Photocatalytic Reduction of Hexavalent Chromium with Nanosized TiO2 in Presence of Formic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Photocatalysts and Chemicals
2.2. Photocatalytic Reduction of Cr(VI)
2.3. Analysis of Hexavalent Chromium
3. Results and Discussion
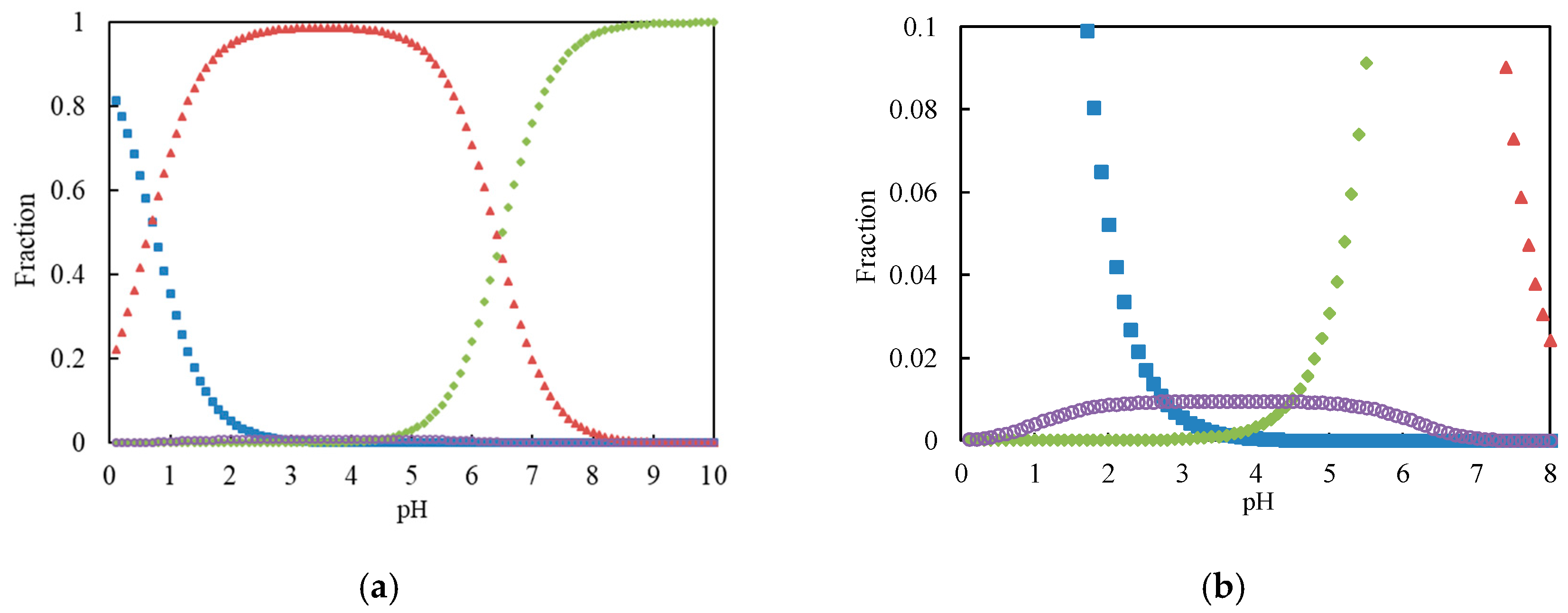
3.1. Chromium(VI) Species with pH
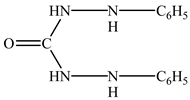
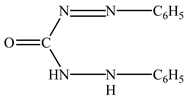
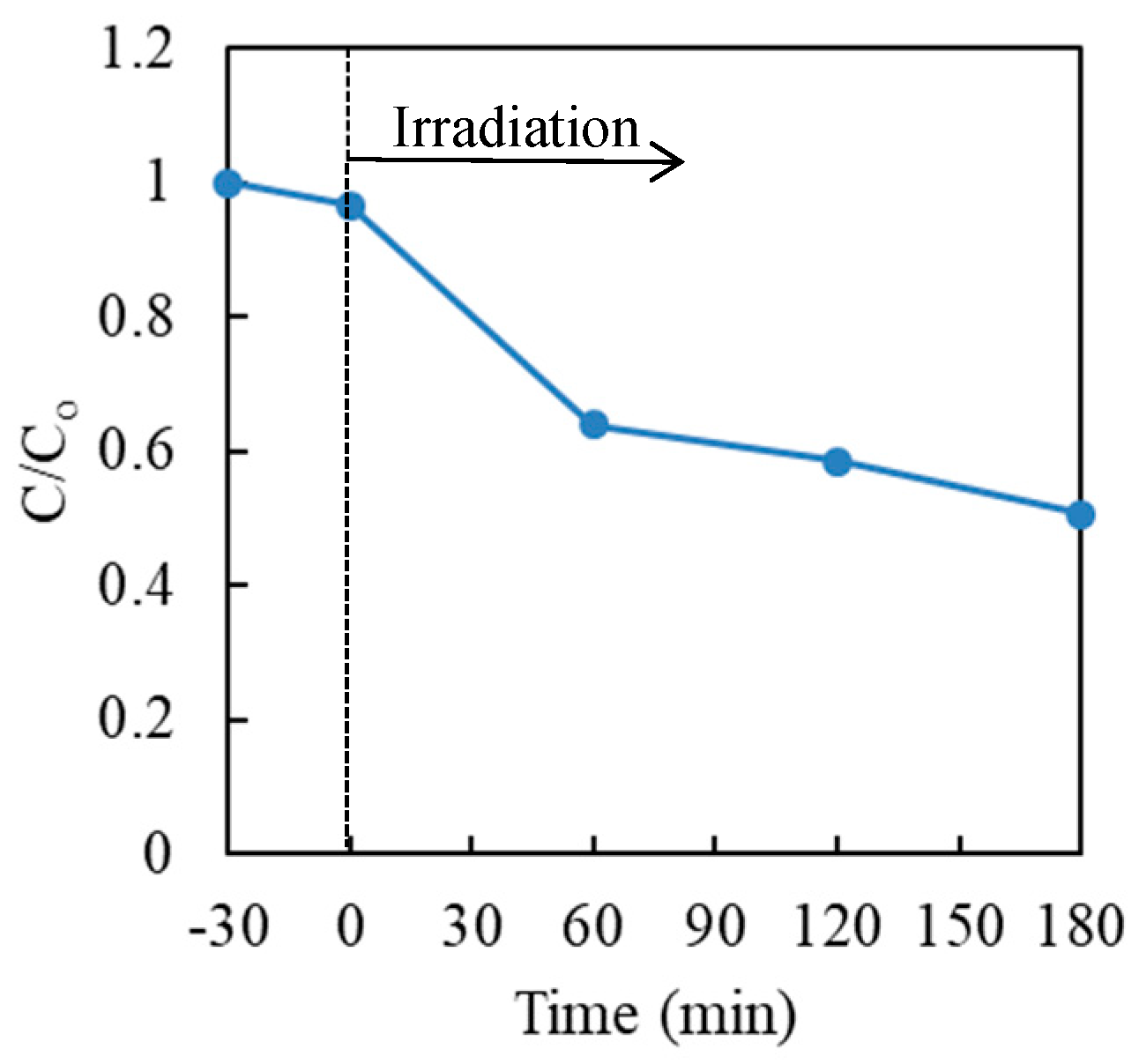
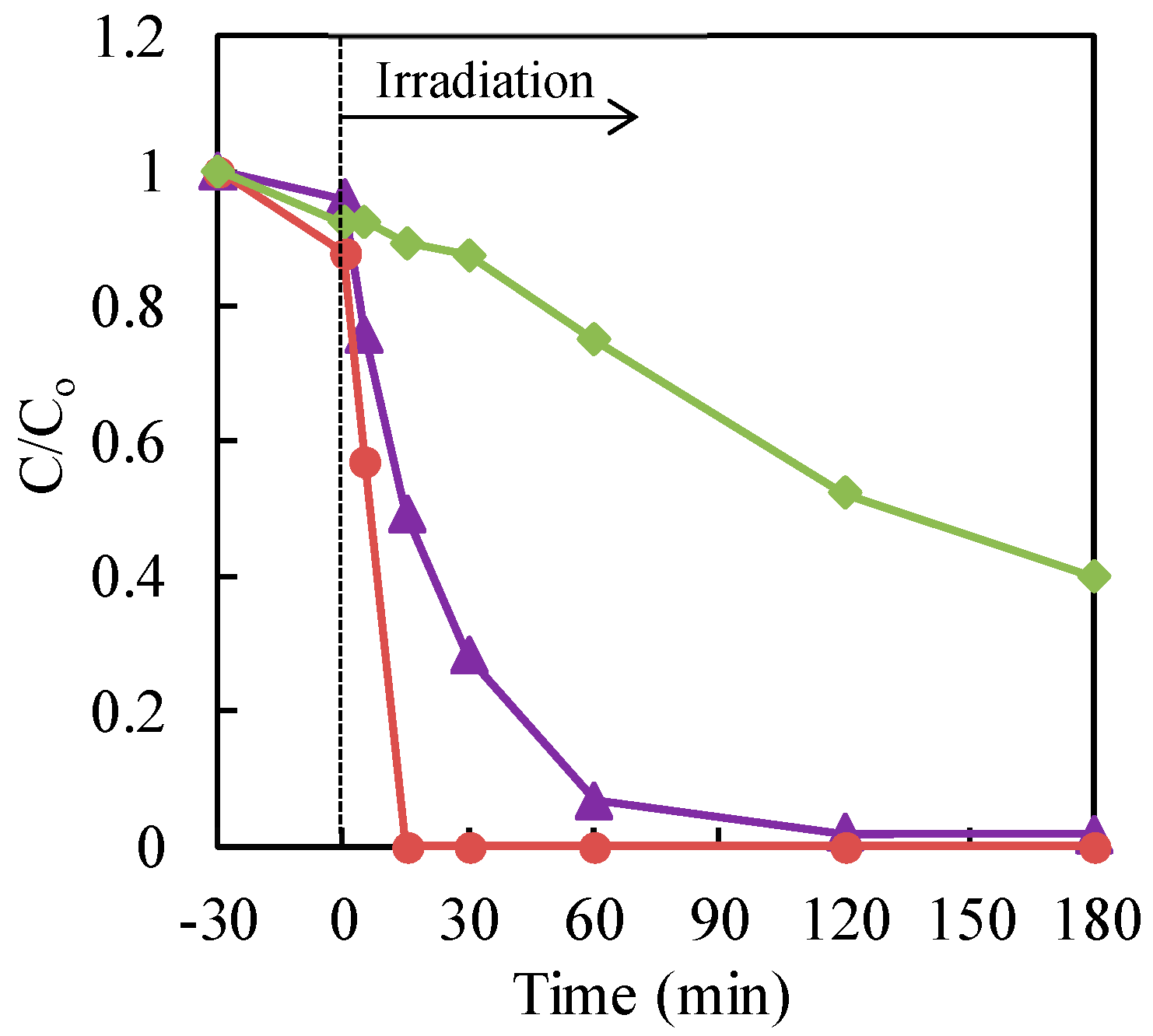
3.2. Effect of Hole Scavengers
3.3. Effect of Commercial TiO2 Type
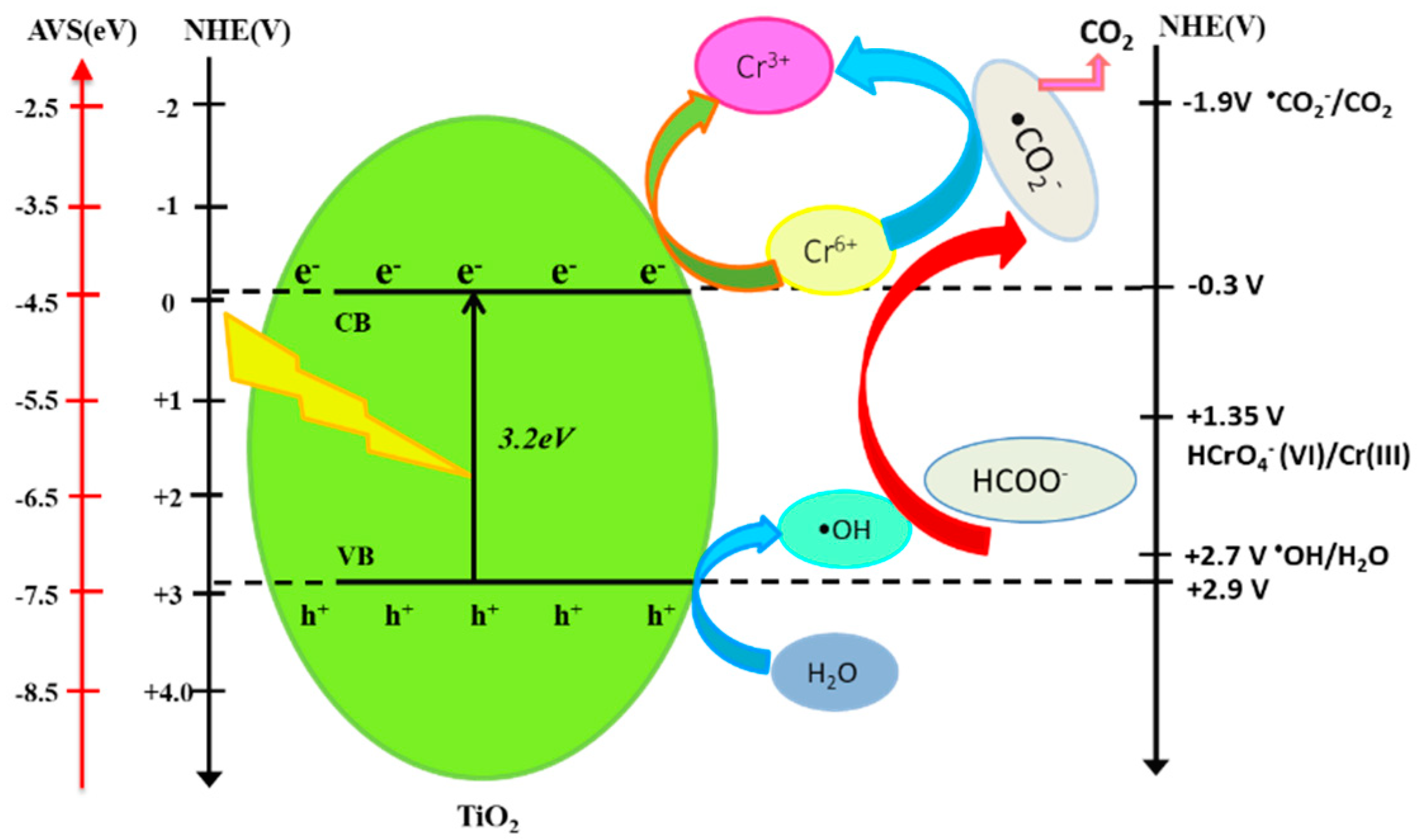
3.4. Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Korak, J.A.; Huggins, R.; Arias-Paic, P. Regeneration of Pilot-Scale Ion Exchange Columns for Hexavalent Chromium Removal. Water Res. 2017, 118, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Du, X.D.; Yi, X.H.; Wang, P.; Zheng, W.; Deng, J.; Wang, C.C. Robust photocatalytic Reduction of Cr(VI) on UiO-66-NH2(Zr/Hf) Metal-Organic Framework Membrane Under Sunlight Irradiation. Chem. Eng. J. 2019, 356, 393–399. [Google Scholar] [CrossRef]
- Siboni, M.S.; Farroki, M.; Soltani, R.D.C.; Khataee, A.; Tajassosi, S. Photocatalytic Reduction of Hexavalent Chromium over ZnO Nanorods Immobilized on Kaolin. Ind. Eng. Chem. Res. 2014, 53, 1079–1087. [Google Scholar] [CrossRef]
- Shih, Y.J.; Chen, C.W.; Hsia, K.F.; Dong, C.D. Granulation for Extended-Release of Nanoscale Zero-Valent Iron Exemplified by Hexavalent Chromium Reduction in Aqueous Solution. Sep. Purif. Technol. 2015, 156, 1073–1081. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Activated Carbons and Low Cost Adsorbents for Remediation of Tri- and Hexavalent Chromium from Water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef]
- Mohan, D.; Rajput, S.; Singh, V.K.; Steele, P.H.; Pittman, C.U., Jr. Modeling and Evaluation of Chromium Remediation from Water Using Low Cost Bio-Char, a Green Adsorbent. J. Hazard. Mater. 2011, 188, 319–333. [Google Scholar] [CrossRef]
- Di Natale, F.; Erto, A.; Lancia, A.; Musmarra, D. Equilibrium and Dynamic Study on Hexavalent Chromium Adsorption onto Activated Carbon. J. Hazard. Mater. 2015, 281, 47–55. [Google Scholar] [CrossRef]
- Qi, H.; Wang, S.; Liu, H.; Gao, Y.; Wang, T.; Huang, Y. Synthesis of an Organic–Inorganic Polypyrrole/Titanium(IV) Biphosphate Hybrid for Cr(VI) Removal. J. Mol. Liq. 2016, 215, 402–409. [Google Scholar] [CrossRef]
- Joubani, M.N.; Siboni, M.S.; Yang, J.K.; Gholami, M.; Farzadkia, M. Photocatalytic Reduction of Hexavalent Chromium with Illuminated ZnO/TiO2 Composite. J. Ind. Eng. Chem. 2015, 22, 317–323. [Google Scholar] [CrossRef]
- Cheng, Q.; Wang, C.; Doudrick, K.; Chan, C.K. Hexavalent Chromium Removal Using Metal Oxide Photocatalysts. Appl. Catal. B 2015, 176–177, 740–748. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, L.; Deng, K.; She, Y.; Yu, Y.; Tang, H. Visible Light Photocatalytic Reduction of Cr(VI) on TiO2 in Situ Modified with Small Molecular Weight Organic Acids. Appl. Catal. B 2010, 95, 400–407. [Google Scholar] [CrossRef]
- Testa, J.J.; Grela, M.A.; Litter, M.I. Heterogeneous Photocatalytic Reduction of Chromium (VI) over TiO2 Particles in the Presence of Oxalate: Involvement of Cr(V) Species. Environ. Sci. Technol. 2004, 38, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K.; Lee, S.M. Removal of Cr(VI) and Humic Acid by Using TiO2 Photocatalysis. Chemosphere 2006, 63, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Reddy, E.P.; Smirniotis, P.G. Visible Light Cr(VI) Reduction and Organic Chemical Oxidation by TiO2 Photocatalysis. Environ. Sci. Technol. 2005, 39, 6251–6259. [Google Scholar] [CrossRef]
- Kaneco, S.; Kurimoto, H.; Ohta, K.; Mizuno, T.; Saji, A. Photocatalytic Reduction of CO2 Using TiO2 Powders in Liquid CO2 Medium. J. Photochem. Photobiol. A Chem. 1997, 109, 59–63. [Google Scholar] [CrossRef]
- Szabó, M.; Kalmár, J.; Ditrói, T.; Bellér, G.; Lente, G.; Simic, N.; Fábián, I. Equilibria and Kinetics of Chromium(VI) Speciation in Aqueous Solution—A Comprehensive Study from pH 2 to 11. Inorg. Chim. Acta 2018, 472, 295–301. [Google Scholar] [CrossRef]
- Tandon, R.K.; Crisp, P.T.; Ellis, J.; Baker, R.S. Effect of pH on Chromium(VI) Species in Solution. Talanta 1984, 31, 227–228. [Google Scholar] [CrossRef]
- Gardner, M.; Comber, S. Determination of Trace Concentration of Hexavalent Chromium. Analyst 2002, 127, 153–156. [Google Scholar] [CrossRef]
- Duffy, G.; Maguire, I.; Heery, B.; Gers, P.; Ducrée, J.; Regan, F. ChromiSense: A Colourimetric Lab-on-a-Disc Sensor for Chromium Speciation in Water. Talanta 2018, 178, 392–399. [Google Scholar] [CrossRef]
- Yang, J.K.; Lee, S.M.; Siboni, M.S. Effect of Different Types of Organic Compounds on the Photocatalytic Reduction of Cr(VI). Environ. Technol. 2012, 33, 2027–2032. [Google Scholar] [CrossRef]
- Turchi, C.S.; Ollis, D.E. Photocatalytic Degradation of Organic Water Contaminants: Mechanisms Involving Hydroxyl Radical Attack. J. Catal. 1990, 122, 178–192. [Google Scholar] [CrossRef]
- Sofi, F.A.; Majid, K.; Mehraj, O. The Visible Light Driven Copper Based Metal-Organic-Framework Heterojunction:HKUST-1@Ag-Ag3PO4 for Plasmon Enhanced Visible Light Photocatalysis. J. Alloys Compd. 2018, 737, 798–808. [Google Scholar] [CrossRef]
- Samad, A.; Ahsan, S.; Tateshi, T.; Furukawa, M.; Katsumata, H.; Suzuki, T.; Kaneco, S. Indirect Photocatalytic Reduction of Arsenate to Arsenite in Aqueous Solution with TiO2 in the Presence of Hole Scavengers. Chin. J. Chem. Eng. 2018, 26, 529–533. [Google Scholar] [CrossRef]
- Shaban, Y.A.; Maradny, A.A.E.; Farawati, R.K.A. Photocatalytic Reduction of Nitrate in Seawater Using C/TiO2 nanoparticles. J. Photochem. Photobiol. A Chem. 2016, 328, 114–121. [Google Scholar] [CrossRef]
- Xu, Q.; Li, R.; Wang, C.; Yuan, D. Visible-Light Photocatalytic Reduction of Cr(VI) Using Nano-Sized Delafossite (CuFeO2) Synthesized by Hydrothermal Method. J. Alloys Compd. 2017, 723, 441–447. [Google Scholar] [CrossRef]
- Wang, L.; Lia, X.; Tenga, W.; Zhaoa, Q.; Shi, Y.; Yue, R.; Chen, Y. Efficient Photocatalytic Reduction of Aqueous Cr(VI) over Flower-like SnIn4S8 Microspheres under Visible Light Illumination. J. Hazard. Mater. 2013, 244–245, 681–688. [Google Scholar] [CrossRef]
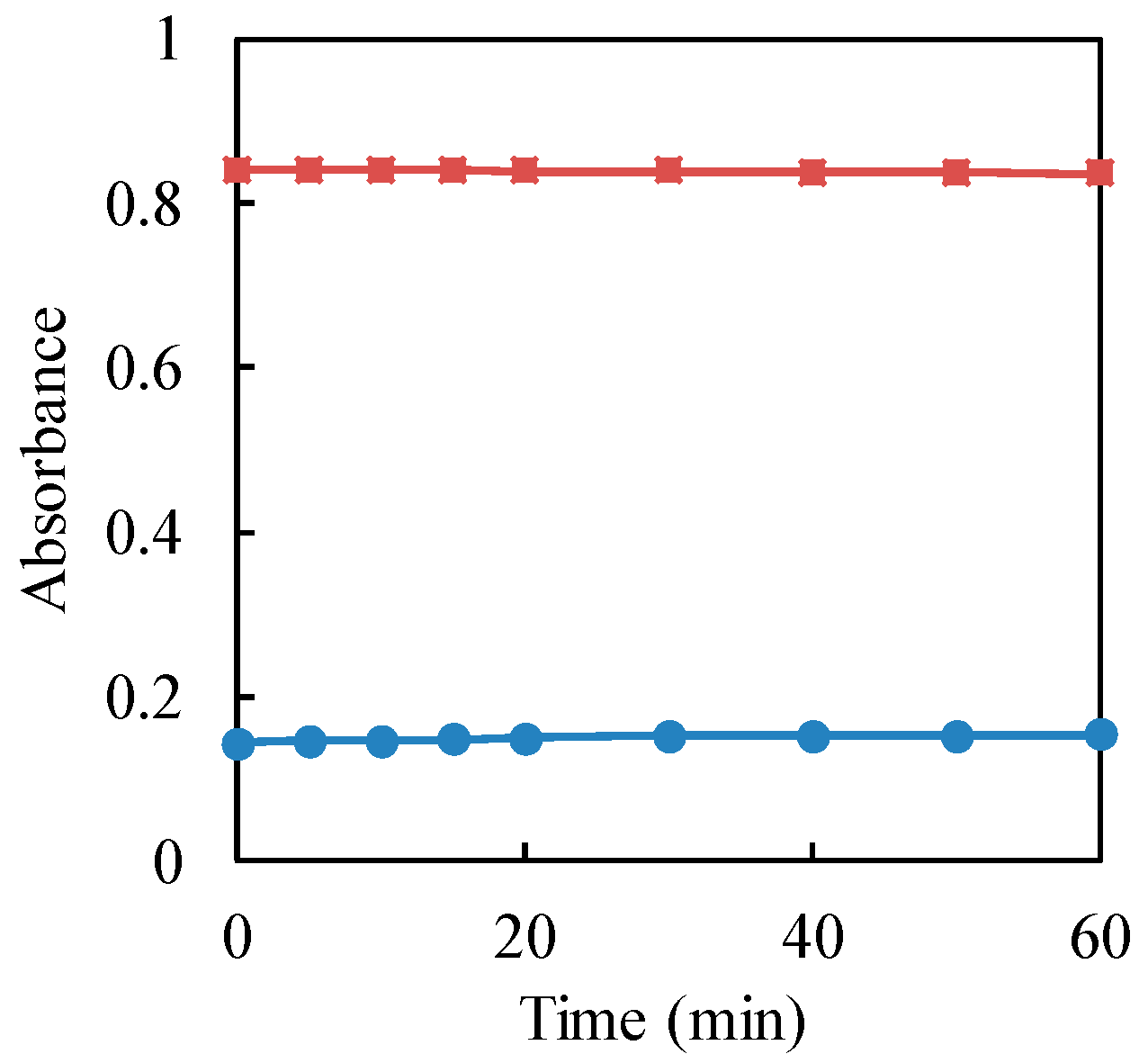

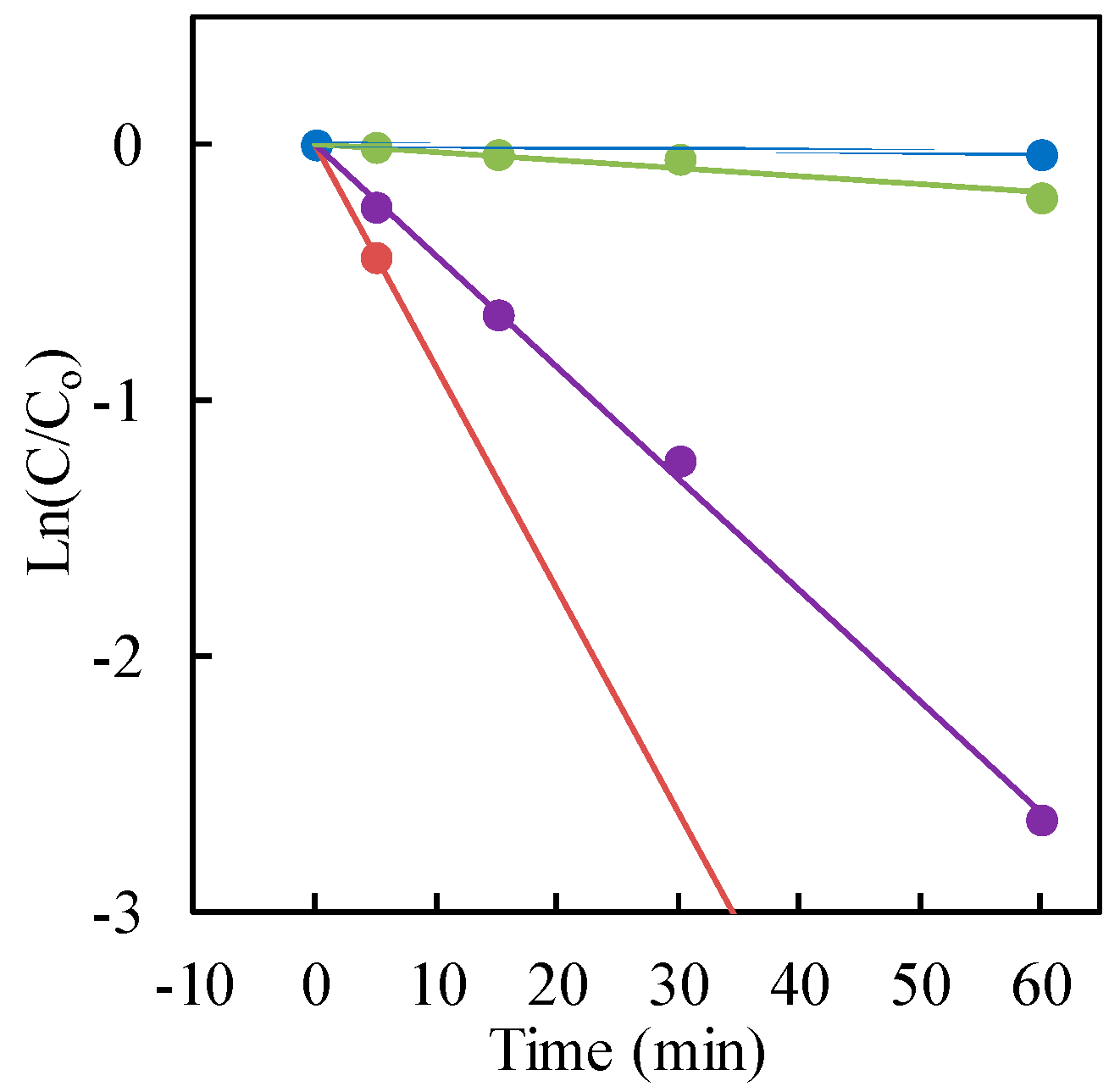
| Commercial TiO2 | Specific Surface Area (m2·g−1) | kobs (min−1) | Surface Area-Normalized ksur (m2·min−1·g−1) | R2 | t1/2 (min) |
|---|---|---|---|---|---|
| FUJIFILM Wako | 8.7 | 0.0031 | 3.6 × 10−4 | 0.999 | 224 |
| AEROXIDE® P25 | 50 | 0.043 | 8.6 × 10−4 | 0.998 | 16.1 |
| Ishihara ST-01 | 300 | 0.087 | 2.9 × 10−4 | 0.999 | 7.97 |
| P25 (Without scavenger) | 50 | 0.00058 | 0.12 × 10−4 | 0.999 | 1190 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, J.B.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneco, S. Photocatalytic Reduction of Hexavalent Chromium with Nanosized TiO2 in Presence of Formic Acid. ChemEngineering 2019, 3, 33. https://doi.org/10.3390/chemengineering3020033
Islam JB, Furukawa M, Tateishi I, Katsumata H, Kaneco S. Photocatalytic Reduction of Hexavalent Chromium with Nanosized TiO2 in Presence of Formic Acid. ChemEngineering. 2019; 3(2):33. https://doi.org/10.3390/chemengineering3020033
Chicago/Turabian StyleIslam, Jahida Binte, Mai Furukawa, Ikki Tateishi, Hideyuki Katsumata, and Satoshi Kaneco. 2019. "Photocatalytic Reduction of Hexavalent Chromium with Nanosized TiO2 in Presence of Formic Acid" ChemEngineering 3, no. 2: 33. https://doi.org/10.3390/chemengineering3020033
APA StyleIslam, J. B., Furukawa, M., Tateishi, I., Katsumata, H., & Kaneco, S. (2019). Photocatalytic Reduction of Hexavalent Chromium with Nanosized TiO2 in Presence of Formic Acid. ChemEngineering, 3(2), 33. https://doi.org/10.3390/chemengineering3020033





