Reactive Extraction of Lactic Acid, Formic Acid and Acetic Acid from Aqueous Solutions with Tri-n-octylamine/1-Octanol/n-Undecane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytics
2.3. Experimental Setup and Procedure
3. Results and Discussion
3.1. Physical Extraction
3.2. Reactive Extraction
3.2.1. Influence of the Solvent Composition on the Distribution Coefficient
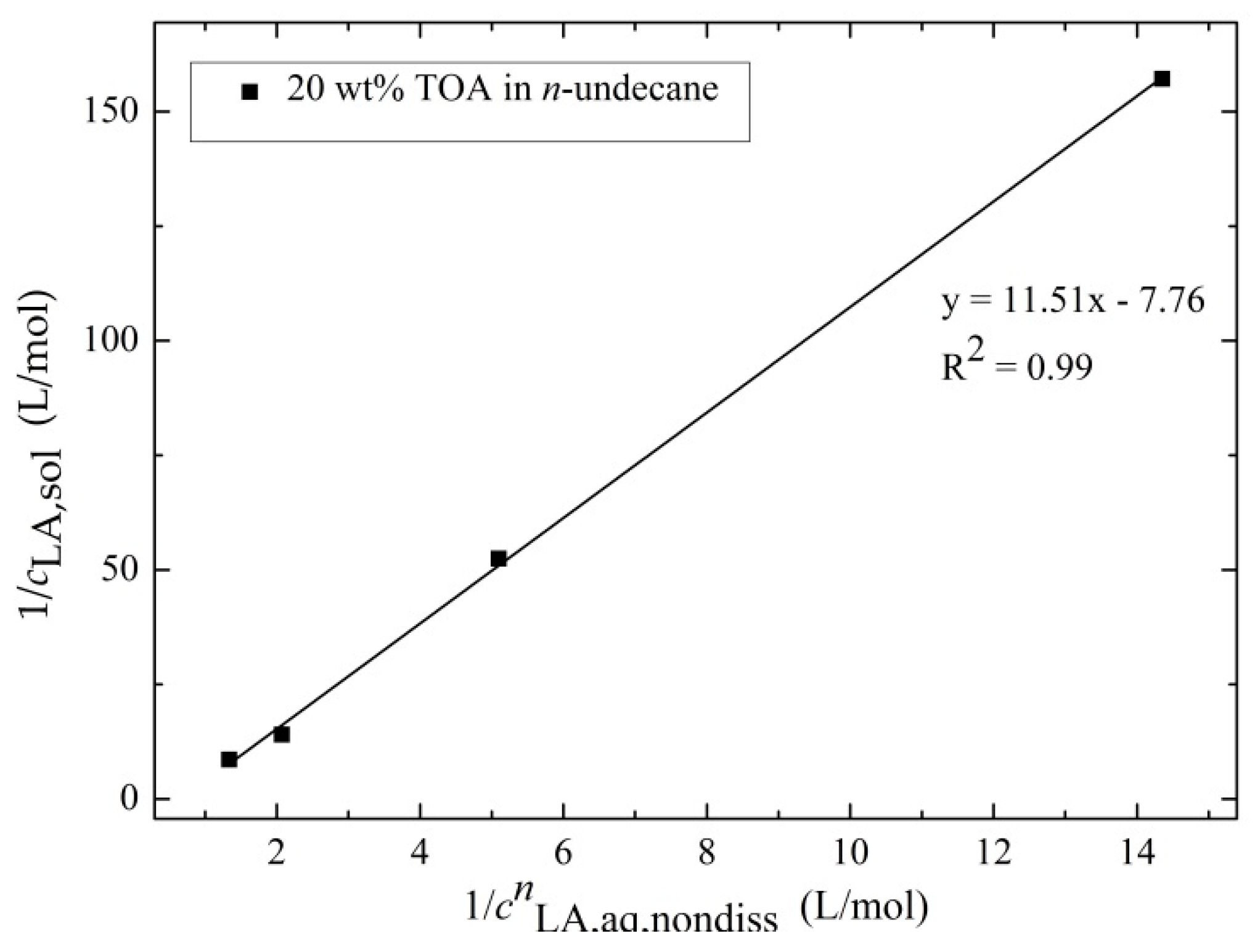
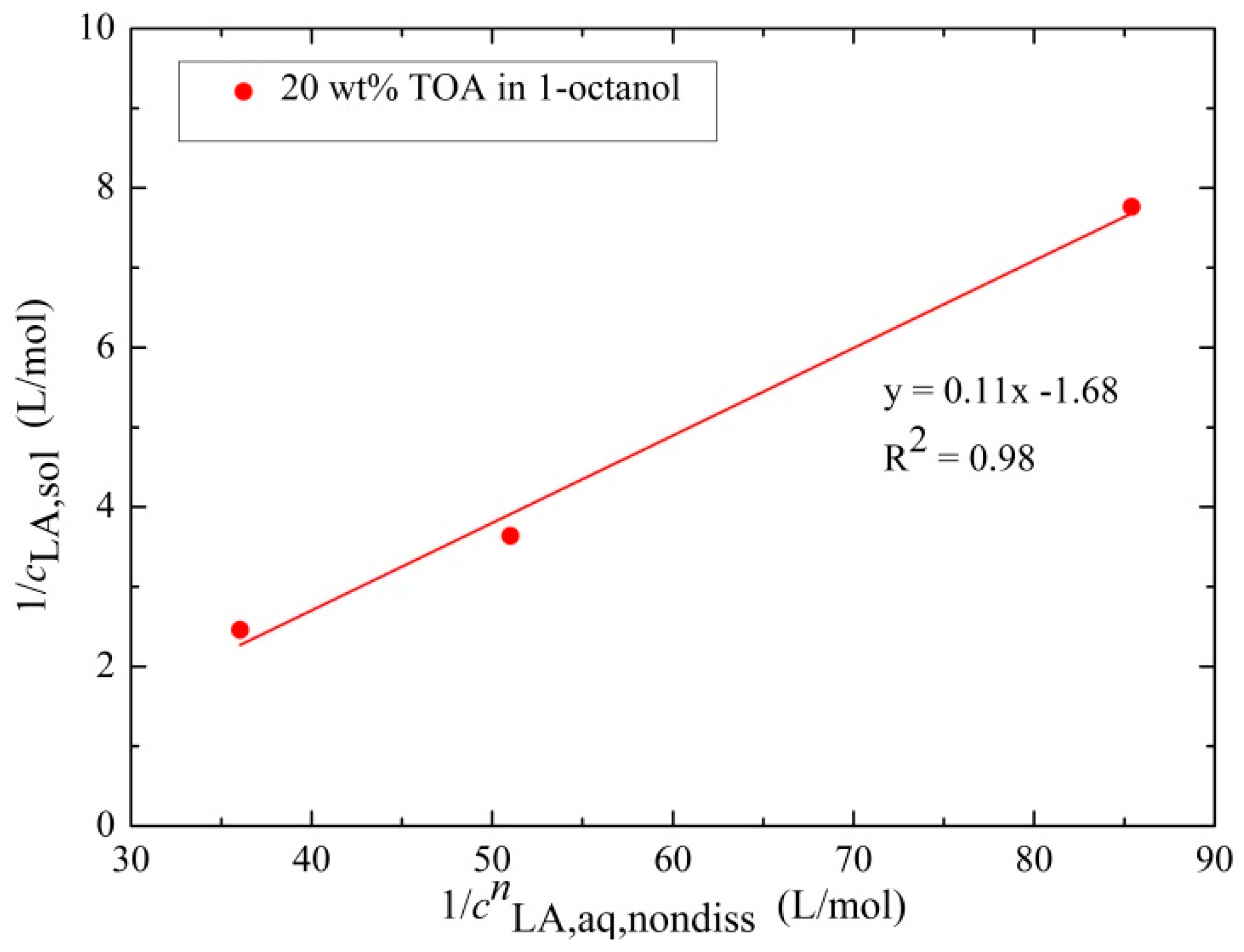
3.2.2. Determination of the Extraction Equilibrium Constant and the Degree of Association
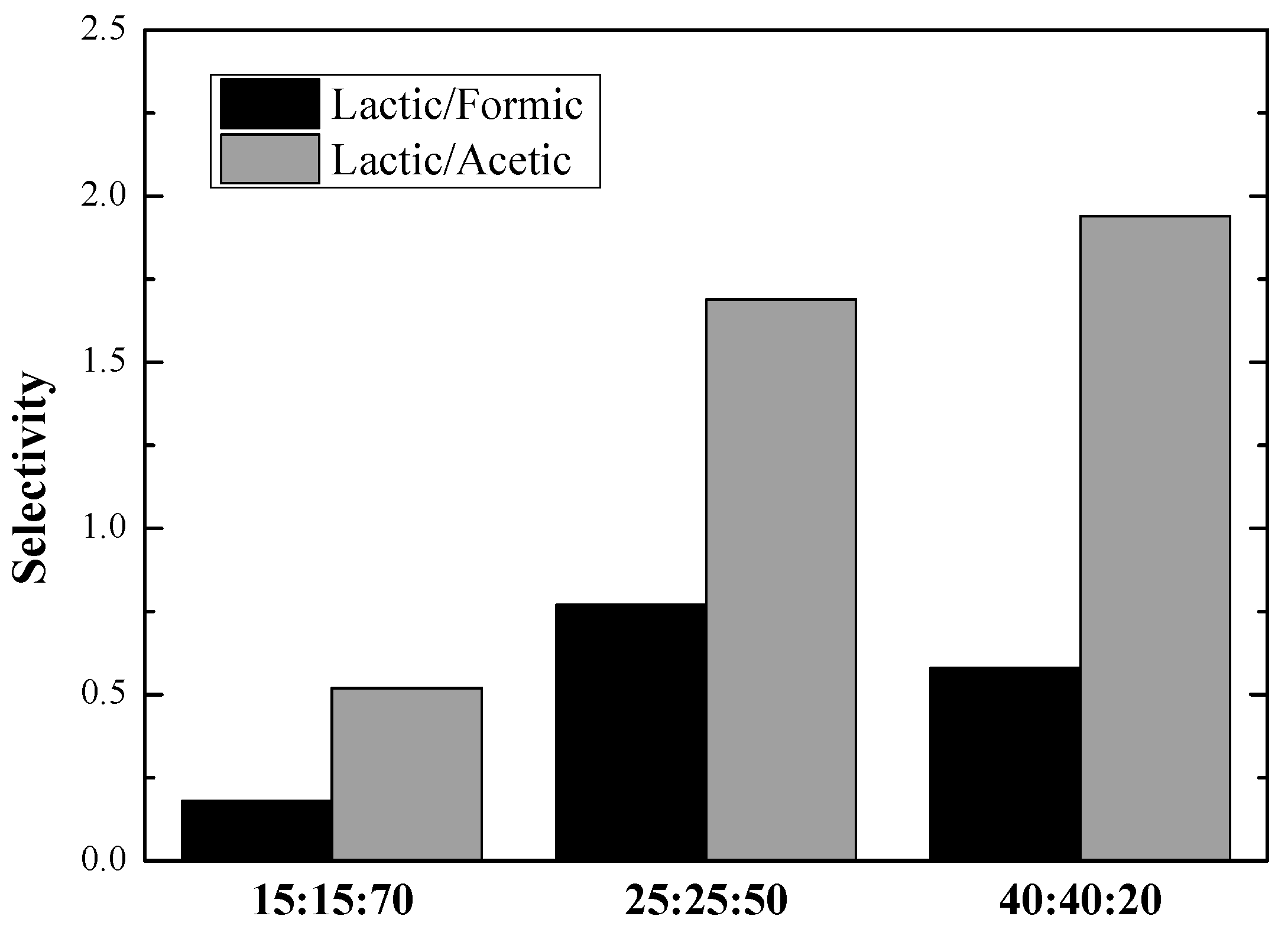
3.3. Selectivity
3.4. Acids Back-Extraction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| aq | aqueous phase |
| c | concentration |
| D | dimerization constant |
| E | extraction efficiency |
| HA | carboxylic acid |
| KD | distribution coefficient |
| Kdiss | Dissociation constant |
| KE | extraction equilibrium constant |
| LA | Lactic acid |
| m | mass |
| n | degree of association |
| nondiss | non-dissociated |
| P | partition coefficient |
| sol | solvent phase |
| Z | loading |
| 0, in | initial concentration |
| α | Degree of dissociation |
References
- Davis, J. Lactic Acid Market Size Worth over 6 Billion Dollars by 2024. Available online: https://globenewswire.com/news-release/2016/11/09/888221/0/en/Lactic-Acid-Market-size-worth-over-6-billion-by-2024-Global-Market-Insights-Inc.html (accessed on 12 June 2017).
- James, S. Lactic Acid and Poly Lactic Acid (PLA) Market to Reach 4,312.2 Million dollars and 2,169.6 Million dollars Respectively by 2020. Available online: http://www.reuters.com/article/idUSnMKWSZm27a+1f0+MKW20150714 (accessed on 12 June 2017).
- Jantasee, S.; Kienberger, M.; Mungma, N.; Siebenhofer, M. Potential and assessment of lactic acid production and isolation—A review. J Chem. Technol. Biotechnol. 2017. [Google Scholar] [CrossRef]
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Dominguez Gonzalez, J.M.; Converti, A.; de Souza Oliveira, R.P. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Wang, Y.; Tashiro, Y.; Sonomoto, K. Fermentative production of lactic acid from renewable materials: Recent achievements, prospects, and limits. J. Biosci. Bioeng. 2015, 119, 10–18. [Google Scholar] [CrossRef]
- López-Garzón, C.S.; Straathof, A.J.J. Recovery of carboxylic acids produced by fermentation. Biotechnol. Adv. 2014, 32, 873–904. [Google Scholar] [CrossRef]
- Taskila, S.; Ojamo, H. The Current Status and Future Expectations in Industrial Production of Lactic Acid by Lactic Acid Bacteria. In Lactic Acid Bacteria—R & D for Food, Health and Livestock Purposes; InTech: Rijeka, Croatia, 2013; Available online: https://www.intechopen.com/books/lactic-acid-bacteria-r-d-for-food-health-and-livestock-purposes/the-current-status-and-future-expectations-in-industrial-production-of-lactic-acid-by-lactic-acid-ba (accessed on 3 December 2018).
- Abdel Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef]
- Lee, H.J.; Xie, Y.; Koo, Y.M.; Wang, N.H.L. Separation of Lactic Acid from Acetic Acid Using a Four-Zone SMB. Biotechnol. Prog. 2004, 20, 179–192. [Google Scholar] [CrossRef]
- Patel, M.; Bassi, A.S.; Zhu, J.J.; Gomaa, H. Investigation of a Dual-Particle Liquid – Solid Circulating Fluidized Bed Bioreactor for Extractive Fermentation of Lactic Acid. Biotechnol. Prog. 2008, 24, 821–831. [Google Scholar] [CrossRef]
- Dey, P.; Linnanen, L.; Pal, P. Separation of lactic acid from fermentation broth by cross flow nanofiltration: Membrane characterization and transport modelling. Desalination 2012, 288, 47–57. [Google Scholar] [CrossRef]
- Habova, V.; Melzoch, K.; Rychtera, M.; Pribyl, L.; Vladimir, M. Application of electrodialysis for lactic acid recovery. Czech J. Food Sci. 2001, 19, 73–80. [Google Scholar] [CrossRef]
- Woodley, J.M.; Bisschops, M.; Straathof, A.J.J.; Ottens, M. Perspective Future directions for in-situ product removal (ISPR). J. Chem. Technol. Biotechnol. 2008, 82, 121–123. [Google Scholar] [CrossRef]
- Anvari, M.; Khayati, G. In situ recovery of 2,3-butanediol from fermentation by liquid-liquid extraction. J. Ind. Microbiol. Biotechnol. 2009, 36, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Bart, H.-J. Reactive Extraction. In Reactive Extraction; Dechema: Frankfurt, Germany, 2001; pp. 10–11. ISBN 978-3-642-07430-1. [Google Scholar]
- Eyal, A.M.; Canari, R. pH Dependence of Carboxylic and Mineral Acid Extraction by Amine-Based Extractants: Effects of pKa, Amine Basicity, and Diluent Properties. Ind. Eng. Chem. Res. 1995, 34, 1789–1798. [Google Scholar] [CrossRef]
- Leis, D.; Lauß, B.; Macher-ambrosch, R.; Pfennig, A.; Nidetzky, B.; Kratzer, R. Integration of whole-cell reaction and product isolation: Highly hydrophobic solvents promote in situ substrate supply and simplify extractive product isolation. J. Biotechnol. 2017, 257, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Kienberger, M.; Hackl, M.; Siebenhofer, M. Emulsion Prevention with Supported Liquid Membrane Permeation. Chem. Eng. Technol. 2018, 41, 504–508. [Google Scholar] [CrossRef]
- Canari, R.; Eyal, A.M. Selectivity in Monocarboxylic Acids Extraction from Their Mixture Solutions Using an Amine-Based Extractant: Effect of pH. Ind. Eng. Chem. Res. 2003, 42, 1301–1307. [Google Scholar] [CrossRef]
- Datta, D.; Kumar, S.; Uslu, H. Status of the Reactive Extraction as a Method of Separation. J. Chem. 2014, 2015, 1–16. [Google Scholar] [CrossRef]
- Tamada, J.A.; Kertes, A.S.; King, C.J. Extraction of Carboxylic Acids with Amine Extractants. 1. Equilibria and Law of Mass Action Modeling. Ind. Eng. Chem. Res. 1990, 29, 1319–1326. [Google Scholar] [CrossRef]
- Udachan, I.S.; Sahoo, A.K. A studyof parameters affecting the solvent extraction of lactic acid from fermentation broth. Braz. J. Chem. Eng. 2014, 31, 821–827. [Google Scholar] [CrossRef]
- Han, D.H.; Hong, Y.K.; Hong, W.H. Separation Characteristics of Lactic Acid in Reactive Extraction and Stripping. Korean J. Chem. Eng. 2000, 17, 528–533. [Google Scholar] [CrossRef]
- Qin, W.; Li, Z.; Dai, Y. Extraction of Monocarboxylic Acids with Trioctylamine: Equilibria and Correlation of Apparent Reactive Equilibrium Constant. Ind. Eng. Chem. Res. 2003, 42, 6196–6204. [Google Scholar] [CrossRef]
- Wasewar, K.L.; Keshav, A. Seema. Physical extraction of propionic acid. Int. J. Res. Rev. Appl. Sci. 2010, 3, 290–302. [Google Scholar]
- Wang, Y.; Cai, D.; He, M.; Wang, Z.; Qin, P.; Tan, T. Open fermentative production of l-lactic acid using white rice bran by simultaneous saccharification and fermentation. Bioresour. Technol. 2015, 198, 664–672. [Google Scholar] [CrossRef]
- Maurer, G. Modeling the liquid-liquid equilibrium for the recovery of carboxylic acids from aqueous solutions. Fluid Phase Equilib. 2006, 241, 86–95. [Google Scholar] [CrossRef]
- Painer, D.; Lux, S.; Grafschafter, A.; Toth, A.; Siebenhofer, M. Isolation of Carboxylic Acids from Biobased Feedstock. Chemie-Ingenieur-Technik 2017, 89, 161–171. [Google Scholar] [CrossRef]
- Labbaci, A.; Kyuchoukov, G.; Albet, J.; Molinier, J. Detailed investigation of lactic acid extraction with tributylphosphate dissolved in dodecane. J. Chem. Eng. Data 2010, 55, 228–233. [Google Scholar] [CrossRef]
- Thakre, N.; Prajapati, A.K.; Mahapatra, S.P.; Kumar, A.; Khapre, A.; Pal, D. Modeling and Optimization of Reactive Extraction of Citric Acid. J. Chem. Eng. Data 2016, 61, 2614–2623. [Google Scholar] [CrossRef]
- Siebenhofer, M.; Marr, R. Acid extraction by amines. In Proceedings of the ISEC, Denver, CO, USA, 28 August 1983. [Google Scholar]
- Kyuchoukov, G.; Labbaci, A.; Albet, J.; Molinier, J. Simultaneous influence of active and “Inert” diluents on the extraction of lactic acid by means of Tri-n-octylamine (TOA) and Tri-wo-octylamine (TIOA). Ind. Eng. Chem. Res. 2006, 45, 503–510. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kojima, K.; Mori, H.; Kawasaki, H.; Sayama, M. Extraction of lactic acid using long chain amines dissolved in non-polar diluents. J. Chem. Eng. Jpn. 2011, 44, 949–956. [Google Scholar] [CrossRef]
- Kislik, V. Solvent Extraction, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 9780444537782. [Google Scholar]
- Schulz, R.; Van Den Bongard, R.; Islam, J.; Zeiner, T. Purification of Terpenyl Amine by Reactive Extraction. Ind. Eng. Chem. Res. 2016, 55, 5763–5769. [Google Scholar] [CrossRef]
- Kyuchoukov, G.; Marinova, M.; Molinier, J.; Albet, J.; Malmary, G. Extraction of lactic acid by means of a mixed extractant. Ind. Eng. Chem. Res. 2001, 40, 5635–5639. [Google Scholar] [CrossRef]
- Lux, S.; Siebenhofer, M. Investigation of liquid-liquid phase equilibria for reactive extraction of lactic acid with organophosphorus solvents. J. Chem. Technol. Biotechnol. 2013, 88, 462–467. [Google Scholar] [CrossRef]
- Keshav, A.; Chand, S.; Wasewar, K.L. Recovery of propionic acid from aqueous phase by reactive extraction using quarternary amine (Aliquat 336) in various diluents. Chem. Eng. J. 2009, 152, 95–102. [Google Scholar] [CrossRef]
- Morales, A.F.; Albet, J.; Kyuchoukov, G.; Malmary, G.; Molinier, J. Influence of extractant (TBP and TOA), diluent, and modifier on extraction equilibrium of monocarboxylic acids. J. Chem. Eng. Data 2003, 48, 874–886. [Google Scholar] [CrossRef]
- Kyuchoukov, G.; Yankov, D. Theoretical and experimental study of lactic acid stripping from loaded organic phase. Ind. Eng. Chem. Res. 2010, 49, 8238–8243. [Google Scholar] [CrossRef]



| Acid | Solvent | P | D (l/mol) |
|---|---|---|---|
| Lactic acid | n-undecane | 0.06 | 6.62 |
| 1-octanol | 0.24 | 0.46 | |
| Acetic acid | n-undecane | 0.01 | 10.42 |
| 1-octanol | 0.49 | 0.30 | |
| Formic acid | n-undecane | 0.03 | 35.65 |
| 1-octanol | 0.25 | 3.39 |
| TOA:-1-octanol:n-undecane (wt%) | mHA,in (g) | mHA,aq (g) | cHA,aq (mol/L) | cHA,sol (mol/L) | KD | E (%) | pH |
|---|---|---|---|---|---|---|---|
| 15:15:70 | 10.01 | 8.97 | 0.092 | 0.123 | 1.35 | 57.5 | 2.42 |
| 10.01 | 9.29 | 0.236 | 0.236 | 1.01 | 50.2 | 2.15 | |
| 9.99 | 9.73 | 0.414 | 0.326 | 0.83 | 45.4 | 1.99 | |
| 9.99 | 9.06 | 0.599 | 0.408 | 0.69 | 40.7 | 1.82 | |
| 25:25:50 | 10.01 | 9.42 | 0.037 | 0.178 | 4.89 | 83.0 | 2.76 |
| 10.01 | 9.42 | 0.099 | 0.372 | 3.75 | 78.9 | 2.44 | |
| 9.99 | 9.29 | 0.189 | 0.551 | 2.91 | 74.4 | 2.24 | |
| 10.01 | 8.85 | 0.324 | 0.683 | 2.11 | 67.8 | 2.11 | |
| 40:40:20 | 9.99 | 9.23 | 0.006 | 0.209 | 34.79 | 97.2 | 3.59 |
| 9.99 | 8.90 | 0.020 | 0.452 | 22.18 | 95.7 | 3.18 | |
| 10.01 | 8.31 | 0.045 | 0.695 | 15.76 | 94.0 | 2.94 | |
| 9.99 | 8.39 | 0.079 | 0.923 | 11.71 | 92.1 | 2.73 |
| TOA (wt%) | 1-octanol (wt%) | n-undecane (wt%) | Viscosity (m·Pas, 20 °C) | Acid | n | KE | Z |
|---|---|---|---|---|---|---|---|
| 15 | 15 | 70 | 2.34 | Lactic | 0.81 | 3.53 | 0.36–1.99 |
| Acetic | 1.11 | 2.16 | 0.23–1.15 | ||||
| Formic | 1.55 | 27.79 | 0.49–1.68 | ||||
| 25 | 25 | 50 | 2.92 | Lactic * | 0.97 | 9.73 | 0.31–1.99 |
| Acetic * | 1.55 | 5.25 | 0.20–1.06 | ||||
| Formic * | 1.99 | 79.35 | 0.32–1.45 | ||||
| 40 | 40 | 20 | 4.85 | Lactic ** | 0.85 | 24.48 | 0.23–1.02 |
| Acetic ** | 1.09 | 6.48 | 0.18–0.84 | ||||
| Formic ** | 1.99 | 142.79 | 0.22–1.02 | ||||
| 20 | 80 | - | 9.56 | Lactic | 0.99 | 20.03 | 0.27–1.42 |
| Acetic | 0.75 | 17.66 | 0.42–1.61 | ||||
| Formic | 1.65 | 281 | 0.41–1.69 | ||||
| 20 | - | 80 | 1.65 | Lactic | 1.42 | 0.13 | 0.01–0.25 |
| Acetic | 1.01 | 0.11 | 0.03–0.38 | ||||
| Formic | 1.19 | 0.57 | 0.09–1.41 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mungma, N.; Kienberger, M.; Siebenhofer, M. Reactive Extraction of Lactic Acid, Formic Acid and Acetic Acid from Aqueous Solutions with Tri-n-octylamine/1-Octanol/n-Undecane. ChemEngineering 2019, 3, 43. https://doi.org/10.3390/chemengineering3020043
Mungma N, Kienberger M, Siebenhofer M. Reactive Extraction of Lactic Acid, Formic Acid and Acetic Acid from Aqueous Solutions with Tri-n-octylamine/1-Octanol/n-Undecane. ChemEngineering. 2019; 3(2):43. https://doi.org/10.3390/chemengineering3020043
Chicago/Turabian StyleMungma, Nuttakul, Marlene Kienberger, and Matthäus Siebenhofer. 2019. "Reactive Extraction of Lactic Acid, Formic Acid and Acetic Acid from Aqueous Solutions with Tri-n-octylamine/1-Octanol/n-Undecane" ChemEngineering 3, no. 2: 43. https://doi.org/10.3390/chemengineering3020043
APA StyleMungma, N., Kienberger, M., & Siebenhofer, M. (2019). Reactive Extraction of Lactic Acid, Formic Acid and Acetic Acid from Aqueous Solutions with Tri-n-octylamine/1-Octanol/n-Undecane. ChemEngineering, 3(2), 43. https://doi.org/10.3390/chemengineering3020043





