Google Scholar] [CrossRef]
- Almasi, A.; Soltanian, M.; Asadi, F.; Nokhasi, P.; Godini, K.; Mohammadi, M.; Azarian, G.; Mohammadi, A. Tetrachloroethylene removal rate from aqueous solutions by pumice doped with copper: An evaluation of the effect of pH. Avicenna J. Environ. Health Eng. 2016, 3. [Google Scholar] [CrossRef]
- Shestakova, M.; Sillanpää, M. Removal of dichloromethane from ground and wastewater: A review. Chemosphere 2013, 93, 1258–1267. [Google Scholar] [CrossRef]
- Wu, H.; Feng, Q. Fabrication of bimetallic Ag/Fe immobilized on modified biochar for removal of carbon tetrachloride. J. Environ. Sci. 2017, 54, 346–357. [Google Scholar] [CrossRef]
- Tongur, S.; Aydin, M.E. Adsorption kinetics of chloroform from aqueous solutions onto activated lignite. Clean Soil Air Water 2013, 41, 32–36. [Google Scholar] [CrossRef]
- Daniel, C.; Guerra, G. Nanoporous crystalline polymer materials for environmental applications. Macromol. Symp. 2016, 369, 19–25. [Google Scholar] [CrossRef]
- Daniel, C.; Antico, P.; Yamaguchi, H.; Kogure, M.; Guerra, G. Microporous-crystalline microfibers by eco-friendly guests: An efficient tool for sorption of volatile organic pollutants. Microporous Mesoporous Mater. 2016, 232, 205–210. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Reverchon, E. Supercritical assisted electrospray: An improved micronization process. Polymers 2019, 11, 244. [Google Scholar] [CrossRef] [Green Version]
- Jung, B.; Deng, W.; Li, Y.; Batchelor, B.; Abdel-Wahab, A. Simulated solar light-driven photocatalytic degradation of trichloroethylene in water using BiOBr promoted by sulfite addition. Environ. Sci. Eur. 2020, 32, 8. [Google Scholar] [CrossRef]
- Williams, C.K.; McCarver, G.A.; Lashgari, A.; Vogiatzis, K.D.; Jiang, J.J. Electrocatalytic dechlorination of dichloromethane in water using a heterogenized molecular copper complex. Inorg. Chem. 2021, 60, 4915–4923. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Che, M.; Chen, Z.; Qiu, S.; Cui, M.; Huang, R.; Qi, W.; He, Z.; Su, R. Efficient removal of chloroform in groundwater by polyethylene glycol-stabilized Fe/Ni nanoparticles. Environ. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Wang, Q.; Song, X.; Tang, S.; Yu, L. Enhanced removal of tetrachloroethylene from aqueous solutions by biodegradation coupled with nZVI modified by layered double hydroxide. Chemosphere 2020, 243, 125260. [Google Scholar] [CrossRef]
- Liu, S.J.; Yang, Q.M.; Yang, Y.K.; Ding, H.; Qi, Y. In situ remediation of tetrachloroethylene and its intermediates in groundwater using an anaerobic/aerobic permeable reactive barrier. Environ. Sci. Pollut. Res. 2017, 24, 26615–26622. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Karim, A.; Gad-Allah, T.A.; Badawy, M.I.; Khalil, A.S.G.; Ulbricht, M. Removal of humic acid and chloroform from drinking water by using commercial nanofiltration and reverse osmosis membranes. Desalin. Water Treat. 2017, 59, 48–54. [Google Scholar] [CrossRef]
- Abbas, A.; Abussaud, B.A.; Al-Baghli, N.A.H.I.; Khraisheh, M.; Atieh, M.A. Benzene removal by iron oxide nanoparticles decorated carbon nanotubes. J. Nanomater. 2016, 2016, 5654129. [Google Scholar] [CrossRef] [Green Version]
- Heydari, M.; Sabbaghi, S.; Zeinali, S. Adsorptive removal of toluene from aqueous solution using metal-organic framework MIL-101(Cr): Removal optimization by response surface methodology. Int. J. Environ. Sci. Technol. 2019, 16, 6217–6226. [Google Scholar] [CrossRef]
- Nefzi, H.; Salaberria, A.M.; Abderrabba, M.; Ayadi, S.; Labidi, J. Cellulose modified diatomite for toluene removal from aqueous solution. Desalin. Water Treat. 2019, 150, 228–236. [Google Scholar] [CrossRef]
- Yuan, R.; Zhou, B.; Ma, L. Removal of toluene from water by photocatalytic oxidation with activated carbon supported Fe3+-doped TiO2 nanotubes. Water Sci. Technol. 2014, 70, 642–648. [Google Scholar] [CrossRef]
- Al-Sabahi, J.; Bora, T.; Al-Abri, M.; Dutta, J. Efficient visible light photocatalysis of benzene, toluene, ethylbenzene and xylene (BTEX) in aqueous solutions using supported zinc oxide nanorods. PLoS ONE 2017, 12, e0189276. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Z.; Yang, Q.; Liu, W. Photocatalytic degradation of phytotoxic substances in waste nutrient solution by various immobilized levels of Nano-TiO2. Water Air Soil Pollut. 2013, 224, 1461. [Google Scholar] [CrossRef]
- Jans, U.; Prasse, C.; Von Gunten, U. Enhanced treatment of municipal wastewater effluents by Fe-Taml/H2O2: Efficiency of micropollutant abatement. Environ. Sci. Technol. 2021, 55, 3313–3321. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.I.; Yi, C.; Zhao, H.; Asilevi, P.J.; Yin, L.; Yi, R.; Javed, Q.; Wang, H. Experimental study of nitrobenzene degradation in water by strong ionization dielectric barrier discharge. Environ. Technol. 2021, 42, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Li, C.; Liu, X.; Wang, L.; Chen, R. UV-activated permanganate process for micro-organic pollutant degradation: Efficiency, mechanism and influencing factors. Water Sci. Technol. 2021, 83, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhu, L.; Sun, N.; Lan, Y. Degradation of nitrobenzene by sodium persulfate activated with zero-valent zinc in the presence of low frequency ultrasound. J. Taiwan Inst. Chem. Eng. 2017, 78, 137–143. [Google Scholar] [CrossRef]
- Farias, M.F.; Domingos, Y.S.; Fernandes, G.J.T.; Castro, F.L.; Fernandes, V.J.; Costa, M.J.F.; Araujo, A.S. Effect of acidity in the removal-degradation of benzene in water catalyzed by Co-MCM-41 in medium containing hydrogen peroxide. Microporous Mesoporous Mater. 2018, 258, 33–40. [Google Scholar] [CrossRef]
- Cesarino, I.; Cesarino, V.; Moraes, F.C.; Ferreira, T.C.R.; Lanza, M.R.V.; Mascaro, L.H.; Machado, S.A.S. Electrochemical degradation of benzene in natural water using silver nanoparticle-decorated carbon nanotubes. Mater. Chem. Phys. 2013, 141, 304–309. [Google Scholar] [CrossRef]
- Agrahari, G.K.; Verma, N.; Bhattacharya, P.K. Removal of benzoic acid from water by reactive extraction using hollow fiber membrane contactor: Experiment and modeling. Clean Soil Air Water 2014, 42, 901–908. [Google Scholar] [CrossRef]
- Al Bsoul, A.; Hailat, M.; Abdelhay, A.; Tawalbeh, M.; Al-Othman, A.; Al-kharabsheh, I.N.; Al-Taani, A.A. Efficient removal of phenol compounds from water environment using Ziziphus leaves adsorbent. Sci. Total Environ. 2021, 761, 143229. [Google Scholar] [CrossRef]
- Demissie, H.; An, G.; Jiao, R.; Ma, G.; Liu, L.; Sun, H.; Wang, D. Removal of phenolic contaminants from water by in situ coated surfactant on Keggin-aluminum nanocluster and biodegradation. Chemosphere 2021, 269, 128692. [Google Scholar] [CrossRef]
- Yu, L.; Yang, X.; Ye, Y.; Peng, X.; Wang, D. Silver nanoparticles decorated anatase TiO2 nanotubes for removal of pentachlorophenol from water. J. Colloid Interface Sci. 2015, 453, 100–106. [Google Scholar] [CrossRef]
- Jay, L.; Chirwa, E.E.M.N. Pathway analysis of phenol degradation by UV/TiO2 photocatalysis utilising the C-13 isotopic labelling technique. Chem. Eng. Trans. 2018, 70, 181–186. [Google Scholar] [CrossRef]
- Yao, Z.; Li, H.; Zhou, X.; Dong, Y.; Hang, Y.; Wang, Y. Preparation and photocatalysis degradation performances for phenol of TiO2/montmorillonite composites. Fuhe Cailiao Xuebao 2015, 32, 1581–1589. [Google Scholar] [CrossRef]
- Horová, D.; Nováková, J.; Pelíšková, L.; Kohout, J.; Šafář, J.; Hrachovcová, K.; Tokarová, V. Synthesis of MFI structured iron silicates and their catalytic performance in phenol removal by wet peroxide oxidation. React. Kinet. Mech. Catal. 2020, 130, 1077–1092. [Google Scholar] [CrossRef]
- Jothinathan, L.; Cai, Q.Q.; Ong, S.L.; Hu, J.Y. Organics removal in high strength petrochemical wastewater with combined microbubble-catalytic ozonation process. Chemosphere 2021, 263, 127980. [Google Scholar] [CrossRef]
- Asgari, G.; Rahmani, A.; Askari, F.B.; Godini, K. Catalytic ozonation of phenol using copper coated pumice and zeolite as catalysts. J. Res. Health Sci. 2012, 12, 93–97. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-Segura, S. Benchmarking recent advances and innovative technology approaches of Fenton, photo-Fenton, electro-Fenton, and related processes: A review on the relevance of phenol as model molecule. Sep. Purif. Technol. 2020, 237, 116337. [Google Scholar] [CrossRef]
- Gernjak, W.; Krutzler, T.; Glaser, A.; Malato, S.; Caceres, J.; Bauer, R.; Fernández-Alba, A.R. Photo-fenton treatment of water containing natural phenolic pollutants. Chemosphere 2003, 50, 71–78. [Google Scholar] [CrossRef]
- Carta, R.; Desogus, F. The enhancing effect of low power microwaves on phenol oxidation by the Fenton process. J. Environ. Chem. Eng. 2013, 1, 1292–1300. [Google Scholar] [CrossRef]
- Azizi, A.; Abouseoud, M.; Amrane, A. Phenol removal by a sequential combined fenton-enzymatic process. Nat. Environ. Pollut. Technol. 2017, 16, 321–330. [Google Scholar]
- Suryaman, D.; Hasegawa, K.; Kagaya, S. Combined biological and photocatalytic treatment for the mineralization of phenol in water. Chemosphere 2006, 65, 2502–2506. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Xia, B.; Zuo, W. Phenol Oxidation by Combined Cavitation Water Jet and Hydrogen Peroxide. Chin. J. Chem. Eng. 2012, 20, 760–767. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Wang, L.; Cheng, X.; Shang, Q. Rapid removal of phenol/antibiotics in water by Fe-(8-hydroxyquinoline-7-carboxylic)/TiO2 flower composite: Adsorption combined with photocatalysis. Chem. Eng. J. 2020, 402. [Google Scholar] [CrossRef]
- Ipek, I.Y.; Kabay, N.; Yüksel, M.; Yapici, D.; Yüksel, Ü. Application of adsorption-ultrafiltration hybrid method for removal of phenol from water by hypercrosslinked polymer adsorbents. Desalination 2012, 306, 24–28. [Google Scholar] [CrossRef]
- Salehi, E.; Shafie, M. Adsorptive removal of acetaldehyde from water using strong anionic resins pretreated with bisulfite: An efficient method for spent process water recycling in petrochemical industry. J. Water Process Eng. 2020, 33, 101025. [Google Scholar] [CrossRef]
- Emerging Pollutants in Water and Wastewater. Available online: https://en.unesco.org/emergingpollutantsinwaterandwastewater (accessed on 8 June 2021).
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field. Environ. Pollut. 2017, 231, 954–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Chaminda, G.G.T.; An, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Gong, R.; Ye, J.; Dai, W.; Yan, X.; Hu, J.; Hu, X.; Li, S.; Huang, H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with finger-citron-residue-based activated carbon. Ind. Eng. Chem. Res. 2013, 52, 14297–14303. [Google Scholar] [CrossRef]
- Chen, S.; Huang, Y.; Han, X.; Wu, Z.; Lai, C.; Wang, J.; Deng, Q.; Zeng, Z.; Deng, S. Simultaneous and efficient removal of Cr(VI) and methyl orange on LDHs decorated porous carbons. Chem. Eng. J. 2018, 352, 306–315. [Google Scholar] [CrossRef]
- Chaukura, N.; Murimba, E.C.; Gwenzi, W. Synthesis, characterisation and methyl orange adsorption capacity of ferric oxide-biochar nano-composites derived from pulp and paper sludge. Appl. Water Sci. 2017, 7, 2175–2186. [Google Scholar] [CrossRef] [Green Version]
- Kisku, G.C.; Markandeya; Shukla, S.P.; Singh, D.S.; Murthy, R.C. Characterization and adsorptive capacity of coal fly ash from aqueous solutions of disperse blue and disperse orange dyes. Environ. Earth Sci. 2015, 74, 1125–1135. [Google Scholar] [CrossRef]
- Markandeya; Dhiman, N.; Shukla, S.P.; Kisku, G.C. Statistical optimization of process parameters for removal of dyes from wastewater on chitosan cenospheres nanocomposite using response surface methodology. J. Clean. Prod. 2017, 149, 597–606. [Google Scholar] [CrossRef]
- Ghanbari, M.; Salavati-Niasari, M. Copper iodide decorated graphitic carbon nitride sheets with enhanced visible-light response for photocatalytic organic pollutant removal and antibacterial activities. Ecotoxicol. Environ. Saf. 2021, 208, 111712. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, Y.; Sabbaghi, S.; Abouzari-Lotf, E.; Jahangirian, H.; Sairi, N.A. A new achievement in green degradation of aqueous organic pollutants under visible-light irradiation. Water Sci. Technol. 2018, 77, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.H.; Fu, C.C.; Juang, R.S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Almaamary, E.A.S.; Abdullah, S.R.S.; Hasan, H.A.; Rahim, R.A.A.; Idris, M. Rawatan metilena biru dalam air sisa menggunakan Scirpus grossus. Malays. J. Anal. Sci. 2017, 21, 182–187. [Google Scholar] [CrossRef]
- Al-Zurfi, S.K.L.; Albdairi, N.A.; Almadany, S.A. Phytoremediation of Congo red and brilliant green using Lemna minor. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 1–10. [Google Scholar]
- Sharma, R.; Saini, H.; Paul, D.R.; Chaudhary, S.; Nehra, S.P. Removal of organic dyes from wastewater using Eichhornia crassipes: A potential phytoremediation option. Environ. Sci. Pollut. Res. 2021, 28, 7116–7122. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, K.; Zhang, W.; Bai, Y.; Sun, Y.; Gu, J. Graphene oxide quantum dots incorporated into a thin film nanocomposite membrane with high flux and antifouling properties for low-pressure nanofiltration. ACS Appl. Mater. Interfaces 2017, 9, 11082–11094. [Google Scholar] [CrossRef]
- Modi, A.; Bellare, J. Efficient removal of dyes from water by high flux and superior antifouling polyethersulfone hollow fiber membranes modified with ZnO/cGO nanohybrid. J. Water Process Eng. 2019, 29, 100783. [Google Scholar] [CrossRef]
- Hu, F.; Wang, Z.; Zhu, B.; Zhu, L.; Xu, Y. Poly (N-vinyl imidazole) gel-filled membrane adsorbers for highly efficient removal of dyes from water. J. Chromatogr. A 2018, 1563, 198–206. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Abdou, A.E.H.; Shehata, A.K.; Header, H.M.A.; Hamed, E.A. Sustainable super fast adsorptive removal of Congo red dye from water by a novel technique based on microwave-enforced sorption process. J. Ind. Eng. Chem. 2018, 57, 28–36. [Google Scholar] [CrossRef]
- Zhang, J.J.; Fang, S.S.; Mei, J.Y.; Zheng, G.P.; Zheng, X.C.; Guan, X.X. High-efficiency removal of rhodamine B dye in water using g-C3N4 and TiO2 co-hybridized 3D graphene aerogel composites. Sep. Purif. Technol. 2018, 194, 96–103. [Google Scholar] [CrossRef]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef]
- Substances Identified as Endocrine Disruptors at EU Level. Available online: https://edlists.org/the-ed-lists/list-i-substances-identified-as-endocrine-disruptors-by-the-eu (accessed on 10 June 2021).
- Xu, J.; Wang, L.; Zhu, Y. Decontamination of bisphenol A from aqueous solution by graphene adsorption. Langmuir 2012, 28, 8418–8425. [Google Scholar] [CrossRef]
- Substances under Evaluation for Endocrine Disruption under an EU Legislation. Available online: https://edlists.org/the-ed-lists/list-ii-substances-under-eu-investigation-endocrine-disruption (accessed on 10 June 2021).
- Phuekphong, A.F.; Imwiset, K.J.; Ogawa, M. Organically modified bentonite as an efficient and reusable adsorbent for triclosan removal from water. Langmuir 2020, 36, 9025–9034. [Google Scholar] [CrossRef] [PubMed]
- Moeini, Z.; Azhdarpoor, A.; Yousefinejad, S.; Hashemi, H. Removal of atrazine from water using titanium dioxide encapsulated in salicylaldehyde–NH2–MIL-101 (Cr): Adsorption or oxidation mechanism. J. Clean. Prod. 2019, 224, 238–245. [Google Scholar] [CrossRef]
- Limmun, W.; Ito, A.; Ishikawa, N.; Momotori, J.; Kawamura, Y.; Majima, Y.; Sasamoto, M.; Umita, T. Removal of nonylphenol and nonylphenol monoethoxylate from water and anaerobically digested sewage sludge by Ferrate(VI). Chemosphere 2019, 236, 124399. [Google Scholar] [CrossRef]
- Akbari-Adergani, B.; Saghi, M.H.; Eslami, A.; Mohseni-Bandpei, A.; Rabbani, M. Removal of dibutyl phthalate from aqueous environments using a nanophotocatalytic Fe, Ag-ZnO/VIS-LED system: Modeling and optimization. Environ. Technol. 2018, 39, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Palharim, P.H.; Graça, C.A.L.; Teixeira, A.C.S.C. Comparison between UVA- and zero-valent iron-activated persulfate processes for degrading propylparaben. Environ. Sci. Pollut. Res. 2020, 27, 22214–22224. [Google Scholar] [CrossRef]
- Orhon, K.B.; Orhon, A.K.; Dilek, F.B.; Yetis, U. Triclosan removal from surface water by ozonation—Kinetics and by-products formation. J. Environ. Manag. 2017, 204, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, S.; Kabay, N.; Yüksel, M. Removal of bisphenol A (BPA) from water by various nanofiltration (NF) and reverse osmosis (RO) membranes. J. Hazard. Mater. 2013, 263, 307–310. [Google Scholar] [CrossRef]
- Rastgar, M.; Shakeri, A.; Karkooti, A.; Asad, A.; Razavi, R.; Sadrzadeh, M. Removal of trace organic contaminants by melamine-tuned highly cross-linked polyamide TFC membranes. Chemosphere 2020, 238, 124691. [Google Scholar] [CrossRef]
- Wei, X.; Shi, Y.; Fei, Y.; Chen, J.; Lv, B.; Chen, Y.; Zheng, H.; Shen, J.; Zhu, L. Removal of trace phthalate esters from water by thin-film composite nanofiltration hollow fiber membranes. Chem. Eng. J. 2016, 292, 382–388. [Google Scholar] [CrossRef]
- López-Ortiz, C.M.; Sentana-Gadea, I.; Varó-Galvañ, P.; Maestre-Pérez, S.E.; Prats-Rico, D. The use of combined treatments for reducing parabens in surface waters: Ion-exchange resin and nanofiltration. Sci. Total Environ. 2018, 639, 228–236. [Google Scholar] [CrossRef]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef]
- Koutník, I.; Vráblová, M.; Bednárek, J. Reynoutria japonica, an invasive herb as a source of activated carbon for the removal of xenobiotics from water. Bioresour. Technol. 2020, 309, 123315. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Mahbub, M.K.B.; Wilhelm, M.; Lima, E.C.; Sampaio, C.H.; Saucier, C.; Dias, S.L.P. Activated carbon from sewage sludge for removal of sodium diclofenac and nimesulide from aqueous solutions. Korean J. Chem. Eng. 2016, 33, 3149–3161. [Google Scholar] [CrossRef]
- Ciğeroğlu, Z.; Özdemir, O.K.; Şahin, S.; Haşimoğlu, A. Naproxen adsorption onto graphene oxide nanopowders: Equilibrium, kinetic, and thermodynamic studies. Water Air Soil Pollut. 2020, 231. [Google Scholar] [CrossRef]
- Ali, I.; Afshinb, S.; Poureshgh, Y.; Azari, A.; Rashtbari, Y.; Feizizadeh, A.; Hamzezadeh, A.; Fazlzadeh, M. Green preparation of activated carbon from pomegranate peel coated with zero-valent iron nanoparticles (nZVI) and isotherm and kinetic studies of amoxicillin removal in water. Environ. Sci. Pollut. Res. 2020, 27, 36732–36743. [Google Scholar] [CrossRef]
- Changanaqui, K.; Alarcón, H.; Brillas, E.; Sirés, I. Blue LED light-driven photoelectrocatalytic removal of naproxen from water: Kinetics and primary by-products. J. Electroanal. Chem. 2020, 867, 114192. [Google Scholar] [CrossRef]
- Martins, M.; Mourato, C.; Sanches, S.; Noronha, J.P.; Crespo, M.T.B.; Pereira, I.A.C. Biogenic platinum and palladium nanoparticles as new catalysts for the removal of pharmaceutical compounds. Water Res. 2017, 108, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Jayasree, P.; Remya, N. Photocatalytic degradation of paracetamol using aluminosilicate supported TiO2. Water Sci. Technol. 2020, 82, 2114–2124. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Q.; Ni, J.; Xu, Y.; Song, N.; Gao, M. Highly efficient removal of amoxicillin from water by three-dimensional electrode system within granular activated carbon as particle electrode. J. Water Process Eng. 2020, 38, 101656. [Google Scholar] [CrossRef]
- Tepe, O.; Tunç, Z.; Yıldız, B.; Şahin, M. Efficient removal of paracetamol by manganese oxide octahedral molecular sieves (OMS-2) and persulfate. Water Air Soil Pollut. 2020, 231, 238. [Google Scholar] [CrossRef]
- Periyasamy, S.; Muthuchamy, M. Electrochemical oxidation of paracetamol in water by graphite anode: Effect of pH, electrolyte concentration and current density. J. Environ. Chem. Eng. 2018, 6, 7358–7367. [Google Scholar] [CrossRef]
- He, Y.; Wang, L.; Chen, Z.; Shen, B.; Wei, J.; Zeng, P.; Wen, X. Catalytic ozonation for metoprolol and ibuprofen removal over different MnO2 nanocrystals: Efficiency, transformation and mechanism. Sci. Total Environ. 2021, 785, 147328. [Google Scholar] [CrossRef] [PubMed]
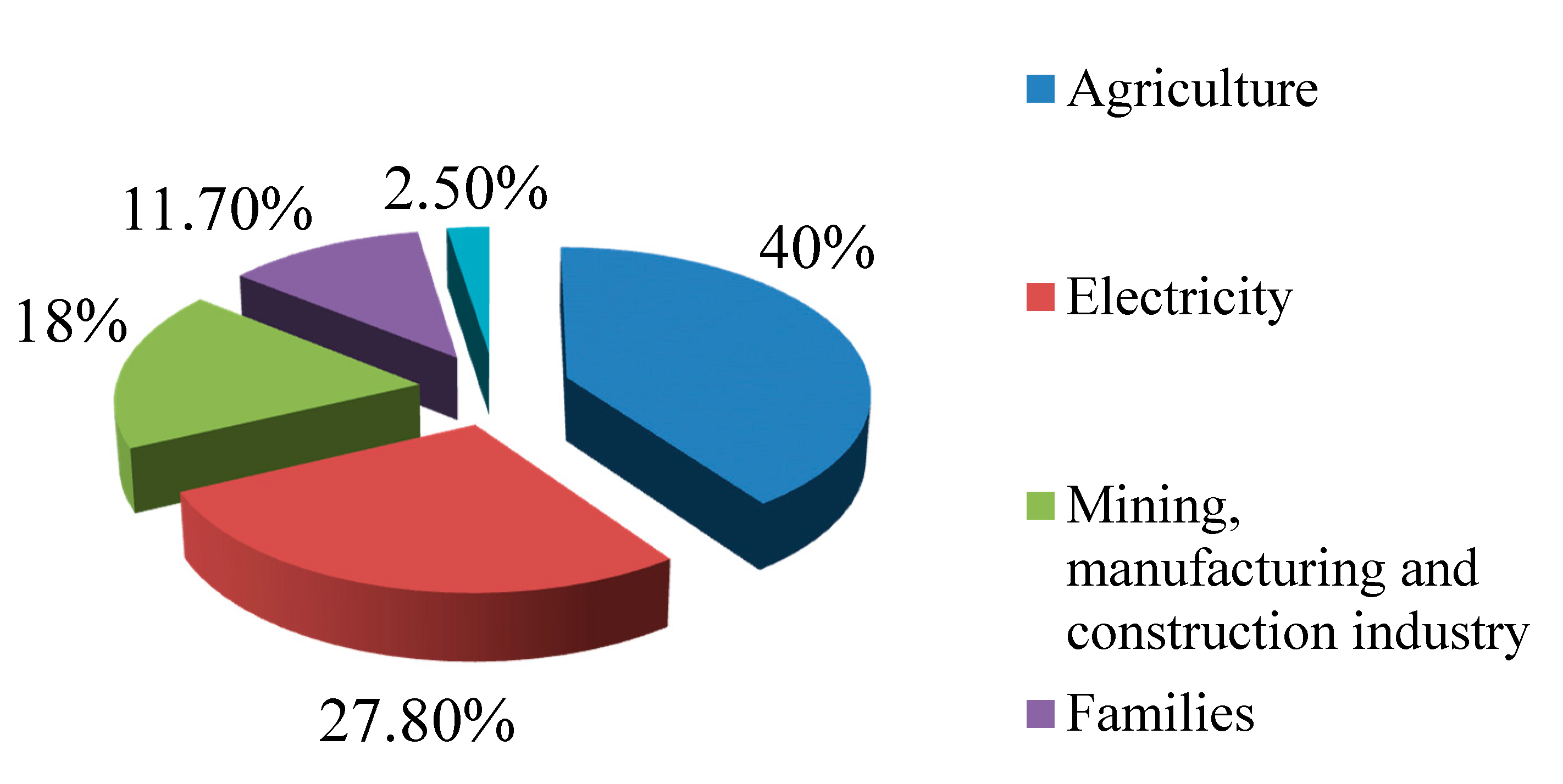
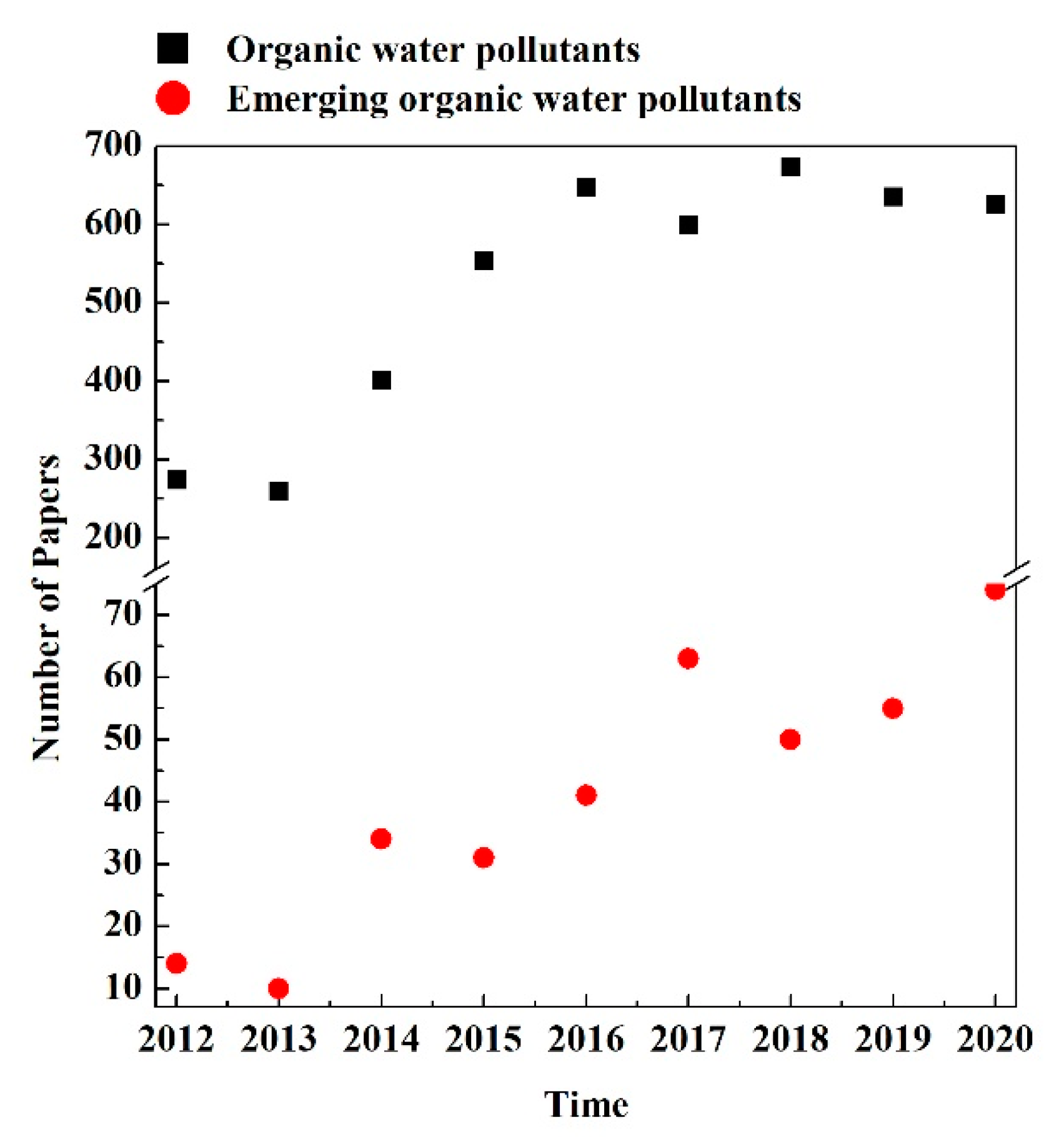
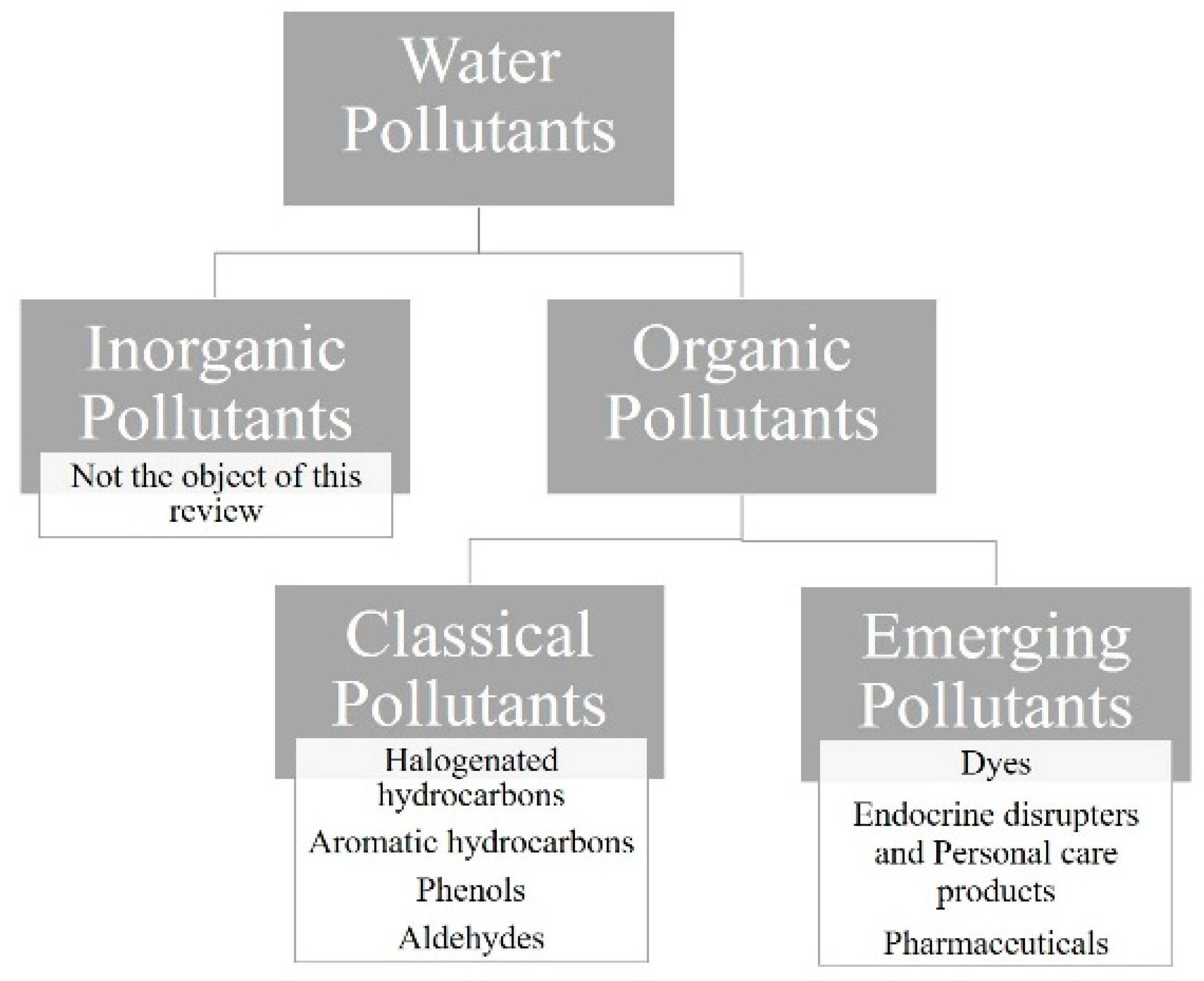
| Family of Compounds | Main Compounds | Main Usage |
|---|---|---|
| Aliphatic hydrocarbons | Propane, butane, hexane, limonene | Fuels, detergents, aerosol propellants, refrigerants, perfume bases, flavorings |
| Halogenated hydrocarbons | Chloroform, methylene chloride, pentachlorophenol | Aerosol propellants, pesticides, refrigerants, degreasers |
| Aromatic hydrocarbons | Benzene, toluene, xylene | Varnishes, paints, glues, enamels, lacquers, detergents |
| Alcohols | Ethyl alcohol, methyl alcohol | Window cleaners, paints, thinners, adhesives, cosmetics |
| Aldehydes | Formaldehyde, acetaldehyde | Fungicides, insulators, germicides, resins, disinfectants |
| Pollutant | Method | Max Removal Efficiency | References |
|---|---|---|---|
| TCE | Adsorption by: spruce and oak-derived biochars GAC herbal pomace biochar | >99.5% 95% 93% | [31] |
| Photocatalysis using BiOBr | 78% | [53] | |
| Abiotic reduction by electrospun polymer nanofibrous mats immobilized Fe/Pd nanoparticles | 99.6% | [36] | |
| Phytoremediation using Zea Mays | 20% | [21] | |
| Biodegradation with H2O2 | 80.6% | [39] | |
| NF membranes RO membranes | 100% 93% | [43] | |
| PCE | Adsorption by stevensite | 88.8% | [32] |
| Adsorption by: granulated pumice pumice doped with copper | 90% 98.4% | [46] | |
| Biodegradation enhanced by modified nZVI | 100% | [56] | |
| Anaerobic/aerobic permeable reactive barrier | 99% | [57] | |
| NF membranes RO membranes | 100% 93% | [43] | |
| DCM | Adsorption by CeO2-NP/AC | 82.72% | [16] |
| Electrocatalytic dechlorination with CuT2 | 70% | [54] | |
| Reductive process with Cu0 and NaBH4 | 100% | [37] | |
| TCM | Adsorption by CeO2-NP/AC | 99.4% | [16] |
| NF membranes RO membranes | 76–92% 94–98.5% | [58] | |
| PEG-Fe/Ni | 100% | [55] | |
| Adsorption by activated lignite | 99.5% | [49] | |
| CTC | Adsorption by CeO2-NP/AC | 89.42% | [16] |
| Adsorption by Ag/Fe/MB | 100% | [48] |
| Pollutant | Method | Max Removal Efficiency | References |
|---|---|---|---|
| 1-methoxy naphthalene | Fe-TAML/H2O2 oxidation | 85% | [65] |
| Nitrobenzene | Dielectric barrier discharge | 100% | [66] |
| Zn0-PS without US Low frequency US with: PS Zn0 Zn0-PS | 50% 5% 22% 96% | [68] | |
| Benzoic acid | Oxidation: UV/PM UV/H2O2 | 52% 80% | [67] |
| LLE with HFMC and TOA | >95% | [71] | |
| Photocatalysis with nano-TiO2 | 33.6% | [64] | |
| Benzene | Degradation with H2O2 and Co-MCM-41 | 82.1% | [69] |
| Adsorption by: CNTs CNTs impregnated with iron oxide nanoparticles | 53% 61% | [59] | |
| Fe-BC/PS system | 100% | [40] | |
| Electrocatalytic oxidation with a GC/MWCNT-Ag electrode | 77.9% | [70] | |
| ZnO nanorods under visible light irradiation | 65% | [63] | |
| Chlorobenzene | Fe-BC/PS system | 100% | [40] |
| Toluene | AC-supported Fe-TNTs with O3/UV | 90.7% | [62] |
| Adsorption by manganese oxide nanowires | 55% | [17] | |
| Adsorption by MIL-101(Cr) | 97% | [60] | |
| Adsorption by: natural cellulose diatomite modified cellulose diatomite | 79.33% 97.45% | [61] | |
| ZnO nanorods under visible light irradiation | 90% | [63] |
| Pollutant | Method | Max Removal Efficiency | References |
|---|---|---|---|
| Phenols | M-O3/Fe/GAC process Photo-Fenton process under UV irradiation | 96% 100% | [78] [81] |
| Phenol | Adsorption by Ziziphus leaves | 37.5% | [72] |
| Fe-TAML/H2O2 oxidation | 79% | [65] | |
| Adsorption by bacterial cellulose/cyclodextrin oligomer composites | 18.1% | [7] | |
| Ozonation with: zeolite modified with copper pumice modified with copper | 51% 63% | [79] | |
| TiO2/MMT under UV irradiation | 63% | [76] | |
| TiO2 under UV irradiation Biodegradation + photocatalysis Water cavitation jet and H2O2 Fenton process with microwaves Fenton process + enzymatic polymerization Adsorption and photocatalysis using Fe-HQLC/TiO2 composite Adsorption + membranes | 75% 98% 99.85% 100% 99.7% 99% 90–100% | [75] [84] [85] [82] [83] [86] [87] | |
| 4-methyl phenol | Fe-TAML/H2O2 oxidation | 100% | [65] |
| 4-chlorophenol | Fe-TAML/H2O2 oxidation | 98% | [65] |
| 2,5-dimethyl phenol | Fe-TAML/H2O2 oxidation | 100% | [65] |
| 2,4,6-trimethyl phenol | Fe-TAML/H2O2 oxidation | 100% | [65] |
| 2,4,6-trichlorophenol | Fe-TAML/H2O2 oxidation | 100% | [65] |
| 2,3-dichlorophenol | Adsorption-sedimentation by SDS-Al30 | 89% | [73] |
| 2,4-dichlorophenol | Adsorption-sedimentation by SDS-Al30 | 85% | [73] |
| 3,4-dichlorophenol | Adsorption-sedimentation by SDS-Al30 | 87% | [73] |
| 2,3,4-trichlorophenol | Adsorption-sedimentation by SDS-Al30 | 88% | [73] |
| 2,4,5-trichlorophenol | Adsorption-sedimentation by SDS-Al30 | 89% | [73] |
| 2,3,4,6-tetrachlorophenol | Adsorption-sedimentation by SDS-Al30 | 89% | [73] |
| Pentachlorophenol | Adsorption-sedimentation by SDS-Al30 | 98% | [73] |
| Photodegradation with: P25 TNTs Ag/TNTs | 54.3% 59.4% 99% | [74] |
| Pollutant | Method | Max Removal Efficiency | References |
|---|---|---|---|
| Formaldehyde | Adsorption by CSH | 99% | [8] |
| Electrolysis with ferrate(VI) UV irradiation UV/ferrate(VI) | 87% 95% 100% | [42] | |
| Acetaldehyde | Adsorption by AMBERLITE IRA 402-OH | 86% | [88] |
| H2O2/UV254/Ultrasonic irradiation | 100% | [41] |
| Pollutant | Method | Max Removal Efficiency | References |
|---|---|---|---|
| CR | Adsorption by Ce-UiO-66 MOF | 99.9% | [10] |
| TA/GOQDs TFN membranes | 99.8% | [104] | |
| MES with: γ-Al2O3 γ-Al2O3-SiCl | 94.86% 99.28% | [107] | |
| Phytoremediation by Lemna minor | 51% | [102] | |
| MB | Adsorption by Ce-UiO-66 MOF | 90% | [10] |
| Adsorption by: AC MAC MMAC | 64% 67% 82% | [9] | |
| Phytoremediation by Scirpus grossus | 86% | [101] | |
| Adsorption by FAC | 48.9% | [93] | |
| TA/GOQDs TFN membranes | 97.6% | [104] | |
| Photocatalysis using Pd-TiO2 | 85.9% | [100] | |
| Phytoremediation by Eichhornia crassipes | 90.8% | [103] | |
| ZOGP-50 HFMs membranes | 98.6% | [105] | |
| RhB | Photocatalysis using CuI/g-C3N4 nanocomposite | 98.5% | [98] |
| Adsorption by Fe3O4/HA | 98.5% | [12] | |
| Adsorption by g-C3N4-TiO2-GA without light irradiation with light irradiation | 96.5% 98.4% | [108] | |
| Phytoremediation by Eichhornia crassipes | 84.8% | [103] | |
| ZOGP-50 HFMs membranes | 98.5% | [105] | |
| MO | Photocatalysis using CuI/g-C3N4 nanocomposite | 98% | [98] |
| Adsorption by: AC MAC MMAC | 66.9% 76% 98.5% | [9] | |
| Adsorption by chitosan microspheres | 98.5% | [11] | |
| Adsorption by Ni/Al@PAB | 75% | [94] | |
| Adsorption by BC and Fe2O3–BC from PPS | 100% | [95] | |
| Photocatalysis using 8% graphene- CaCu3Ti4O12 | 89% | [99] | |
| Photocatalysis using Pd-TiO2 | 77.1% | [100] | |
| Phytoremediation by Eichhornia crassipes | 62.8% | [103] | |
| DO 25 | Adsorption by CFA | 75% | [96] |
| Adsorption by cenospheres nanosyntactic foam | 90% | [13] | |
| Adsorption by chitosan cenospheres nanocomposite | 97.3% | [97] | |
| CrR | Phytoremediation by Eichhornia crassipes | 33.3% | [103] |
| RB | Phytoremediation by Eichhornia crassipes | 87.4% | [103] |
| CV | Phytoremediation by Eichhornia crassipes | 87.2% | [103] |
| AO | Phytoremediation by Eichhornia crassipes | 79% | [103] |
| XO | Phytoremediation by Eichhornia crassipes | 46.2% | [103] |
| PR | Phytoremediation by Eichhornia crassipes | 44.4% | [103] |
| SY | PVI membranes | >99% | [106] |
| Pollutant | Method | Max Removal Efficiency | References |
|---|---|---|---|
| BPA | Adsorption by bacterial cellulose/cyclodextrin oligomer composites | 34% | [7] |
| NF and RO Membranes | ≥98% | [119] | |
| Adsorption by graphene | 86% | [111] | |
| Adsorption by Fe3O4@SiO2/CTAB-SiO2 | 93.2% | [6] | |
| Triclosan | Ozonation | 100% | [118] |
| Adsorption by 2C18-BT | 100% | [113] | |
| Atrazine | Adsorption by TS-MIL | 90% | [114] |
| FOTFC membranes | 97.3% | [120] | |
| Nonylphenol | Oxidation by K2FeO4 containing Fe(VI) | 98% | [115] |
| DBP | Fe,Ag-ZnO/VIS-LED | 95% | [116] |
| NF membranes | 91.5% | [121] | |
| Propylparaben | Removal by: DOC resin + NF-90 membrane DOC resin + DESAL-HL membrane | 100% 100% | [122] |
| UVA/PS process ZVI/PS process | 94.8% 98.5% | [117] | |
| Methylparaben | Removal by: DOC resin + NF-90 membrane DOC resin + DESAL-HL membrane | 91% 92% | [122] |
| Butylparaben | Removal by: DOC resin + NF-90 membrane DOC resin + DESAL-HL membrane | 100% 100% | [122] |
| Ethylparaben | Removal by: DOC resin + NF-90 membrane DOC resin + DESAL-HL membrane | 96% 97% | [122] |
| DMP | NF membranes | 82.3% | [121] |
| DnOP | NF membranes | 95.1% | [121] |
| DEP | NF membranes | 86.7% | [121] |
| DEHP | NF membranes | 95.4% | [121] |
| Pollutant | Method | Max Removal Efficiency | References |
|---|---|---|---|
| DCF | Adsorption by Ce-UiO-66 MOF | 99.9% | [10] |
| Adsorption by AC from Reynoutria japonica | 58% | [124] | |
| Adsorption by Moringa oleifera pods | 72.4% | [14] | |
| Adsorption by AC from sewage sludge | 96.34% | [125] | |
| Amoxicillin | Adsorption by: AC-nZVI | 97.9% | [127] |
| 3DES | 98.8% | [131] | |
| Ibuprofen | Catalytic ozonation | 99% | [134] |
| Adsorption by iron nano adsorbent | 92% | [15] | |
| Biotransformation by Desulfovibrio vulgaris | 100% | [129] | |
| Ciprofloxacin | Catalytic removal with Bio-Pt | 70% | [129] |
| Naproxen | PEC with ZnO/TiO2/Ag2Se and visible light | 100% | [128] |
| Adsorption by GON | 65.28% | [126] | |
| Paracetamol | Oxidation with OMS-2/PS | 99.5% | [132] |
| Electrooxidation with graphite as anode | >90% | [133] | |
| Photocatalysis with ATiO2 under UV irradiation | 99% | [130] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somma, S.; Reverchon, E.; Baldino, L. Water Purification of Classical and Emerging Organic Pollutants: An Extensive Review. ChemEngineering 2021, 5, 47. https://doi.org/10.3390/chemengineering5030047
Somma S, Reverchon E, Baldino L. Water Purification of Classical and Emerging Organic Pollutants: An Extensive Review. ChemEngineering. 2021; 5(3):47. https://doi.org/10.3390/chemengineering5030047
Chicago/Turabian StyleSomma, Simona, Ernesto Reverchon, and Lucia Baldino. 2021. "Water Purification of Classical and Emerging Organic Pollutants: An Extensive Review" ChemEngineering 5, no. 3: 47. https://doi.org/10.3390/chemengineering5030047
APA StyleSomma, S., Reverchon, E., & Baldino, L. (2021). Water Purification of Classical and Emerging Organic Pollutants: An Extensive Review. ChemEngineering, 5(3), 47. https://doi.org/10.3390/chemengineering5030047








