Understanding Catalysis—A Simplified Simulation of Catalytic Reactors for CO2 Reduction
Abstract
1. Introduction
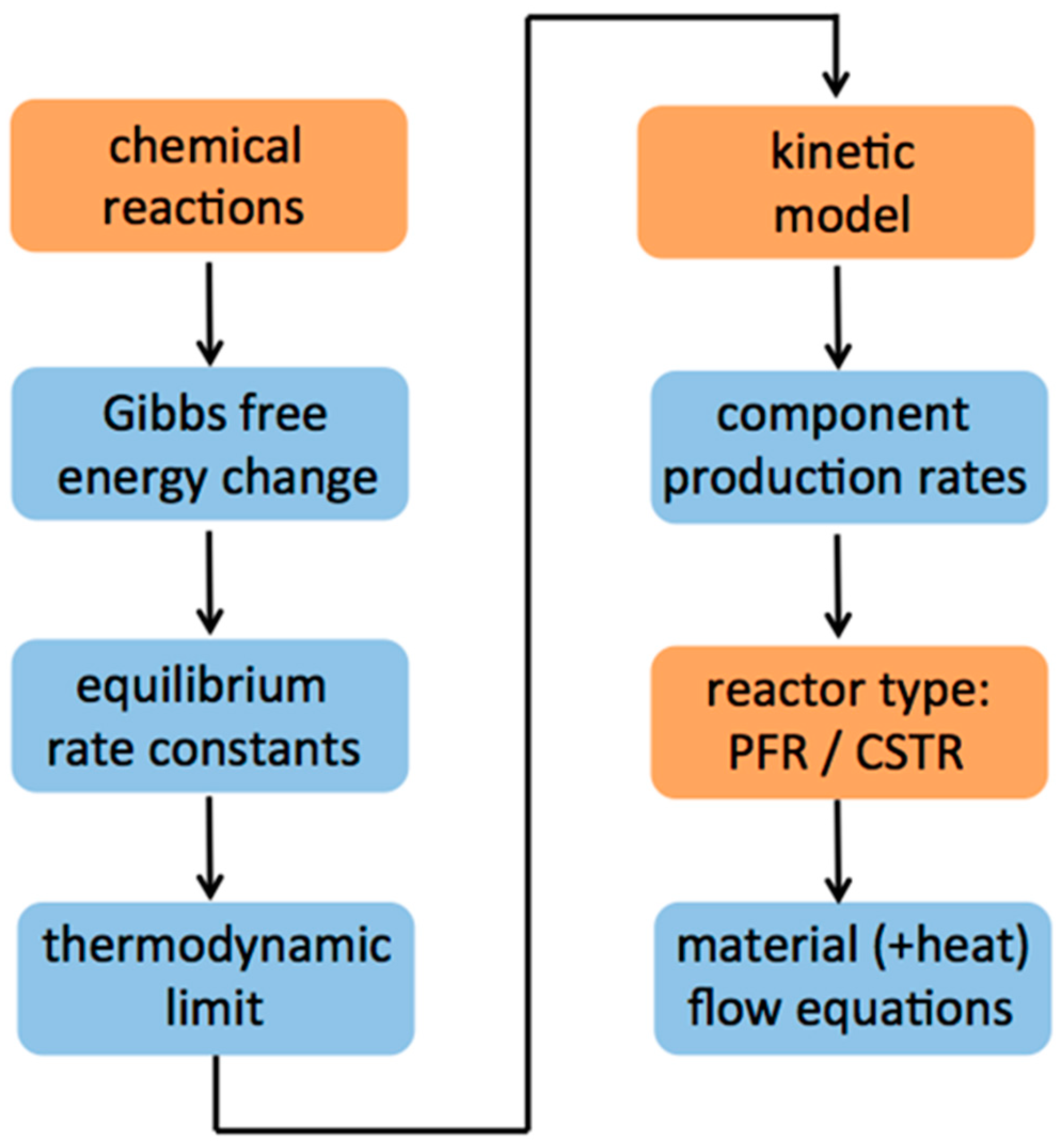
2. Materials and Methods
3. Results and Discussion
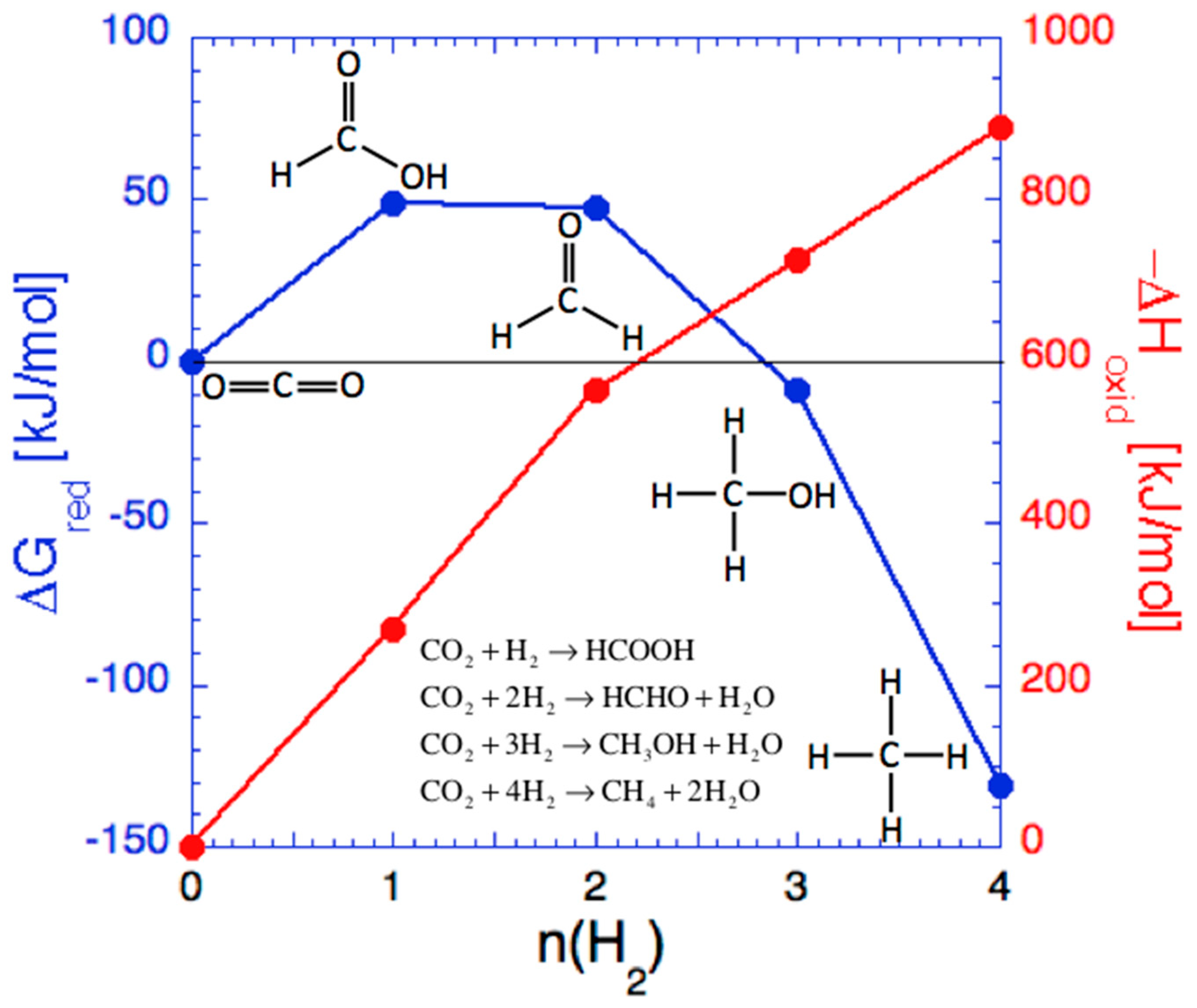
3.1. CO2 Hydrogenation to Methanol and Methane
3.2. Thermodynamic Equilibrium
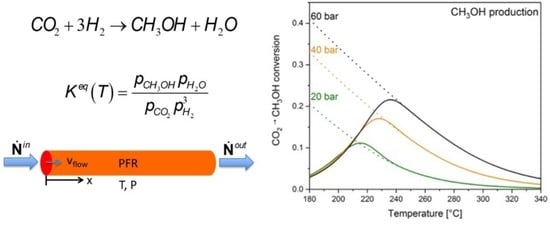
3.3. Kinetic Behavior in a Continuous Flow Catalytic Reactor
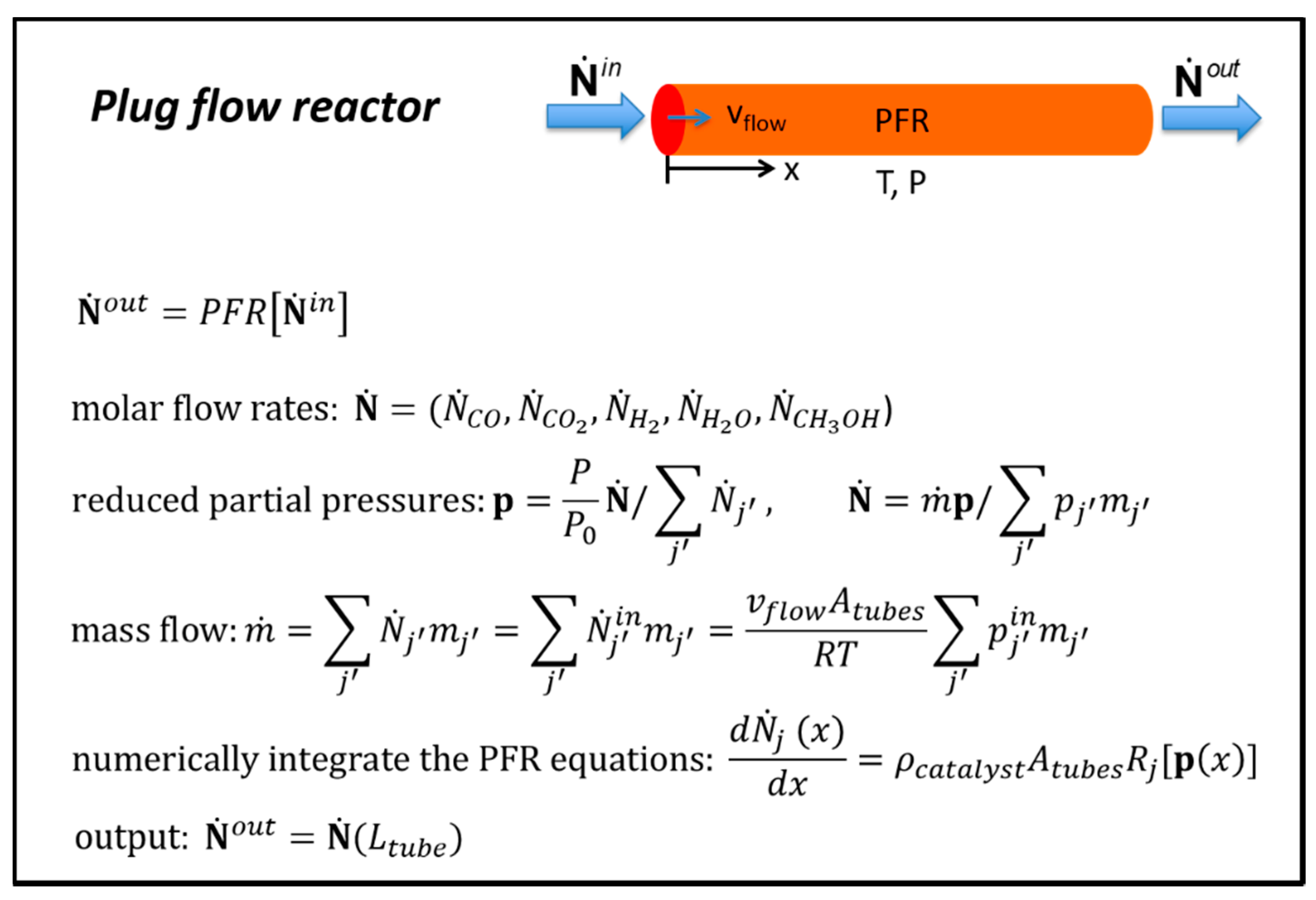
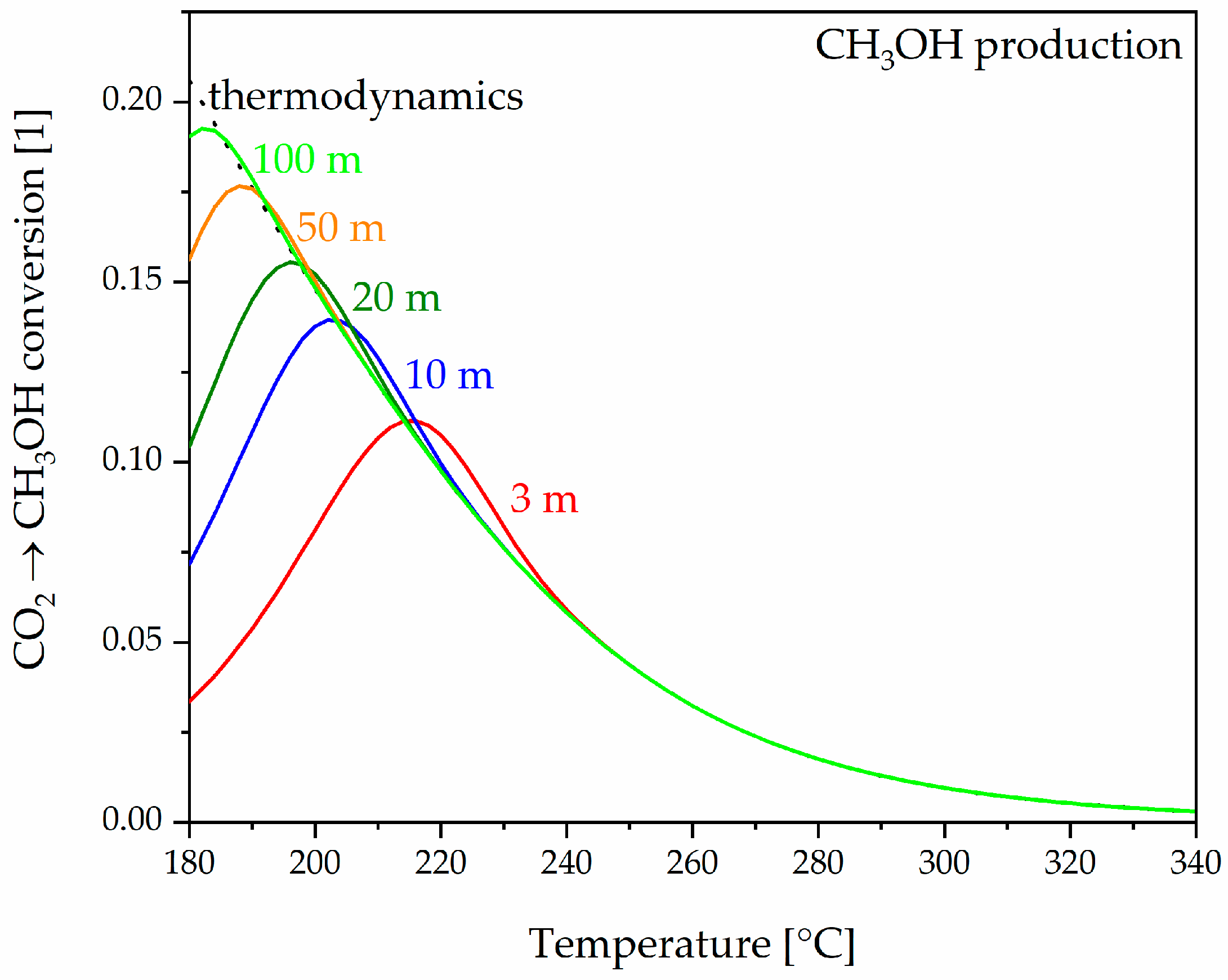
3.3.1. Plug Flow Reactor
3.3.2. Continuously Stirred Tank Reactor
- The volume of the reactor tank is given by Vtank;
- The reactants enter the tank with a flow velocity vflow through an inlet aperture, the area of which is Ainlet;
- The remaining variables have the same meaning as for the PFR described in Figure 6;
- The solution of the CSTR equations is self-consistent and requires the component production rates Rj to be evaluated at the exit of the reactor [41].
3.4. Modified Plug Flow Reactor
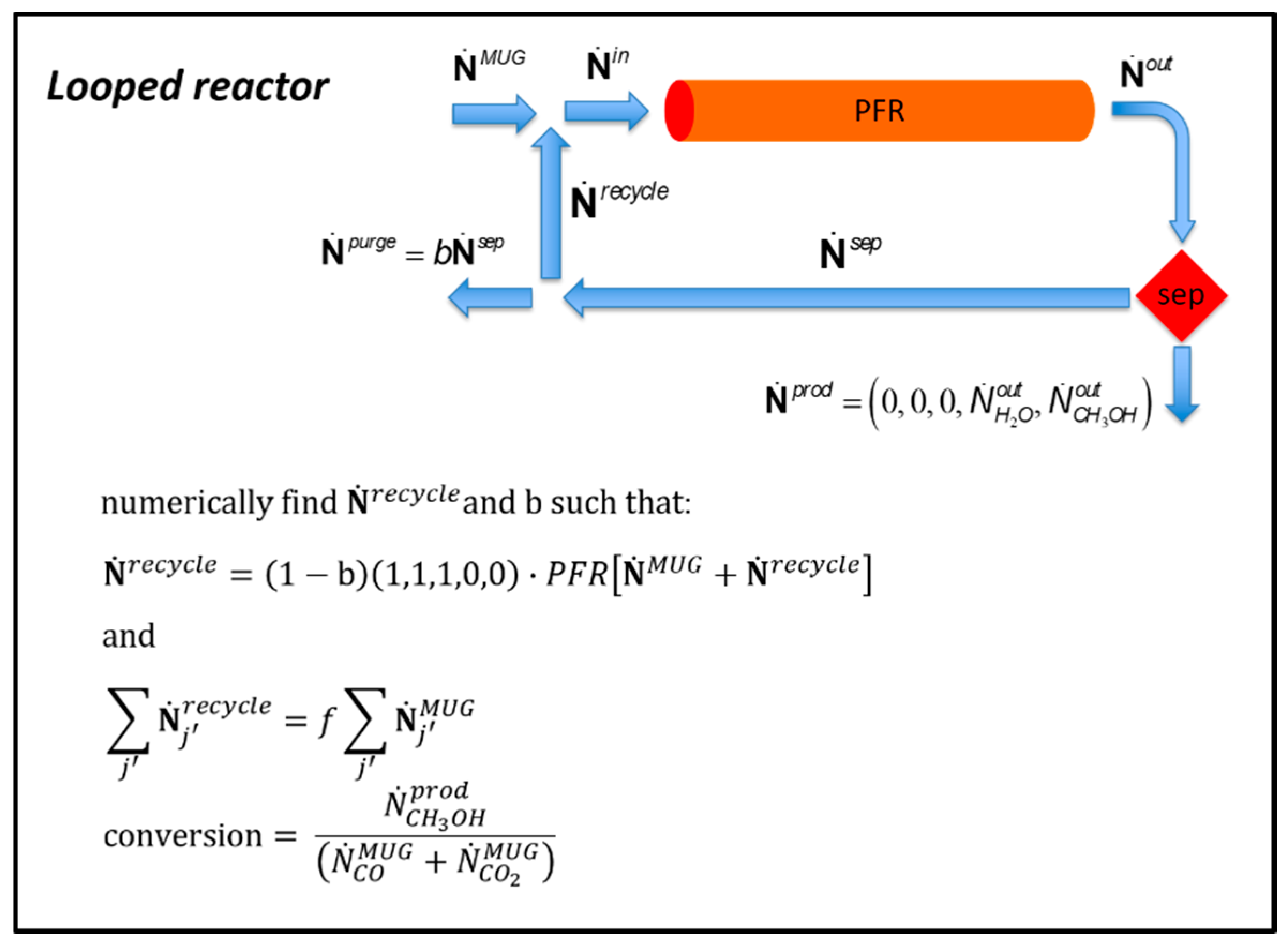
3.4.1. Looped Plug Flow Reactor with Recycling
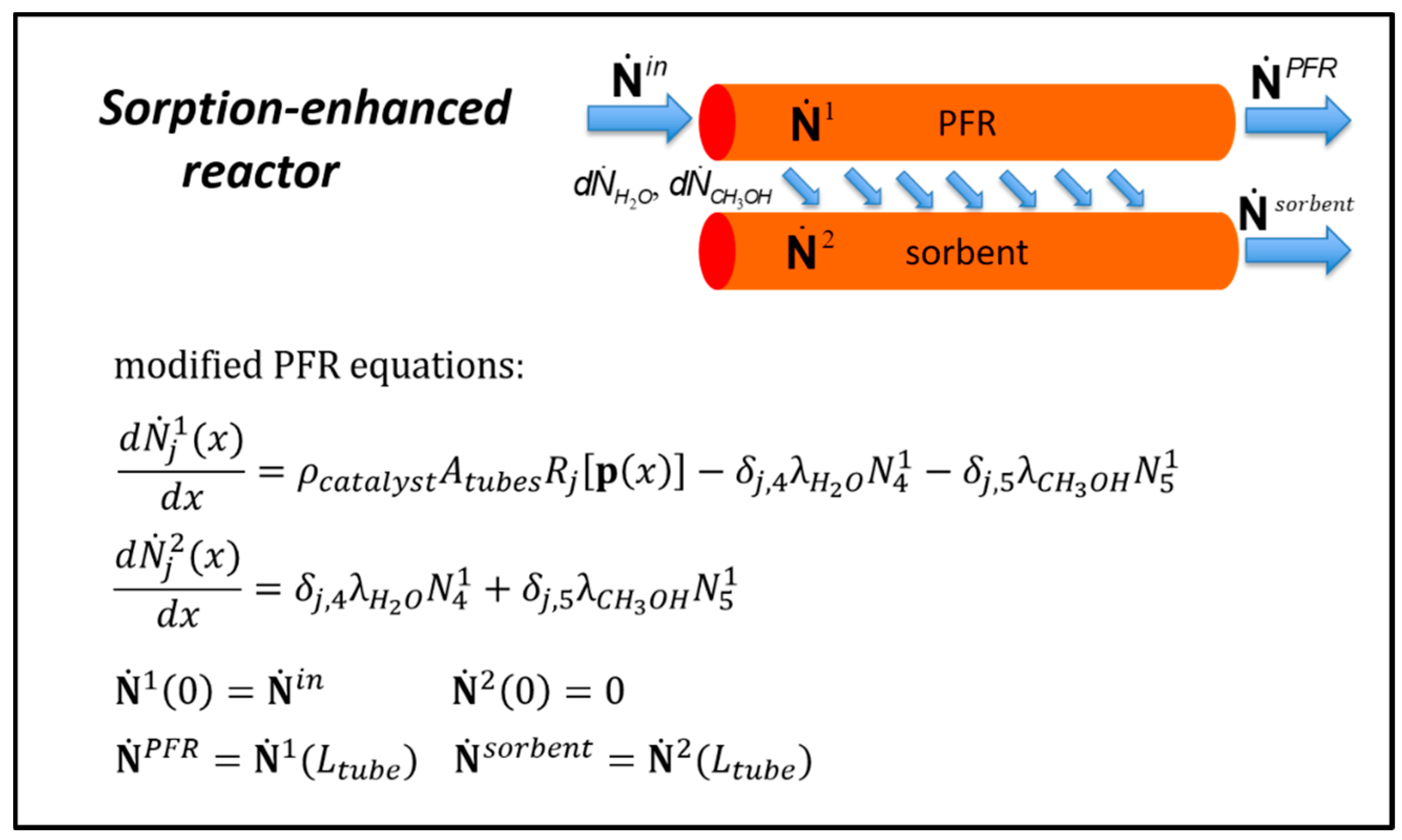
3.4.2. Sorption-Enhanced Plug Flow Reactor
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tripodi, A.; Compagnoni, M.; Martinazzo, R.; Ramis, G.; Rossetti, I. Process simulation for the design and scale up of heterogeneous catalytic process: Kinetic modeling issues. Catalysis 2017, 7, 159. [Google Scholar] [CrossRef]
- Haydary, J. Chemical Process Design and Simulation: Aspen Plus and Aspen HYSYS Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Savelski, M.J.; Hesketh, R.P. Issues encountered with students using process simulators. Age 2002, 8, 1. [Google Scholar]
- Fogler, S.H. Elements of Chemical Reaction Engineering; Pearson Education Inc.: Upper Saddle River, NJ, USA, 1987. [Google Scholar]
- Davis, M.E.; Davis, R.J. Fundamentals of Chemical Reaction Engineering; McGraw Hill: New York, NY, USA, 2003. [Google Scholar]
- Manos, G. Introduction to Chemical Reaction Engineering. In Concepts of Chemical Engineering 4 Chemists; Simons, S., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2007. [Google Scholar]
- Nauman, E.B. Chemical Reactor Design, Optimization, and Scaleup; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Hill, C.G.; Root, T.W. Introduction to Chemical Engineering Kinetics and Reactor Design; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Hagen, J. Industrial Catalysis; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar]
- Press, W.H.; Flannery, B.P.; Teukolsky, S.A.; Vetterling, W.T. Numerical Recipes: The Art of Scientific Computing; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Carvill, B.T.; Hufton, J.R.; Anand, M.; Sircar, S. Sorption-Enhanced Reaction Process. AIChE J. 1996, 42, 2765–2772. [Google Scholar] [CrossRef]
- Swaddle, T.W. Inorganic Chemistry—An Industrial and Environmental Perspective; Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Graaf, G.H.; Stamhuis, E.J.; Beenackers, A.A.C.M. Kinetics of low-pressure Methanol Synthesis. Chem. Eng. Sci. 1988, 43, 3185–3195. [Google Scholar] [CrossRef]
- Graaf, G.H.; Scholtens, H.; Stamhuis, E.J.; Beenackers, A.A.C.M. Intra-particle Diffusion Limitations in low-pressure Methanol Synthesis. Chem. Eng. Sci. 1990, 45, 773–783. [Google Scholar] [CrossRef]
- Xu, J.; Froment, G.F. Methane Steam Reforming, Methanation and Water-gas Shift: I. Intrinsic Kinetics. AlChE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- Wolfram, S. Mathematica: A System for Doing Mathematics by Computer; Addison-Wesley: Reading, MA, USA, 1991. [Google Scholar]
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 conversion: A key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 2003, 6, 1711. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef]
- Abas, N.; Kalair, A.; Khan, N. Review of fossil fuels and future energy technologies. Futures 2015, 69, 31–49. [Google Scholar] [CrossRef]
- Patterson, B.D.; Mo, F.; Borgschulte, A.; Hillestad, M.; Joos, F.; Kristiansen, T.; Sunde, S.; van Bokhoven, J.A. Renewable CO2 recycling and synthetic fuel production in a marine environment. Proc. Natl. Acad. Sci. USA 2019, 116, 12212–12219. [Google Scholar] [CrossRef]
- Miguel, C.V.; Soria, M.A.; Mendes, A.; Madeira, L.M. Direct CO2 hydrogenation to methane or methanol from post-combustion exhaust streams—A thermodynamic study. J. Nat. Gas Sci. Eng. 2015, 22, 1–8. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62. [Google Scholar] [CrossRef]
- Moioli, E.; Mutschler, R.; Züttel, A. Renewable energy storage via CO2 and H2 conversion to methane and methanol: Assessment for small scale applications. Renew. Sustain. Energy Rev. 2019, 107, 497–506. [Google Scholar]
- Atkins, P.W.; de Paula, J. Physikalische Chemie; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Schüth, F. Chemical Compounds for Energy Storage. Chem. Ing. Tech. 2011, 83, 1984–1993. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Rush, J.D. Reduction potential of the carbon dioxide/carbon dioxide radical anion: A comparison with other C1 radicals. J. Phys. Chem. 1987, 91, 4429–4430. [Google Scholar] [CrossRef]
- Rumble, J. CRC Handbook of Chemistry and Physics; Taylor & Francis: Abdingdon, UK, 2020. [Google Scholar]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Kustov, A.L.; Frey, A.M.; Larsen, K.E.; Johannessen, T.; Nørskov, J.K.; Christensen, C.H. CO methanation over supported bimetallic Ni-Fe catalysts: From computational studies towards catalyst optimization. Appl. Catal. A Gen. 2007, 320, 98–104. [Google Scholar] [CrossRef]
- Skrzypek, J.; Lachowska, M.; Serafin, D. Methanol Synthesis from CO2 and H2: Dependence of equilibrium conversions and exit equilibrium concentrations of components on the main process variables. Chem. Eng. Sci. 1990, 45, 89–96. [Google Scholar] [CrossRef]
- Stangeland, K.; Li, H.; Yu, Z. Thermodynamic analysis of chemical and phase equilibria in CO2 hydrogenation to methanol, dimethyl ether, and higher alcohols. Ind. Eng. Chem. Res. 2018, 57, 4081–4094. [Google Scholar] [CrossRef]
- Schlereth, D.; Hinrichsen, O. A fixed-bed reactor modeling study on the methanation of CO2. Chem. Eng. Res. Des. 2014, 92, 702–712. [Google Scholar]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar]
- Hanefeld, U.; Lefferts, L. Catalysis: An Integrated Textbook for Students; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Nørskov, J.K.; Studt, F.; Abild-Pedersen, F.; Bligaard, T. Fundamental Concepts in Heterogeneous Catalysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Toyir, J.; Miloua, R.; Elkadri, N.E.; Nawdali, M.; Toufik, H.; Miloua, F.; Saito, M. Sustainable process for the production of methanol from CO2 and H2 using Cu/ZnO-based multicomponent catalyst. Phys. Procedia 2009, 2, 1075–1079. [Google Scholar] [CrossRef]
- Gaikwad, R.; Bansode, A.; Urakawa, A. High-pressure advantages in stoichiometric hydrogenation of carbon dioxide to methanol. J. Catal. 2016, 343, 127–132. [Google Scholar]
- Slotboom, Y.; Bos, M.J.; Pieper, J.; Vrieswijk, V.; Likozar, B.; Kersten, S.R.A.; Brilman, D.W.F. Critical assessment of steady-state kinetic models for the synthesis of methanol over an industrial Cu/ZnO/Al2O3 catalyst. Chem. Eng. J. 2020, 389, 124181. [Google Scholar]
- Cao, H.; Wang, W.; Cui, T.; Zhu, G.; Ren, X. Enhancing CO2 hydrogenation to methane by Ni-based catalyst with V species using 3D-mesoporous KIT-6 as support. Energies 2020, 13, 2235. [Google Scholar] [CrossRef]
- Van-Dal, E.S.; Bouallou, C. Design and simulation of a methonal production plant from CO2 hydrogenation. J. Clean. Prod. 2013, 57, 38–45. [Google Scholar]
- Falconer, J.L. Comparing CSTR and PFR Mass Balances, LearnChemE Video Presentation, Univ. Colorado, Boulder, and Private Communication. 2019. Available online: www.youtube.com/watch?v=xrOdRKzlkcE (accessed on 15 November 2020).
- Terreni, J.; Trottmann, M.; Franken, T.; Heel, A.; Borgschulte, A. Sorption-Enhanced Methanol Synthesis. Energy Technol. 2019, 7, 1801093. [Google Scholar] [CrossRef]









| ΔH0 [kJ/mol] | S0 [J/mol K] | |
|---|---|---|
| CO | −110.52 | 197.67 |
| CO2 | −393.51 | 213.74 |
| H2 | 0 | 130.68 |
| H2O | −241.82 | 188.82 |
| CH3OH | −200.66 | 239.81 |
| CH4 | −74.81 | 186.26 |
| Variable | a | b |
|---|---|---|
| KCO | 2.16 | 0.468 |
| KCO2 | 7.05 | 0.617 |
| KH2O/KH21/2 | 6.37 | 0.840 |
| Parameter | Methanol Synthesis | Methane Synthesis |
|---|---|---|
| Catalyst | Cu/ZnO/Al2O3 | Ni/MgAl2O4 |
| Catalyst density ρcatalyst | 1000 kg/m3 | 1000 kg/m3 |
| Nr of parallel tubes ntubes | 10,000 | 10 |
| Tube diameter d | 2 cm | 2 cm |
| Tube area Atube = ntube × πd2/4 | 3.14 m2 | 0.00314 m2 |
| Tube length Ltube | 3 m | 1 m |
| Initial flow velocity vflow | 0.05 m/s | 5 m/s |
| Stoichiometric number SN | 2 | 3 |
| Temperature range T | 180–340 °C | 100–1000 °C |
| Pressures P | 20, 40, 60 bar | 1, 10, 20 bar |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terreni, J.; Borgschulte, A.; Hillestad, M.; Patterson, B.D. Understanding Catalysis—A Simplified Simulation of Catalytic Reactors for CO2 Reduction. ChemEngineering 2020, 4, 62. https://doi.org/10.3390/chemengineering4040062
Terreni J, Borgschulte A, Hillestad M, Patterson BD. Understanding Catalysis—A Simplified Simulation of Catalytic Reactors for CO2 Reduction. ChemEngineering. 2020; 4(4):62. https://doi.org/10.3390/chemengineering4040062
Chicago/Turabian StyleTerreni, Jasmin, Andreas Borgschulte, Magne Hillestad, and Bruce D. Patterson. 2020. "Understanding Catalysis—A Simplified Simulation of Catalytic Reactors for CO2 Reduction" ChemEngineering 4, no. 4: 62. https://doi.org/10.3390/chemengineering4040062
APA StyleTerreni, J., Borgschulte, A., Hillestad, M., & Patterson, B. D. (2020). Understanding Catalysis—A Simplified Simulation of Catalytic Reactors for CO2 Reduction. ChemEngineering, 4(4), 62. https://doi.org/10.3390/chemengineering4040062