Sol-Gel Processes in Micro-Environments of Black Shale: Learning from the Industrial Production of Nanometer-Sized TiO2 Polymorphs
Abstract
1. Introduction
2. Geochemical Micro-Environments in General and in Black Shale—A Brief Review
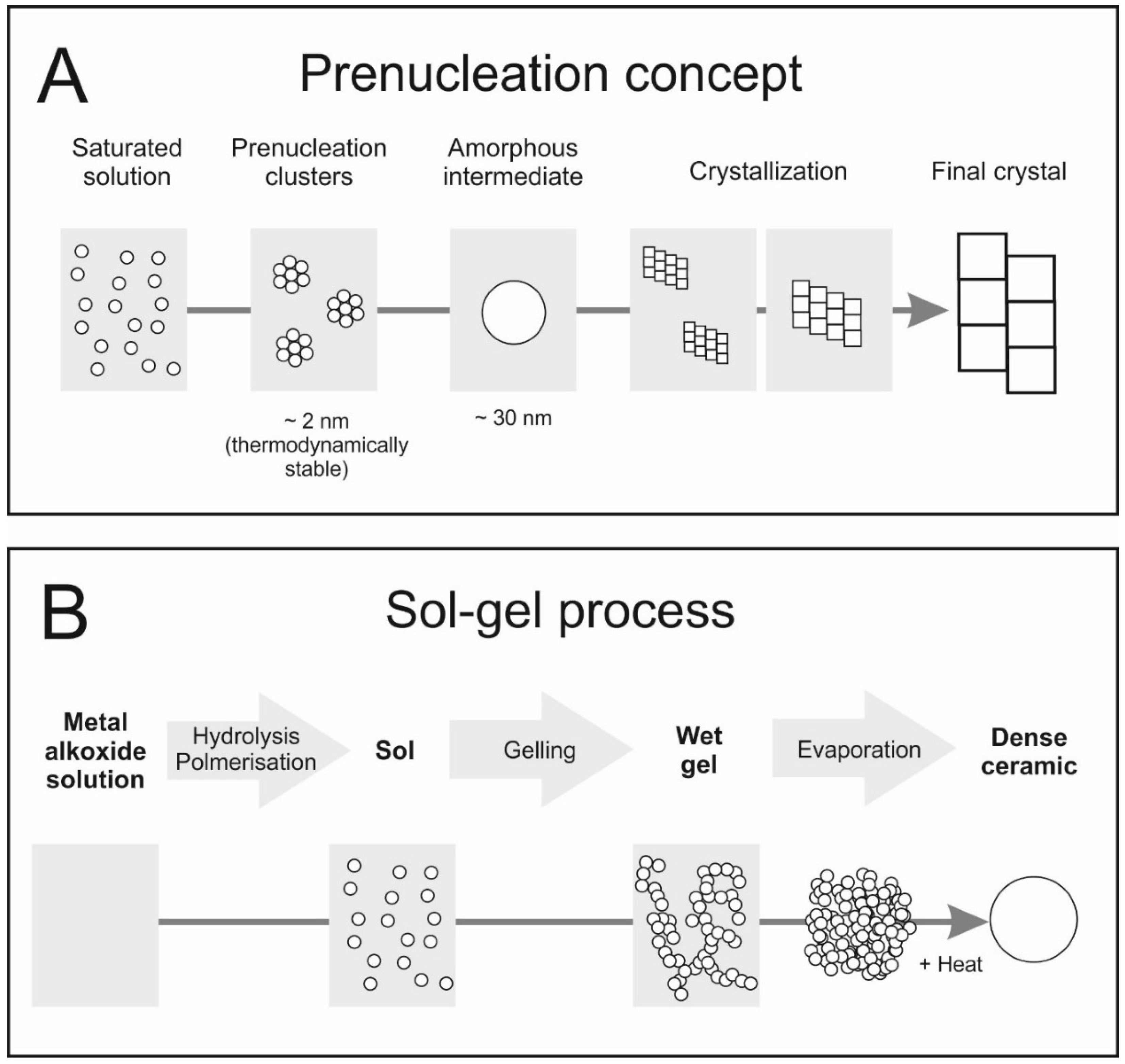
3. Formation of Inorganic Materials from Solutions
4. Methodologies to Characterize and Analyze Micro-Environments
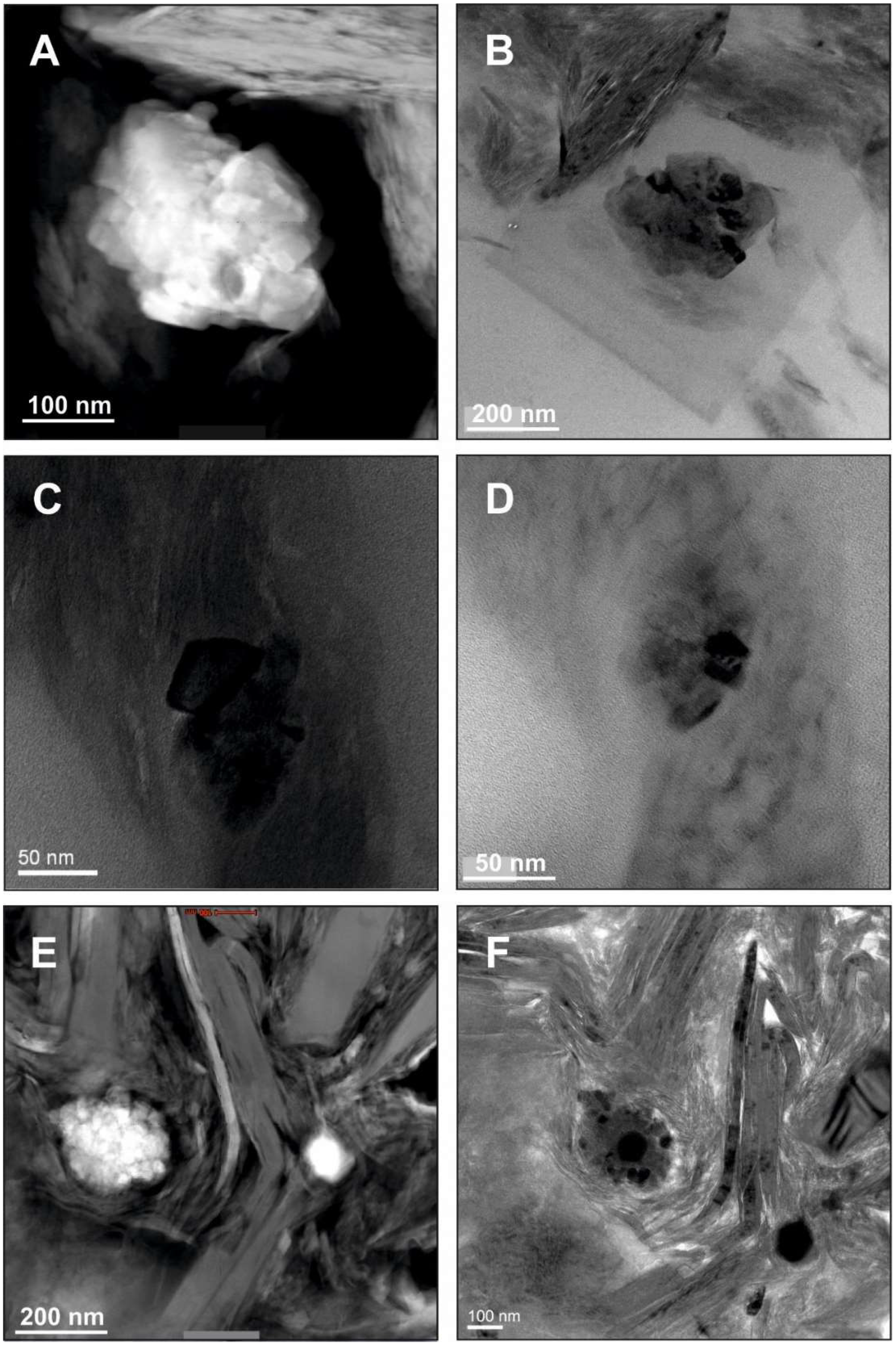
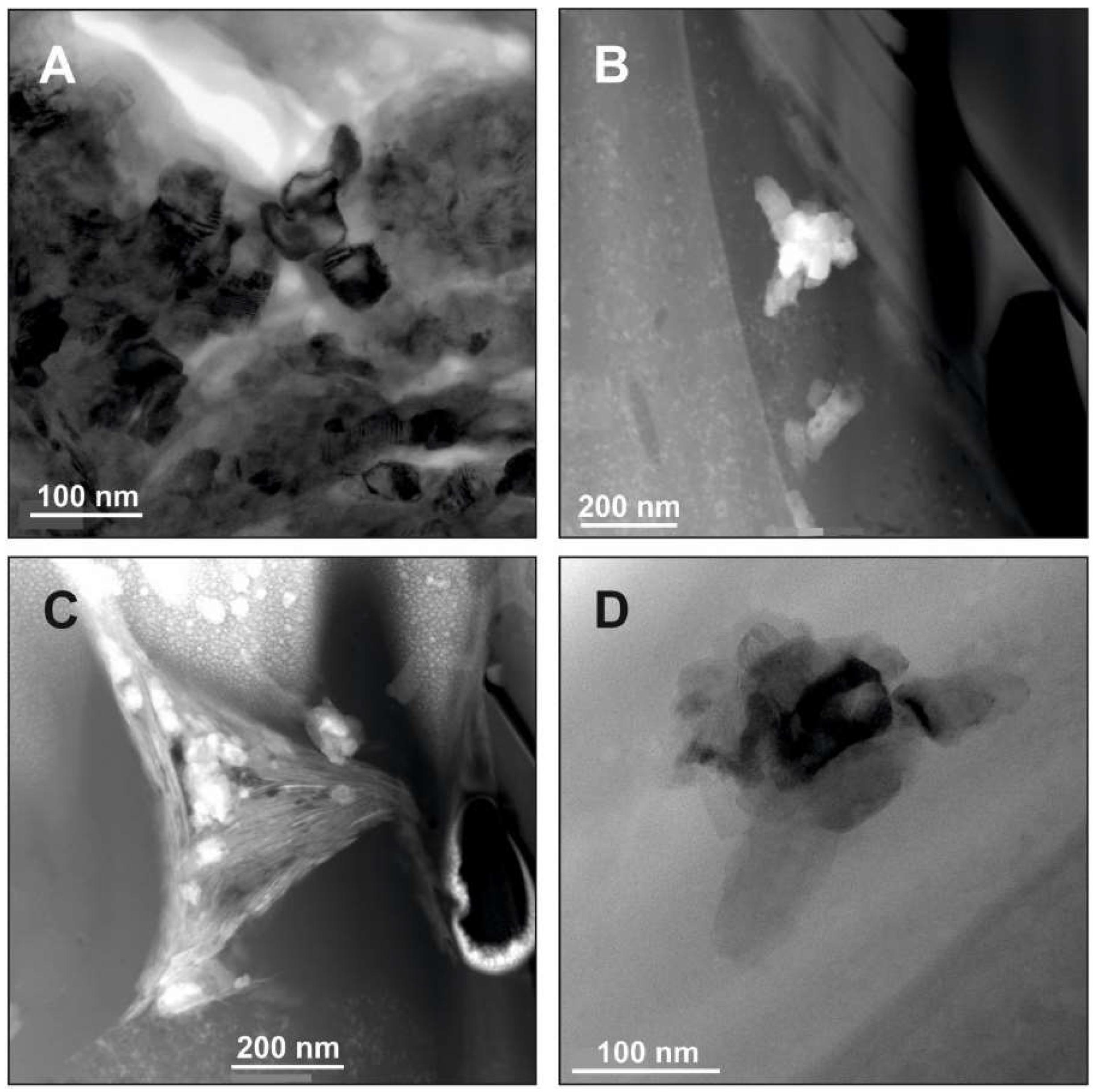
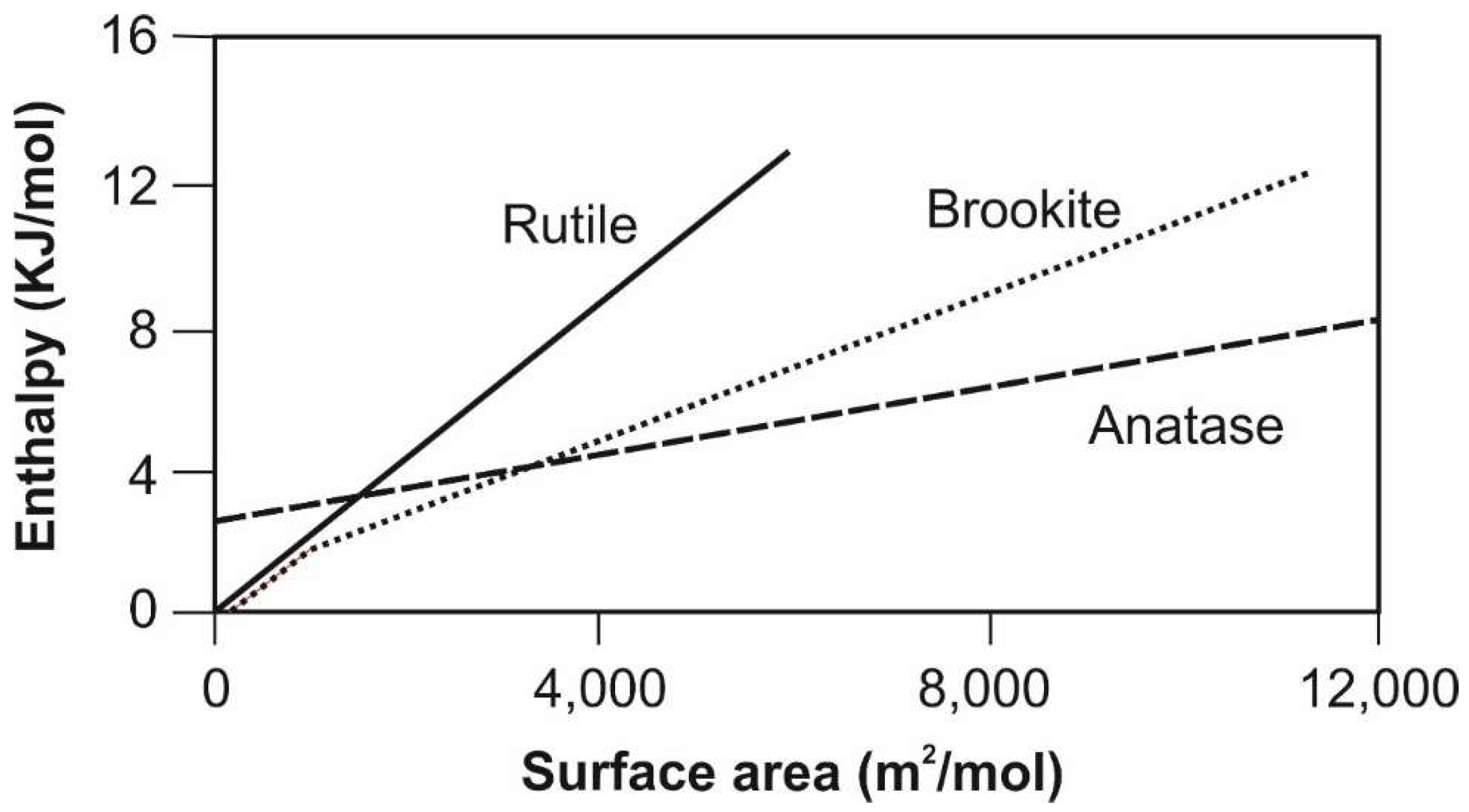
5. Example Brookite (and Anatase): Formation of Unstable Titania Polymorphs in Micro-Environments of Black Shale
6. Industrial Production of Titania Polymorphs
7. Conclusions and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Mondol, N.H.; Bjørlykke, K.; Jahren, J.; Høeg, K. Experimental mechanical compaction of clay mineral aggregates—Changes in physical properties of mudstones during burial. Mar. Petrol. Geol. 2007, 24, 289–311. [Google Scholar] [CrossRef]
- Ross, D.A.; Degens, E.T. Recent sediments of the Black Sea. In Black Sea-Geology, Chemistry, and Biology; Degens, E.T., Ross, D.A., Eds.; American Association of Petroleum Geologist Memoir: Tulsa, Ok, USA, 1974; Volume 20, pp. 183–199. [Google Scholar]
- Slatt, R.M.; O’Brien, N.R. Pore types in the Barnett and Woodford gas shales: Contribution to understanding gas storage and migration pathways in fine-grained rocks. AAPG Bull. 2011, 95, 2017–2030. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Bernard, S.; Horsfield, B.; Schulz, H.-M.; Wirth, R.; Schreiber, A.; Sherwood, N. Geochemical evolution of organic-rich shales with increasing maturity: A STXM and TEM study of the Posidonia Shale (Lower Toarcian, northern Germany). Mar. Petrol. Geol. 2012, 31, 70–89. [Google Scholar] [CrossRef]
- Bernard, S.; Wirth, R.; Schreiber, A.; Schulz, H.-M.; Horsfield, B. Formation of nanoporous pyrobitumen residues during maturation of the Barnett Shale (Fort Worth Basin). Int. J. Coal Geol. 2012, 103, 3–11. [Google Scholar] [CrossRef]
- Van Berk, W.; Schulz, H.-M.; Fu, Y. Controls on CO2 fate and behavior in the Gullfaks oilfield (Norway): How hydrogeochemical modeling can help to decipher organic–inorganic interactions. AAPG Bull. 2013, 97, 2233–2255. [Google Scholar] [CrossRef]
- Fu, Y.; van Berk, W.; Schulz, H.-M.; Mu, N. Berthierine formation in reservoir rocks from the Siri oilfield (Danish North Sea) as result of fluid-rock interactions: Part II. Deciphering organic–inorganic processes by hydrogeochemical modeling. Mar. Petrol. Geol. 2015, 65, 317–326. [Google Scholar] [CrossRef]
- Schulz, H.-M.; Biermann, S.; van Berk, W.; Krüger, M.; Straaten, N.; Bechtel, A.; Wirth, R.; Lüders, V.; Schovsbo, N.H.; Crabtree, S. From shale oil to biogenic shale gas: Retracing organic–inorganic interactions in the Alum Shale (Furongian–Lower Ordovician) in southern Sweden. AAPG Bull. 2015, 99, 927–956. [Google Scholar] [CrossRef]
- Schulz, H.-M.; Wirth, R.; Schreiber, A. Nano-crystal formation of TiO2 polymorphs brookite and anatase due to organic–inorganic rock-fluid interactions. J. Sediment. Res. 2016, 86, 59–72. [Google Scholar] [CrossRef]
- Ruiz Cruz, M.D.; Reyes, E. Kaolinite and dickite formation during shale diagenesis: Isotopic data. Appl. Geochem. 1998, 13, 95–104. [Google Scholar] [CrossRef]
- Cockell, C.S.; Pybus, D.; Olsson-Francis, K.; Kelly, L.; Petley, D.; Rosser, N.; Howard, K.; Mosselmans, F. Molecular Characterization and Geological Microenvironment of a Microbial Community Inhabiting Weathered Receding Shale Cliffs. Microb. Ecol. 2011, 61, 166–181. [Google Scholar] [CrossRef] [PubMed]
- Schieber, J. Iron Sulfide Formation. Encyclopedia of Geobiology; Reitner, J., Thiel, V., Eds.; Springer: Amsterdam, The Netherlands, 2011; pp. 486–502. [Google Scholar]
- Wilson, M.A.; Palmer, T.J.; Guensburg, T.E.; Finton, C.D.; Kaufman, L.E. The development of an Early Ordovician hardground community in response to rapid sea-floor calcite precipitation. Lethaia 1992, 25, 19–34. [Google Scholar] [CrossRef]
- Ellis, A.G.; Weis, A.E. Coexistence and differentiation of ‘flowering stones’: The role of local adaptation to soil microenvironment. J. Ecol. 2006, 94, 322–335. [Google Scholar] [CrossRef]
- Jiang, N.; Cai, D.; He, L.; Zhong, N.; Wen, H.; Zhang, X.; Wu, Z. A Facile Approach to Remediate the Microenvironment of Saline–Alkali Soil. ACS Sustain. Chem. Eng. 2015, 3, 374–380. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Fernández-Remolar, D.; Amils, R.; Sánchez-Navas, A.; Schmid, T.; San Martin-Uriz, P.; Rodríguez, N.; McKenzie, J.A.; Vasconcelos, C. Microbial mediated formation of Fe-carbonate minerals under extreme acidic conditions. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Stief, P.; de Beer, D. Probing the microenvironment of freshwater sediment macrofauna: Implications of deposit-feeding and bioirrigation for nitrogen cycling. Limnol. Oceanogr. 2006, 51, 2538–2548. [Google Scholar] [CrossRef]
- Wolf-Gladrow, D.A.; Bijma, J.; Zeebe, R.E. Model simulation of the carbonate chemistry in the microenvironment of symbiont bearing foraminifera. Mar. Chem. 1999, 64, 181–198. [Google Scholar] [CrossRef]
- Bennett, R.H.; Bryant, W.R.; Hulbert, M.H. Microstructure of Fine-Grained Sediments, from Mud to Shale; Springer: New York, NY, USA, 1990. [Google Scholar]
- Haese, R.R. The Biogeochemistry of Iron. In Marine Geochemistry; Schulz, H.D., Zabel, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; Volume 7, pp. 241–270. [Google Scholar]
- Raiswell, R.; Benning, L.G.; Davidson, L.; Tranter, M.; Tulaczyk, S. Schwertmannite in wet, acid, and oxic microenvironments beneath polar and polythermal glaciers. Geology 2009, 37, 431–434. [Google Scholar] [CrossRef]
- Schwenzer, S.P.; Bridges, J.C.; McAdam, A.; Steer, E.D.; Conrad, P.G.; Kelley, S.P.; Wiens, R.C.; Mangold, N.; Grotzinger, J.; Eigenbrode, J.L.; et al. Modeling of sulphide microenvironments on Mars. In Proceedings of the 47th Lunar and Planetary Science Conference, Houston, TX, USA, 21–25 March 2016. [Google Scholar]
- Steefel, C.I.; DePaolo, D.J.; Lichtner, P.C. Reactive transport modeling: An essential tool and a new research approach for the Earth sciences. Earth Planet. Sci. Lett. 2005, 240, 539–558. [Google Scholar] [CrossRef]
- Kaszuba, J.P.; Yardley, B.W.D.; Andreani, M. Experimental Perspectives of Mineral Dissolution and Precipitation due to Carbon Dioxide-Water-Rock Interactions. Rev. Mineral. Geochem. 2013, 77, 153–188. [Google Scholar] [CrossRef]
- Mu, N.; Schulz, H.-M.; Fu, Y.; Schovsbo, N.H.; Wirth, R.; Rhede, D.; van Berk, W. Berthierine formation in reservoir rocks from the Siri oilfield (Danish North Sea) as result of fluid-rock interactions: Part I. Characterization. Mar. Petrol. Geol. 2015, 65, 302–316. [Google Scholar] [CrossRef]
- McMahon, S.; Anderson, R.P.; Saupe, E.E.; Briggs, D.E.G. Experimental evidence that clay inhibits bacterial decomposers: Implications for preservation of organic fossils. Geology 2016, 44, 867–870. [Google Scholar] [CrossRef]
- Van Berk, W.; Fu, Y.; Schulz, H.-M. Creation of pre-oil-charging porosity by migration of source-rock-derived corrosive fluids through carbonate reservoirs: One-dimensional reactive mass transport modelling. Petrol. Geosci. 2015, 21, 35–42. [Google Scholar] [CrossRef]
- Stawski, T.M.; Benning, L.G. Chapter Five—SAXS in Inorganic and Bioinspired Research. Research Methods in Biomineralization Science. In Methods in Enzymology; De Yoreo, J.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 532, pp. 95–127. [Google Scholar]
- Niederberger, M.; Pinna, N. Chapter 2: Aqueous and Nonaqueous Sol-Gel Chemistry. In Metal Oxide Nanoparticles in Organic Solvents; Niederberger, M., Pinna, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 7–18. [Google Scholar]
- Hoover, S.E.; Lehmann, D. The expansive effects of concentrated pyritic zones within the Devonian Marcellus Shale Formation of North America. Q. J. Eng. Geol. Hydrogeol. 2009, 42, 157–164. [Google Scholar] [CrossRef]
- Aller, R.C. Quantifying solute distributions in the bioturbated zone of marine sediments by defining an average microenvironment. Geochim. Cosmochim. Acta 1965, 44, 1955–1965. [Google Scholar] [CrossRef]
- Wirth, R. Focused Ion Beam (FIB) combined with SEM and TEM: Advanced analytical tools for studies of chemical composition, microstructure and crystal structure in geomaterials on a nanometre scale. Chem. Geol. 2009, 261, 217–229. [Google Scholar] [CrossRef]
- Navrotsky, A. Energetic clues to pathways to biomineralization: Precursors, clusters, and nanoparticles. Proc. Natl. Acad. Sci. USA 2004, 101, 12096–12101. [Google Scholar] [CrossRef]
- Ranade, M.R.; Navrotsky, A.; Zhang, H.Z.; Banfield, J.F.; Elder, S.H.; Zaban, A.; Borse, P.H.; Kulkarni, S.K.; Doran, G.S.; Whitfield, H.J. Energetics of nanocrystalline TiO2 2002, Energetics of nanocrystalline TiO2. Proc. Natl. Acad. Sci. USA 2002, 99 (Suppl. 2), 6476–6481. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Thermodynamic analysis of phase stability of nanocrystalline titania. J. Mater. Chem. 1998, 8, 2073–2076. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The Sol-Gel Process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, M. Nonaqueous Sol–Gel Routes to Metal Oxide Nanoparticles. Acc. Chem. Res. 2007, 40, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Pottier, A.; Chané, C.; Tronc, E.; Mazerolles, L.; Jolivet, J.-P. Synthesis of brookite TiO2 nanoparticles by thermolysis of TiCl4 in strongly acidic aqueous media. J. Mater. Chem. 2001, 11, 1116–1121. [Google Scholar] [CrossRef]
- Li, J.-G.; Ishigaki, T.; Sun, X. Anatase, Brookite, and Rutile Nanocrystals via Redox Reactions under Mild Hydrothermal Conditions? Phase-Selective Synthesis and Physicochemical Properties. J. Phys. Chem. C 2007, 111, 4969–4976. [Google Scholar] [CrossRef]
- Bhave, R.C.; Lee, B.I. Experimental variables in the synthesis of brookite phase TiO2 nanoparticles. Mater. Sci. Eng. A 2007, 467, 146–149. [Google Scholar] [CrossRef]
- Domingos, R.F.; Tufenkji, N.; Wilkinson, K.J. Aggregation of Titanium Dioxide Nanoparticles: Role of a Fulvic Acid. Environ. Sci. Technol. 2009, 43, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Lin, D.; Xing, B. Interactions of Humic Acid with Nanosized Inorganic Oxides. Langmuir 2009, 25, 3571–3576. [Google Scholar] [CrossRef] [PubMed]
- Hada, R.; Kabra, S.; Katara, S.; Rani, A.; Devra, V.; Amriphale, S.S. Synthesis of Nanosized Titania by sol Gel Route. Indian J. Appl. Res. 2013, 3, 49–50. [Google Scholar] [CrossRef]
- Riaz, S.; Naseem, S. Controlled Nanostructuring of TiO2 Nanoparticles—A Sol Gel Approach. J. Sol-Gel Sci. Technol. 2015, 74, 299–309. [Google Scholar] [CrossRef]
- Karimipour, M. Comparison of Acidic and Polymeric Agents in Synthesis of TiO2 Nanoparticles via a Modified Sol-Gel Method. World J. Nano Sci. Eng. 2013, 3, 87–92. [Google Scholar] [CrossRef]
- Khitab, A.; Ahmad, S.; Munir, M.J.; Kazmi, S.M.S.; Arshad, T.; Khushnood, R.A. Synthesis and applications of nano titania particles: A review. Rev. Adv. Mater. Sci. 2018, 53, 90–105. [Google Scholar]
- Isley, S.L.; Penn, L.R. Relative brookite and anatase content in sol-gel-synthesized titanium dioxide nanoparticles. J. Phys. Chem. B 2006, 110, 15134–15139. [Google Scholar] [CrossRef] [PubMed]
- Isley, S.L.; Penn, L.R. Titanium Dioxide Nanoparticles: Effect of Sol−Gel pH on Phase Composition, Particle Size, and Particle Growth Mechanism. J. Phys. Chem. C 2008, 112, 4469–4474. [Google Scholar] [CrossRef]
- Isley, S.L.; Anderson, E.R.; Penn, R.L. Influence of ionic strength on brookite content in sol-gel synthesized titania before and after hydrothermal aging. ECS Trans. 2006, 3, 37–46. [Google Scholar]
- Isley, S.L.; Jordan, D.S.; Penn, L.R. Titanium dioxide nanoparticles: Impact of increasing ionic strength during synthesis, reflux, and hydrothermal aging. Mater. Res. Bull. 2009, 44, 119–125. [Google Scholar] [CrossRef]
- French, R.A.; Jacobson, A.R.; Kim, B.; Isley, S.L.; Penn, R.L.; Baveye, P.C. Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles. Environ. Sci. Technol. 2009, 43, 1354–1359. [Google Scholar] [CrossRef]
- Arning, E.T.; Gaucher, E.C.; van Berk, W.; Schulz, H.-M. Hydrogeochemical models locating sulfate-methane transition zone in marine sediments overlying black shales: A new tool to locate biogenic methane? Marine and Petroleum Geology 2015, 59, 563–574. [Google Scholar] [CrossRef]
- Bräuer, K.; Kämpf, H.; Faber, E.; Koch, U.; Nitzsche, H.M.; Strauch, G. Seismically triggered microbial methane production relating to the Vogtland—NW Bohemia earthquake swarm period 2000, Central Europe. Geochem. J. 2005, 39, 441–450. [Google Scholar]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, H.-M. Sol-Gel Processes in Micro-Environments of Black Shale: Learning from the Industrial Production of Nanometer-Sized TiO2 Polymorphs. ChemEngineering 2019, 3, 28. https://doi.org/10.3390/chemengineering3010028
Schulz H-M. Sol-Gel Processes in Micro-Environments of Black Shale: Learning from the Industrial Production of Nanometer-Sized TiO2 Polymorphs. ChemEngineering. 2019; 3(1):28. https://doi.org/10.3390/chemengineering3010028
Chicago/Turabian StyleSchulz, Hans-Martin. 2019. "Sol-Gel Processes in Micro-Environments of Black Shale: Learning from the Industrial Production of Nanometer-Sized TiO2 Polymorphs" ChemEngineering 3, no. 1: 28. https://doi.org/10.3390/chemengineering3010028
APA StyleSchulz, H.-M. (2019). Sol-Gel Processes in Micro-Environments of Black Shale: Learning from the Industrial Production of Nanometer-Sized TiO2 Polymorphs. ChemEngineering, 3(1), 28. https://doi.org/10.3390/chemengineering3010028



