Advances in Photoreactive Tissue Adhesives Derived from Natural Polymers
Abstract
1. Introduction
2. Photoreactive Tissue Adhesives Derived from Naturally Occurring Polymers
2.1. Photoreactive Tissue Adhesives Derived from Naturally Occurring Proteins
2.1.1. Gelatin-Derived Photoreactive Tissue Adhesives
2.1.2. Fibrinogen for Photoreactive Tissue Adhesives
2.1.3. Recombinant Protein-Derived Photoreactive Tissue Adhesives
2.2. Photoreactive Tissue Adhesives Derived from Polysaccharides
2.2.1. Alginate-Derived Photoreactive Tissue Adhesives
2.2.2. Photoreactive Tissue Adhesives Derived from Chitosan
2.2.3. Photoreactive Tissue Adhesives Derived from Chondroitin Sulfate
2.2.4. Photoreactive Tissue Adhesives Derived from Dextran
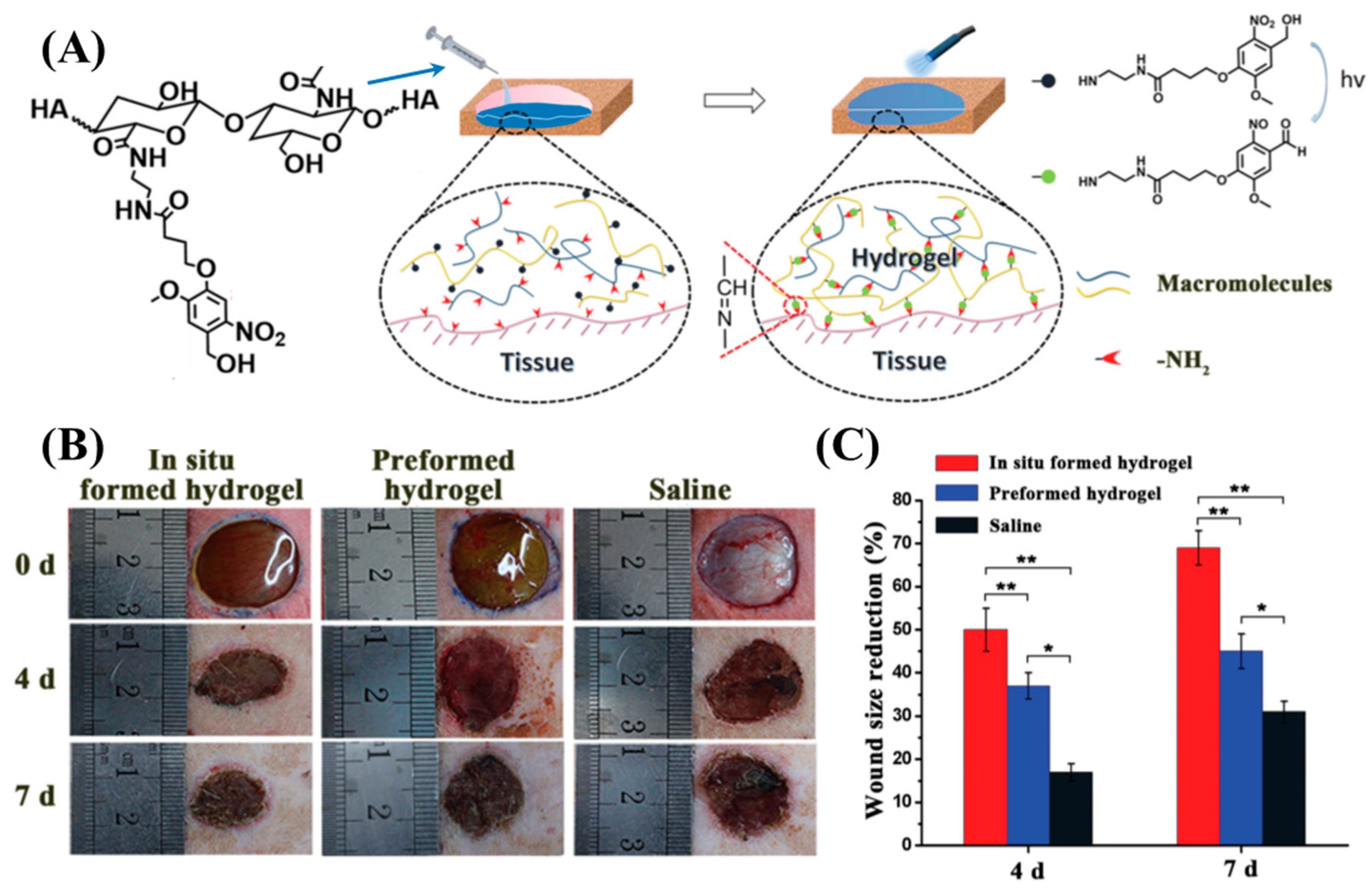
2.2.5. Photoreactive Tissue Adhesives Derived from Hyaluronic Acid
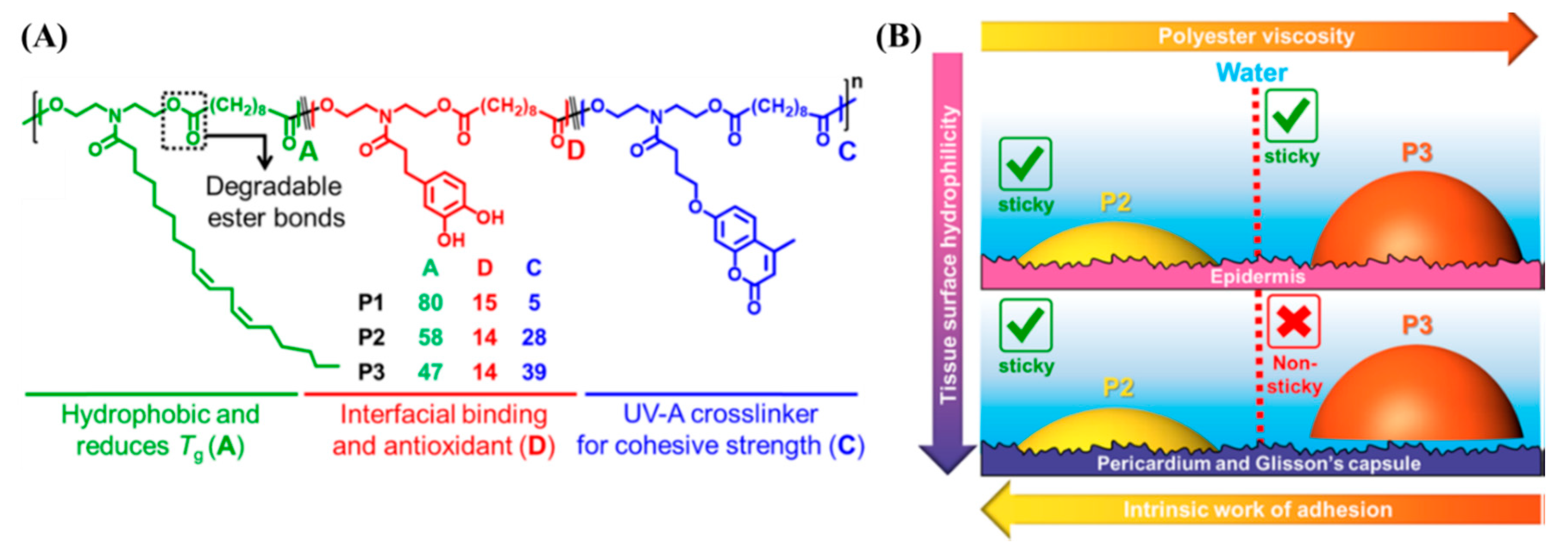
2.3. Natural Oil-Derived Photoreactive Tissue Adhesives
3. Conclusion and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Hall, M.J.; Schwartzman, A.; Zhang, J.; Liu, X. Ambulatory Surgery Data from Hospitals and Ambulatory Surgery Centers: United States, 2010. Natl. Health Stat. Rep. 2017, 102, 1–15. [Google Scholar]
- Forsch, R. Essentials of skin laceration repair. Am. Fam. Physician 2008, 78, 945–951. [Google Scholar] [PubMed]
- Owen, J.; Andrews, W.W. Wound Complications after Cesarean Sections. Clin. Obstet. Gynecol. 1994, 37, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.; Tsao, H.; Henry, F.P.; Kochevar, J.; Redmond, R.; Kochevar, I.; Yao, M.; Zhao, Y. Light-activated tissue bonding for excisional wound closure: A split-lesion clinical trial. Br. J. Dermatol. 2012, 166, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Yao, M.; Farinelli, W.; Hajjarian, Z.; Wang, Y.; Redmond, R.W.; Kochevar, I.E. Light-activated sealing of skin wounds. Lasers Surg. Med. 2014, 47, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Spotnitz, W.D. Hemostats, sealants, and adhesives: A practical guide for the surgeon. Am. Surg. 2012, 78, 1305–1321. [Google Scholar]
- Coselli, J.S.; Bavaria, J.E.; Fehrenbacher, J.; Stowe, C.L.; Macheers, S.K.; Gundry, S.R. Prospective randomized study of a protein-based tissue adhesive used as a hemostatic and structural adjunct in cardiac and vascular anastomotic repair procedures. J. Am. Coll. Surg. 2003, 197, 243–252. [Google Scholar] [CrossRef]
- Schenk, W.G.; Spotnitz, W.D.; Burks, S.G.; Lin, P.H.; Lin, P.; Lumsden, A.B. Absorbable cyanoacrylate as a vascular hemostatic sealant: A preliminary trial. Am. Surg. 2005, 71, 658–661. [Google Scholar]
- LeMaire, S.A.; Carter, S.A.; Won, T.; Wang, X.; Conklin, L.D.; Coselli, J.S. The Threat of Adhesive Embolization: BioGlue Leaks through Needle Holes in Aortic Tissue and Prosthetic Grafts. Ann. Thorac. Surg. 2005, 80, 106–111. [Google Scholar] [CrossRef]
- Alameddine, A.; Alimov, V.K.; Rousou, J.A.; Freeman, J. Aorto-Pulmonary Artery Disruption Following Acute Type-A Aortic Dissection Repair with the Use of BioGlue. J. Card. Surg. 2012, 27, 371–373. [Google Scholar] [CrossRef]
- Spotnitz, W.D.; Burks, S. Hemostats, sealants, and adhesives III: A new update as well as cost and regulatory considerations for components of the surgical toolbox. Transfusion 2012, 52, 2243–2255. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sajiki, T.; Nakayama, Y.; Fukui, M.; Matsuda, T. Novel visible-light-induced photocurable tissue adhesive composed of multiply styrene-derivatized gelatin and poly(ethylene glycol) diacrylate. J. Biomed. Mater. Res. 2003, 66, 439–446. [Google Scholar] [CrossRef]
- O’Rorke, R.; Pokholenko, O.; Gao, F.; Cheng, T.; Shah, A.; Mogal, V.; Steele, T.W.J. Addressing Unmet Clinical Needs with UV Bioadhesives. Biomacromolecules 2017, 18, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Djordjevic, I.; Mogal, V.; O’Rorke, R.; Pokholenko, O.; Steele, T.W.J. Elastic Light Tunable Tissue Adhesive Dendrimers. Macromol. Biosci. 2016, 16, 1072–1082. [Google Scholar] [CrossRef]
- Kloxin, A.M.; Kasko, A.M.; Salinas, C.N.; Anseth, K.S. Photodegradable Hydrogels for Dynamic Tuning of Physical and Chemical Properties. Science 2009, 324, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.G.; Santappa, M.; Uri, N. Photoinitiated free radical polymerization of vinyl compounds in aqueous solution. J. Polym. Sci. 1951, 7, 243–260. [Google Scholar] [CrossRef]
- Nakayama, Y.; Matsuda, T. Newly Designed Hemostatic Technology Based on Photocurable Gelatin. ASAIO J. 1995, 41, M374–M378. [Google Scholar] [CrossRef]
- Lang, N.; Pereira, M.J.; Lee, Y.; Friehs, I.; Vasilyev, N.V.; Feins, E.N.; Ablasser, K.; O’Cearbhaill, E.; Xu, C.; Fabozzo, A.; et al. A Blood-Resistant Surgical Glue for Minimally Invasive Repair of Vessels and Heart Defects. Sci. Transl. Med. 2014, 6, 218ra6. [Google Scholar] [CrossRef]
- Fancy, D.A.; Kodadek, T. Chemistry for the analysis of protein-protein interactions: Rapid and efficient cross-linking triggered by long wavelength light. Proc. Natl. Acad. Sci. USA 1999, 96, 6020–6024. [Google Scholar] [CrossRef]
- Elvin, C.M.; Brownlee, A.G.; Huson, M.G.; Tebb, T.A.; Kim, M.; Lyons, R.E.; Vuocolo, T.; Liyou, N.E.; Hughes, T.; Ramshaw, J.A.M.; et al. The development of photochemically crosslinked native fibrinogen as a rapidly formed and mechanically strong surgical tissue sealant. Biomaterials 2009, 30, 2059–2065. [Google Scholar] [CrossRef]
- Matsuda, T.; Moghaddam, M.J.; MlWA, H.; Sakurai, K.; Iida, F. Photoinduced Prevention of Tissue Adhesion. ASAIO J. 1992, 38, M154–M157. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.D.; Bowen, C.M.; Liotta, F. [2 + 2] Photocycloaddition/Fragmentation Strategies for the Synthesis of Natural and Unnatural Products. Chem. Rev. 1995, 95, 2003–2020. [Google Scholar] [CrossRef]
- Maddipatla, M.V.S.N.; Wehrung, D.; Tang, C.; Fan, W.; Oyewumi, M.O.; Miyoshi, T.; Joy, A. Photoresponsive Coumarin Polyesters That Exhibit Cross-Linking and Chain Scission Properties. Macromolecules 2013, 46, 5133–5140. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Duarte, A.P.; Coelho, J.F.J.; Bordado, J.; Cidade, M.T.; Gil, M.H. Surgical adhesives: Systematic review of the main types and development forecast. Prog. Polym. Sci. 2012, 37, 1031–1050. [Google Scholar] [CrossRef]
- Scognamiglio, F.; Travan, A.; Rustighi, I.; Tarchi, P.; Palmisano, S.; Marsich, E.; Borgogna, M.; Donati, I.; De Manzini, N.; Paoletti, S. Adhesive and sealant interfaces for general surgery applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 104, 626–639. [Google Scholar] [CrossRef]
- Bré, L.P.; Zheng, Y.; Pêgo, A.P.; Wang, W. Taking tissue adhesives to the future: From traditional synthetic to new biomimetic approaches. Biomater. Sci. 2013, 1, 239–253. [Google Scholar] [CrossRef]
- Santiago, G.T.-D.; Sharifi, R.; Yue, K.; Sani, E.S.; Kashaf, S.S.; Alvarez, M.M.; Leijten, J.; Khademhosseini, A.; Dana, R.; Annabi, N. Ocular adhesives: Design, chemistry, crosslinking mechanisms, and applications. Biomaterials 2019, 197, 345–367. [Google Scholar] [CrossRef]
- Annabi, N.; Yue, K.; Tamayol, A.; Khademhosseini, A. Elastic sealants for surgical applications. Eur. J. Pharm. Biopharm. 2015, 95, 27–39. [Google Scholar] [CrossRef]
- Bouten, P.J.; Zonjee, M.; Bender, J.; Yauw, S.T.; Van Goor, H.; Van Hest, J.C.M.; Hoogenboom, R. The chemistry of tissue adhesive materials. Prog. Polym. Sci. 2014, 39, 1375–1405. [Google Scholar] [CrossRef]
- Stenzel, K.H.; Miyata, T.; Rubin, A.L. Collagen as a Biomaterial. Annu. Rev. Biophys. Bioeng. 1974, 3, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, M.; Akbari, M.; Paul, A.; Memic, A.; Dolatshahi-Pirouz, A.; Khademhosseini, A. Gelatin-Based Biomaterials for Tissue Engineering And Stem Cell Bioengineering. In Biomaterials from Nature for Advanced Devices and Therapies; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 37–62. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yan, M.; Wang, Y.; Fu, J.; Suo, H. 3D Bioprinting of Low-Concentration Cell-Laden Gelatin Methacrylate (GelMA) Bioinks with a Two-Step Cross-linking Strategy. ACS Appl. Mater. Interfaces 2018, 10, 6849–6857. [Google Scholar] [CrossRef] [PubMed]
- Draye, J.-P.; Delaey, B.; Van De Voorde, A.; Bulcke, A.V.D.; De Reu, B.; Schacht, E. In vitro and in vivo biocompatibility of dextran dialdehyde cross-linked gelatin hydrogel films. Biomaterials 1998, 19, 1677–1687. [Google Scholar] [CrossRef]
- Nakayama, Y.; Matsuda, T. Photocurable Surgical Tissue Adhesive Glues Composed of Photoreactive Gelatin and Poly(Ethylene Glycol) Diacrylate. J. Biomed. Mater. Res. 1999, 48, 511–521. [Google Scholar] [CrossRef]
- Kito, H.; Matsuda, T. Biocompatible Coatings for Luminal and Outer Surfaces of Small-Caliber Artificial Grafts. J. Biomed. Mater. Res. 1996, 30, 321–330. [Google Scholar] [CrossRef]
- Okino, H.; Nakayama, Y.; Tanaka, M.; Matsuda, T. In situ hydrogelation of photocurable gelatin and drug release. J. Biomed. Mater. Res. 2002, 59, 233–245. [Google Scholar] [CrossRef]
- Okino, H.; Maeyama, R.; Manabe, T.; Matsuda, T.; Tanaka, M. Trans-tissue, sustained release of gemcitabine from photocured gelatin gel inhibits the growth of heterotopic human pancreatic tumor in nude mice. Clin. Cancer Res. 2003, 9, 5786–5793. [Google Scholar]
- Okino, H.; Manabe, T.; Tanaka, M.; Matsuda, T. Novel therapeutic strategy for prevention of malignant tumor recurrence after surgery: Local delivery and prolonged release of adenovirus immobilized in photocured, tissue-adhesive gelatinous matrix. J. Biomed. Mater. Res. 2003, 66, 643–651. [Google Scholar] [CrossRef]
- Elvin, C.M.; Vuocolo, T.; Brownlee, A.G.; Sando, L.; Huson, M.G.; Liyou, N.E.; Stockwell, P.R.; Lyons, R.E.; Kim, M.; Edwards, G.A.; et al. A highly elastic tissue sealant based on photopolymerised gelatin. Biomaterials 2010, 31, 8323–8331. [Google Scholar] [CrossRef] [PubMed]
- Vuocolo, T.; Haddad, R.; Edwards, G.A.; Lyons, R.E.; Liyou, N.E.; Werkmeister, J.A.; Ramshaw, J.A.M.; Elvin, C.M. A Highly Elastic and Adhesive Gelatin Tissue Sealant for Gastrointestinal Surgery and Colon Anastomosis. J. Gastrointest. Surg. 2011, 16, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ng, S.C.; Yu, J.; Tsai, W.-B. Modification and crosslinking of gelatin-based biomaterials as tissue adhesives. Colloids Surf. B Biointerfaces 2019, 174, 316–323. [Google Scholar] [CrossRef]
- Assmann, A.; Vegh, A.; Ghasemi-Rad, M.; Bagherifard, S.; Cheng, G.; Sani, E.S.; Ruiz-Esparza, G.U.; Noshadi, I.; Lassaletta, A.D.; Gangadharan, S.P.; et al. A highly adhesive and naturally derived sealant. Biomaterials 2017, 140, 115–127. [Google Scholar] [CrossRef] [PubMed]
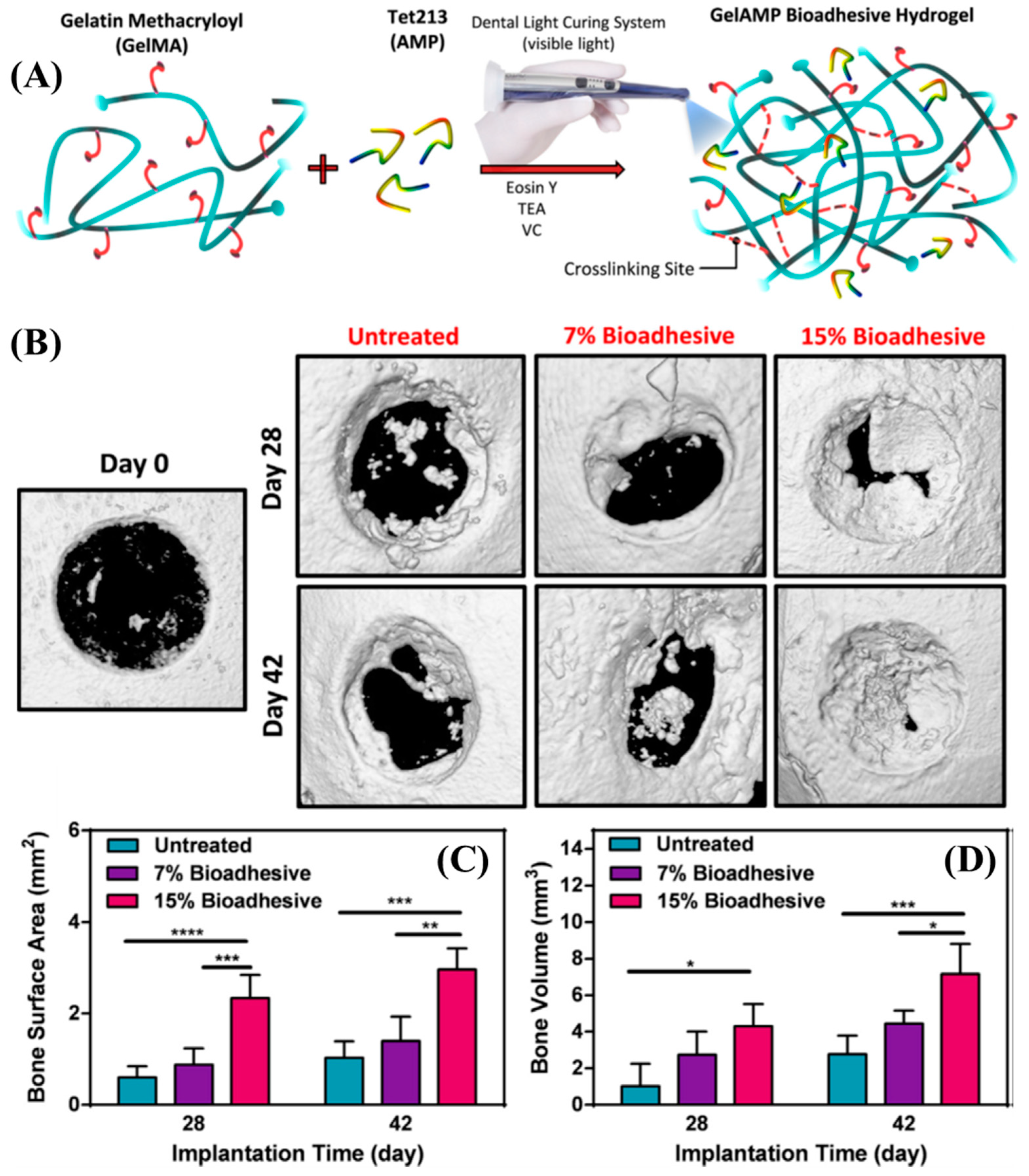
- Sani, E.S.; Lara, R.P.; Aldawood, Z.; Bassir, S.H.; Nguyen, D.; Kantarci, A.; Intini, G.; Annabi, N. An Antimicrobial Dental Light Curable Bioadhesive Hydrogel for Treatment of Peri-Implant Diseases. Matter 2019, 1, 926–944. [Google Scholar] [CrossRef] [PubMed]
- Herrick, S.; Blanc-Brude, O.; Gray, A.; Laurent, G. Fibrinogen. Int. J. Biochem. Cell Biol. 1999, 31, 741–746. [Google Scholar] [CrossRef]
- Wolberg, A.S. Thrombin generation and fibrin clot structure. Blood Rev. 2007, 21, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Elvin, C.M.; Danon, S.J.; Brownlee, A.G.; White, J.; Hickey, M.; Liyou, N.E.; Edwards, G.A.; Ramshaw, J.A.M.; Werkmeister, J.A. Evaluation of photo-crosslinked fibrinogen as a rapid and strong tissue adhesive. J. Biomed. Mater. Res. Part A 2009, 9999, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H. Mussel adhesion-essential footwork. J. Exp. Biol. 2017, 220, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.J.; Wang, C.S.; Song, I.T.; Jones, J.P. The role of coacervation and phase transitions in the sandcastle worm adhesive system. Adv. Colloid Interface Sci. 2016, 239, 88–96. [Google Scholar] [CrossRef]
- Yu, J.; Wei, W.; Menyo, M.S.; Masic, A.; Waite, J.H.; Israelachvili, J.N. Adhesion of Mussel Foot Protein-3 to TiO2 Surfaces: The Effect of pH. Biomacromolecules 2013, 14, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yu, J.; Broomell, C.; Israelachvili, J.N.; Waite, J.H. Hydrophobic Enhancement of Dopa-Mediated Adhesion in a Mussel Foot Protein. J. Am. Chem. Soc. 2012, 135, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.H.; Yu, J.; Estrada, A.; Hammer, M.U.; Waite, J.H.; Israelachvili, J.N. The Contribution of DOPA to Substrate-Peptide Adhesion and Internal Cohesion of Mussel-Inspired Synthetic Peptide Films. Adv. Funct. Mater. 2010, 20, 4196–4205. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Narayanan, A.; Dalvi, S.; Liu, Q.; Joy, A.; Dhinojwala, A. Direct Observation of the Interplay of Catechol Binding and Polymer Hydrophobicity in a Mussel-Inspired Elastomeric Adhesive. ACS Cent. Sci. 2018, 4, 1420–1429. [Google Scholar] [CrossRef]
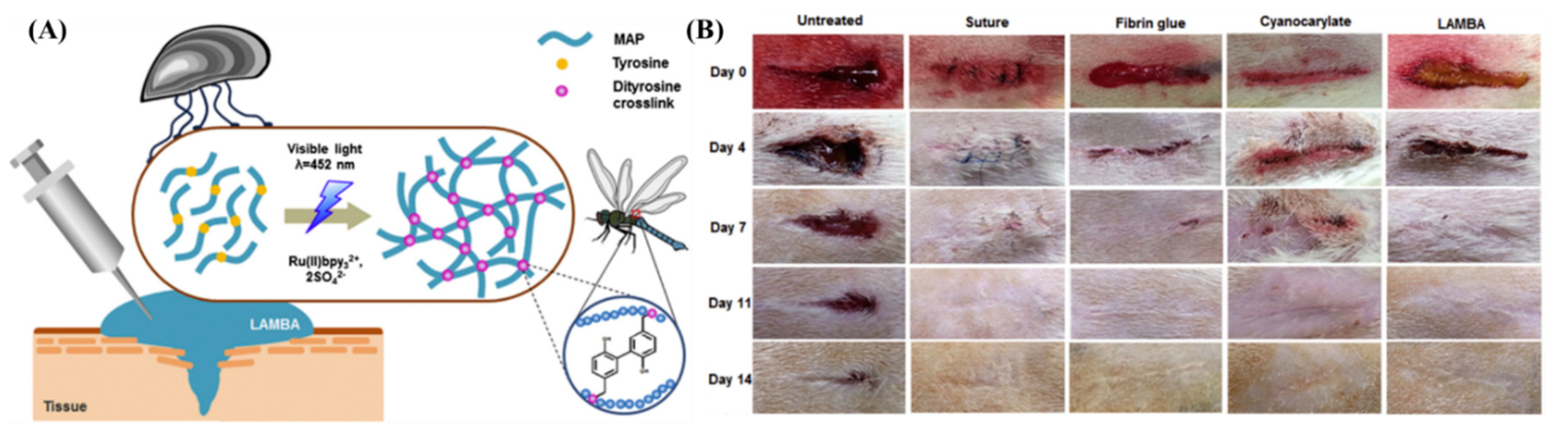
- Jeon, E.Y.; Hwang, B.H.; Yang, Y.J.; Kim, B.J.; Choi, B.-H.; Jung, G.Y.; Cha, H.J. Rapidly light-activated surgical protein glue inspired by mussel adhesion and insect structural crosslinking. Biomaterials 2015, 67, 11–19. [Google Scholar] [CrossRef]
- Wise, S.G.; Weiss, A.S. Tropoelastin. Int. J. Biochem. Cell Biol. 2009, 41, 494–497. [Google Scholar] [CrossRef]
- Annabi, N.; Zhang, Y.-N.; Assmann, A.; Sani, E.S.; Cheng, G.Z.; Lassaletta, A.D.; Vegh, A.; Dehghani, B.; Ruiz-Esparza, G.U.; Wang, X.; et al. Engineering a highly elastic human protein–based sealant for surgical applications. Sci. Transl. Med. 2017, 9, eaai7466. [Google Scholar] [CrossRef]
- Annabi, N.; Rana, D.; Sani, E.S.; Portillo-Lara, R.; Gifford, J.L.; Fares, M.M.; Mithieux, S.; Weiss, A.S. Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials 2017, 139, 229–243. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Orive, G.; Ponce, S.; Bayat, A.; Gascón, A.R.; Igartua, M.; Pedraz, J.L. Biocompatibility of microcapsules for cell immobilization elaborated with different type of alginates. Biomaterials 2002, 23, 3825–3831. [Google Scholar] [CrossRef]
- Chan, A.W.; Neufeld, R.J. Tuneable semi-synthetic network alginate for absorptive encapsulation and controlled release of protein therapeutics. Biomaterials 2010, 31, 9040–9047. [Google Scholar] [CrossRef] [PubMed]
- Smeds, K.A.; Pfister-Serres, A.; Miki, D.; Dastgheib, K.; Inoue, M.; Hatchell, D.L.; Grinstaff, M.W. Photocrosslinkable polysaccharides for in situ hydrogel formation. J. Biomed. Mater. Res. 2001, 54, 115–121. [Google Scholar] [CrossRef]
- Charron, P.N.; Fenn, S.; Poniz, A.; Oldinski, R.A. Mechanical properties and failure analysis of visible light crosslinked alginate-based tissue sealants. J. Mech. Behav. Biomed. Mater. 2016, 59, 314–321. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Li, J.; Celiz, A.D.; Yang, J.; Yang, Q.; Wamala, I.; Whyte, W.; Seo, B.R.; Vasilyev, N.V.; Vlassak, J.J.; Suo, Z.; et al. Tough adhesives for diverse wet surfaces. Science 2017, 357, 378–381. [Google Scholar] [CrossRef]
- Ono, K.; Saito, Y.; Yura, H.; Ishikawa, K.; Kurita, A.; Akaike, T.; Ishihara, M. Photocrosslinkable Chitosan as a Biological Adhesive. J. Biomed. Mater. Res. 2000, 49, 289–295. [Google Scholar] [CrossRef]
- Ishihara, M.; Ono, K.; Saito, Y.; Yura, H.; Hattori, H.; Matsui, T.; Kurita, A. Photocrosslinkable chitosan: An effective adhesive with surgical applications. Int. Congr. Ser. 2001, 1223, 251–257. [Google Scholar] [CrossRef]
- Ishihara, M.; Nakanishi, K.; Ono, K.; Sato, M.; Kikuchi, M.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; Uenoyama, M.; et al. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials 2002, 23, 833–840. [Google Scholar] [CrossRef]
- Ishihara, M. Photocrosslinkable Chitosan Hydrogel as a Wound Dressing and a Biological Adhesive. Trends Glycosci. Glycotechnol. 2002, 14, 331–341. [Google Scholar] [CrossRef]
- Zeng, Z.; Mo, X.-M.; He, C.; Morsi, Y.; El-Hamshary, H.; El-Newehy, M.H. An in situ forming tissue adhesive based on poly(ethylene glycol)-dimethacrylate and thiolated chitosan through the Michael reaction. J. Mater. Chem. B 2016, 4, 5585–5592. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Yang, K.; Fu, Y.V.; Xu, T.; Li, S.; Zhang, D.; Wang, L.; Lee, C.-S. A Novel Double-Crosslinking-Double-Network Design for Injectable Hydrogels with Enhanced Tissue Adhesion and Antibacterial Capability for Wound Treatment. Adv. Funct. Mater. 2020, 30, 1904156. [Google Scholar] [CrossRef]
- Amsden, B.G.; Sukarto, A.; Knight, D.K.; Shapka, S.N. Methacrylated Glycol Chitosan as a Photopolymerizable Biomaterial. Biomacromolecules 2007, 8, 3758–3766. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Liu, Y.; Huang, Y.-C.; Huang, C.-J.; Tsai, W.-B. Fabrication of photo-crosslinkable glycol chitosan hydrogel as a tissue adhesive. Carbohydr. Polym. 2018, 181, 668–674. [Google Scholar] [CrossRef]
- Sugahara, K.; Mikami, T.; Uyama, T.; Mizuguchi, S.; Nomura, K.; Kitagawa, H. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr. Opin. Struct. Biol. 2003, 13, 612–620. [Google Scholar] [CrossRef]
- Clegg, D.O.; Reda, M.J.; Harris, C.L.; Klein, M.A.; O’Dell, J.R.; Hooper, M.M.; Bradley, J.D.; Bingham, C.O.; Weisman, M.H.; Jackson, C.G.; et al. Glucosamine, Chondroitin Sulfate, and the Two in Combination for Painful Knee Osteoarthritis. N. Engl. J. Med. 2006, 354, 795–808. [Google Scholar] [CrossRef]
- Wang, D.-A.; Varghese, S.; Sharma, B.; Strehin, I.; Fermanian, S.; Gorham, J.; Fairbrother, D.H.; Cascio, B.; Elisseeff, J.H. Multifunctional chondroitin sulphate for cartilage tissue–biomaterial integration. Nat. Mater. 2007, 6, 385–392. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T.; Heublein, B.; Hornig, S. Functional Polymers Based on Dextran. Adv. Polym. Sci. 2006, 205, 199–291. [Google Scholar] [CrossRef]
- Hovgaard, L.; Brøndsted, H. Dextran hydrogels for colon-specific drug delivery. J. Control. Release 1995, 36, 159–166. [Google Scholar] [CrossRef]
- Fleming, J.W.; Bloom, W.L. Further Observations on the Hemodynamic Effect of Plasma Volume Expansion by Dextran1. J. Clin. Investig. 1957, 36, 1233–1238. [Google Scholar] [CrossRef]
- Bonnar, J.; Walsh, J. Prevention of Thrombosis after Pelvic Surgery by British Dextran 70. Lancet 1972, 299, 614–616. [Google Scholar] [CrossRef]
- Li, H.; Niu, R.; Yang, J.; Nie, J.; Yang, D. Photocrosslinkable tissue adhesive based on dextran. Carbohydr. Polym. 2011, 86, 1578–1585. [Google Scholar] [CrossRef]
- Wang, T.; Nie, J.; Yang, D. Dextran and gelatin based photocrosslinkable tissue adhesive. Carbohydr. Polym. 2012, 90, 1428–1436. [Google Scholar] [CrossRef]
- Wang, T.; Mu, X.; Li, H.; Wu, W.; Nie, J.; Yang, D. The photocrosslinkable tissue adhesive based on copolymeric dextran/HEMA. Carbohydr. Polym. 2013, 92, 1423–1431. [Google Scholar] [CrossRef]
- Li, C.; Wang, T.; Hu, L.; Wei, Y.; Liu, J.; Mu, X.; Nie, J.; Yang, D. Photocrosslinkable bioadhesive based on dextran and PEG derivatives. Mater. Sci. Eng. C 2014, 35, 300–306. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Luo, Y.; Kirker, K.R.; Prestwich, G.D. Cross-linked hyaluronic acid hydrogel films: New biomaterials for drug delivery. J. Control. Release 2000, 69, 169–184. [Google Scholar] [CrossRef]
- Miki, D.; Dastgheib, K.; Kim, T.; Pfister-Serres, A.; Smeds, K.A.; Inoue, M.; Hatchell, D.L.; Grinstaff, M.W. A Photopolymerized Sealant for Corneal Lacerations. Cornea 2002, 21, 393–399. [Google Scholar] [CrossRef] [PubMed]
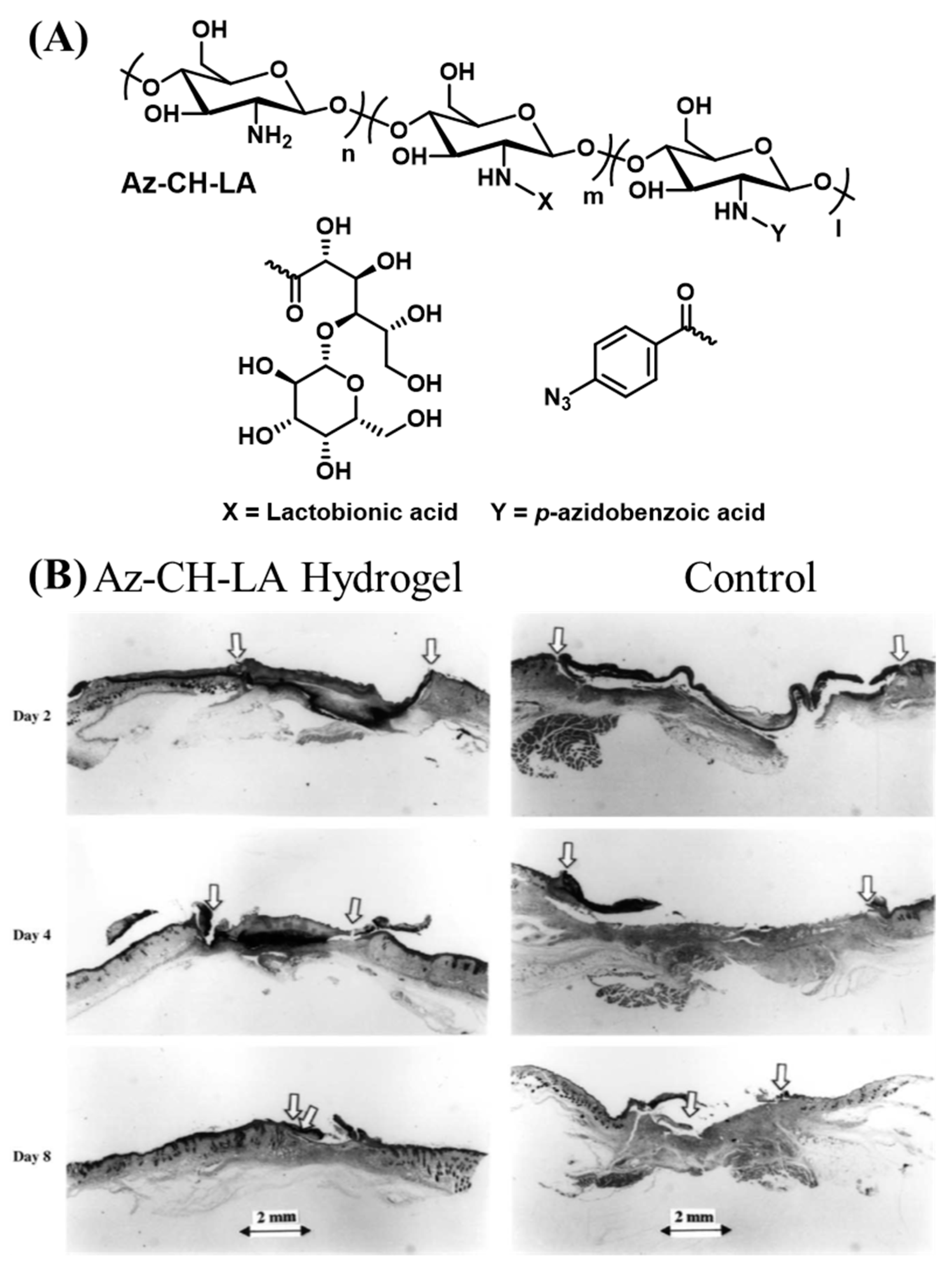
- Chandrasekharan, A.; Seong, K.-Y.; Yim, S.-G.; Kim, S.; Seo, S.; Yoon, J.; Yang, S.Y. In situ photocrosslinkable hyaluronic acid-based surgical glue with tunable mechanical properties and high adhesive strength. J. Polym. Sci. Part A Polym. Chem. 2018, 57, 522–530. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Liu, Z.; Lin, Q.; Liu, X.; Bao, C.; Wang, Y.; Zhu, L. Tissue-Integratable and Biocompatible Photogelation by the Imine Crosslinking Reaction. Adv. Mater. 2016, 28, 2724–2730. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Niu, X.; Lin, Q.; Zhao, B.; Wang, Y.; Zhu, L. An in Situ Photocrosslinkable Platelet Rich Plasma–Complexed Hydrogel Glue with Growth Factor Controlled Release Ability to Promote Cartilage Defect Repair. Acta Biomater. 2017, 62, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, X.; Li, Y.; Wang, Y.; Bao, C.; Chen, Y.; Lin, Q.; Zhu, L. A postoperative anti-adhesion barrier based on photoinduced imine-crosslinking hydrogel with tissue-adhesive ability. Acta Biomater. 2017, 62, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zhou, F.; Hua, Y.; Zhang, X.; Ni, C.; Pan, D.; Zhang, Y.; Jiang, D.; Yang, L.; Lin, Q.; et al. A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat. Commun. 2019, 10, 2060. [Google Scholar] [CrossRef]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Q.; Narayanan, A.; Jain, D.; Dhinojwala, A.; Joy, A. Mussel-Inspired Polyesters with Aliphatic Pendant Groups Demonstrate the Importance of Hydrophobicity in Underwater Adhesion. Adv. Mater. Interfaces 2017, 4, 1700506. [Google Scholar] [CrossRef]
- Narayanan, A.; Kaur, S.; Peng, C.; Debnath, D.; Mishra, K.; Liu, Q.; Dhinojwala, A.; Joy, A. Viscosity Attunes the Adhesion of Bioinspired Low Modulus Polyester Adhesive Sealants to Wet Tissues. Biomacromolecules 2019, 20, 2577–2586. [Google Scholar] [CrossRef]
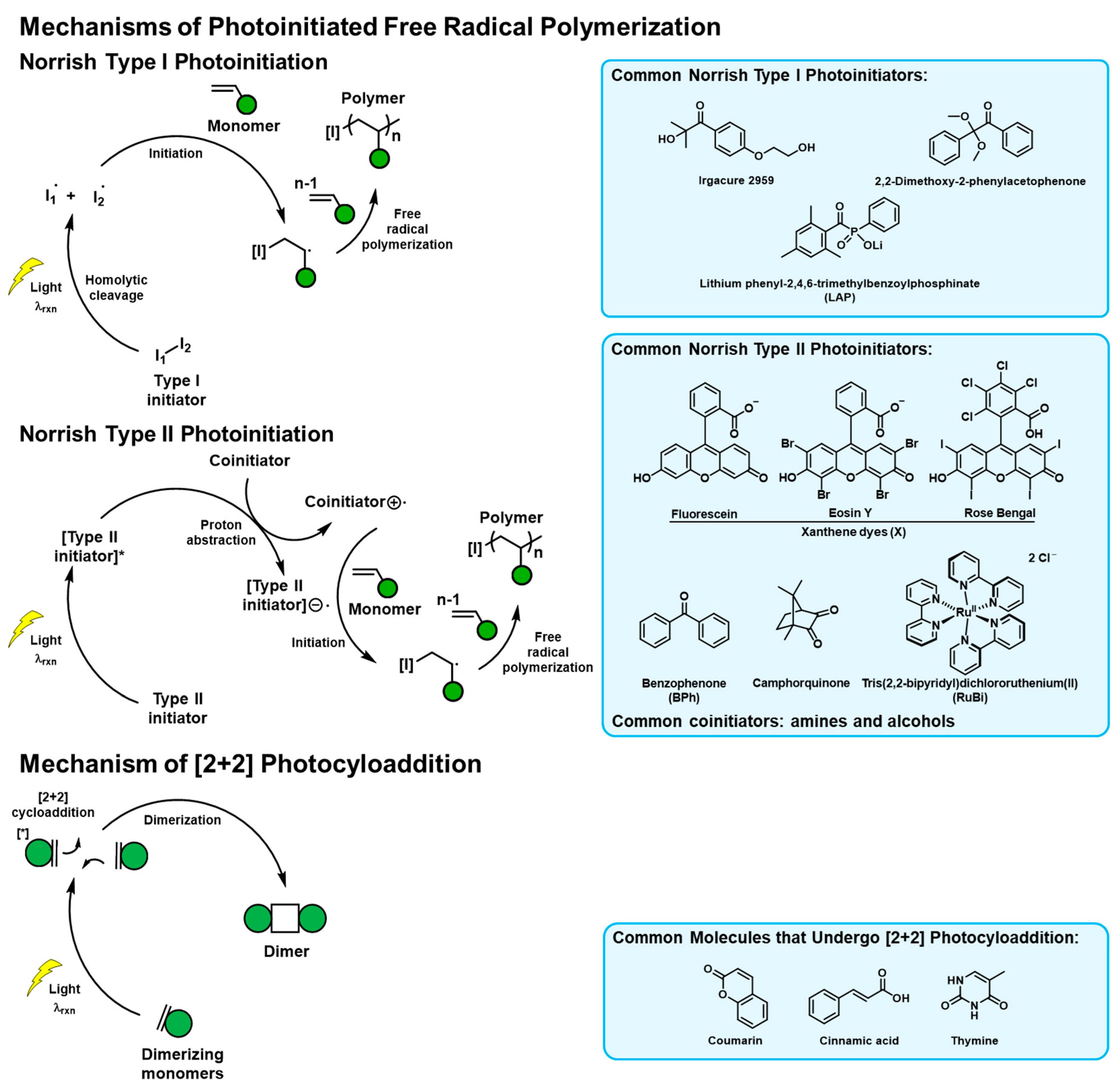
| Natural Polymer | Subclass of Natural Polymer | [a] Gelation System | Photoinitiator System | Mechanism of Crosslinking | Ref. |
|---|---|---|---|---|---|
| [a] Proteins | Gelatin (Gel) Section 2.1.1 | Benzophenone (BPh) and xanthene (X) dyes conjugated Gel mixed with PEGDA | BPh and X pendant groups | Free radical polymerization (FRP) using Norrish type II photoinitiator | 17,37 |
| Styrene pendant Gel mixed with PEGDA and carboxylated camphorquinone (CQ) | CQ | FRP using Norrish type II photoinitiator | 12,39–41 | ||
| Phenol pedant Gel mixed with [Ru(II)bpy3]2+ (RuBi) and sodium persulfate (SPS) | RuBi and SPS | Electron transfer (ET) reaction using Norrish type II photoinitiator | 42–44 | ||
| Methacrylamide pendant Gel (GelMA) mixed different photoinitiators | Irgacure 2959 | FRP using Norrish type I photoinitiator | 45 | ||
| Eosin-Y, TEA, N-vinylcaprolactam | FRP using Norrish type II photoinitiator | 46 | |||
| Fibrinogen Section 2.1.2 | Fibrinogen mixed with RuBi and SPS | RuBi and SPS | ET reaction using Norrish type II photoinitiator | 20,49 | |
| Recombinant proteins Section 2.1.3 | Recombinant mussel protein adhesive mixed with RuBi and SPS | RuBi and SPS | ET reaction using Norrish type II photoinitiator | 56 | |
| Recombinant tropoelastin with pendant methacrylamide (MeTro) and MeTro mixed with GelMA | Irgacure 2959 | FRP using Norrish type I photoinitiator | 58,59 | ||
| [a] Polysaccharides | Alginate (Alg) Section 2.2.1 | Methacrylate pendant native and oxidized Alg with Norrish type II photoinitiator | Eosin-Y, TEA, and 1-vinyl-pyrrilidinone | FRP using Norrish type II photoinitiator | 63,64 |
| Chitosan (CH) Section 2.2.2 | CH with pendant benzyl azide and lactobionic acid | Benzyl azide pedant groups | Nucleophilic attack of nitrene groups to various functional groups | 67–70 | |
| CH modified with thiol pendant groups mixed with PEG-dimethacrylate and Irgacure 2959 | Irgacure 2959 | Thiol-ene reaction initiated by Norrish type I photoinitiator | 71 | ||
| Mixture of CH with pendant methacrylate (CH-MA), CH with pendant methacrylate and catechol (CH-MA-Cat), 2,4,6-trimethylbenzoylphosphinate, and FeCl3 | 2,4,6-trimethylbenzoylphosphinate (LAP) | FRP using Norrish type I photoinitiator | 73 | ||
| Glycol chitosan modified with pedant phenol groups | RuBi and SPS | ET reaction using Norrish type II photoinitiator | 75 | ||
| Chondroitin sulfate (CS) Section 2.2.3 | Methacrylate pendant CS oxidized and mixed with Irgacure 2959 and other acrylates | Irgacure 2959 | FRP using Norrish type I photoinitiator | 78 | |
| Dextran Section 2.2.4 | Native or oxidized dextran mixed with Gel, HEMA, or catechol pendant multiarm PEG | Irgacure 2959 | FRP using Norrish type I photoinitiator | 83–86 | |
| Hyaluronic acid (HA) Section 2.2.5 | Methacrylate pendant HA mixed with type I and type II initiators | 2,2-dimethoxy-2-phenylacetephenone or Irgacure 2959 or Eosin-Y, 1-vinyl-pyrrilidinone, and TEA | FRP using Norrish type I or type II photoinitiator | 63,89,90 | |
| 2-Nitrobenzaldehyde conjugated HA mixed with polymers with multiple amine groups | 2-Nitrobenzaldehyde | Schiff-base reaction between the amine and photo-released benzaldehyde | 91–94 | ||
| Natural oil | Soybean oil Section 2.3 | Melt copolyester with long hydrocarbon from soy oil, catechol, and coumarin | Coumarin | Photodimerization of coumarins | 96,97 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanan, A.; Xu, Y.; Dhinojwala, A.; Joy, A. Advances in Photoreactive Tissue Adhesives Derived from Natural Polymers. ChemEngineering 2020, 4, 32. https://doi.org/10.3390/chemengineering4020032
Narayanan A, Xu Y, Dhinojwala A, Joy A. Advances in Photoreactive Tissue Adhesives Derived from Natural Polymers. ChemEngineering. 2020; 4(2):32. https://doi.org/10.3390/chemengineering4020032
Chicago/Turabian StyleNarayanan, Amal, Ying Xu, Ali Dhinojwala, and Abraham Joy. 2020. "Advances in Photoreactive Tissue Adhesives Derived from Natural Polymers" ChemEngineering 4, no. 2: 32. https://doi.org/10.3390/chemengineering4020032
APA StyleNarayanan, A., Xu, Y., Dhinojwala, A., & Joy, A. (2020). Advances in Photoreactive Tissue Adhesives Derived from Natural Polymers. ChemEngineering, 4(2), 32. https://doi.org/10.3390/chemengineering4020032






