Synthesis and Characterization of Reduced Graphene Oxide and Their Application in Dye-Sensitized Solar Cells
Abstract
1. Introduction
2. Experimental Details
2.1. Preparation of Reduced Graphene Oxide
2.2. AFM Measurements
2.3. Raman Measurements
2.4. Other Characterization Techniques
2.5. Solar Cell Fabrication
3. Results and Discussion
3.1. Synthesis of Reduced Graphene Oxide
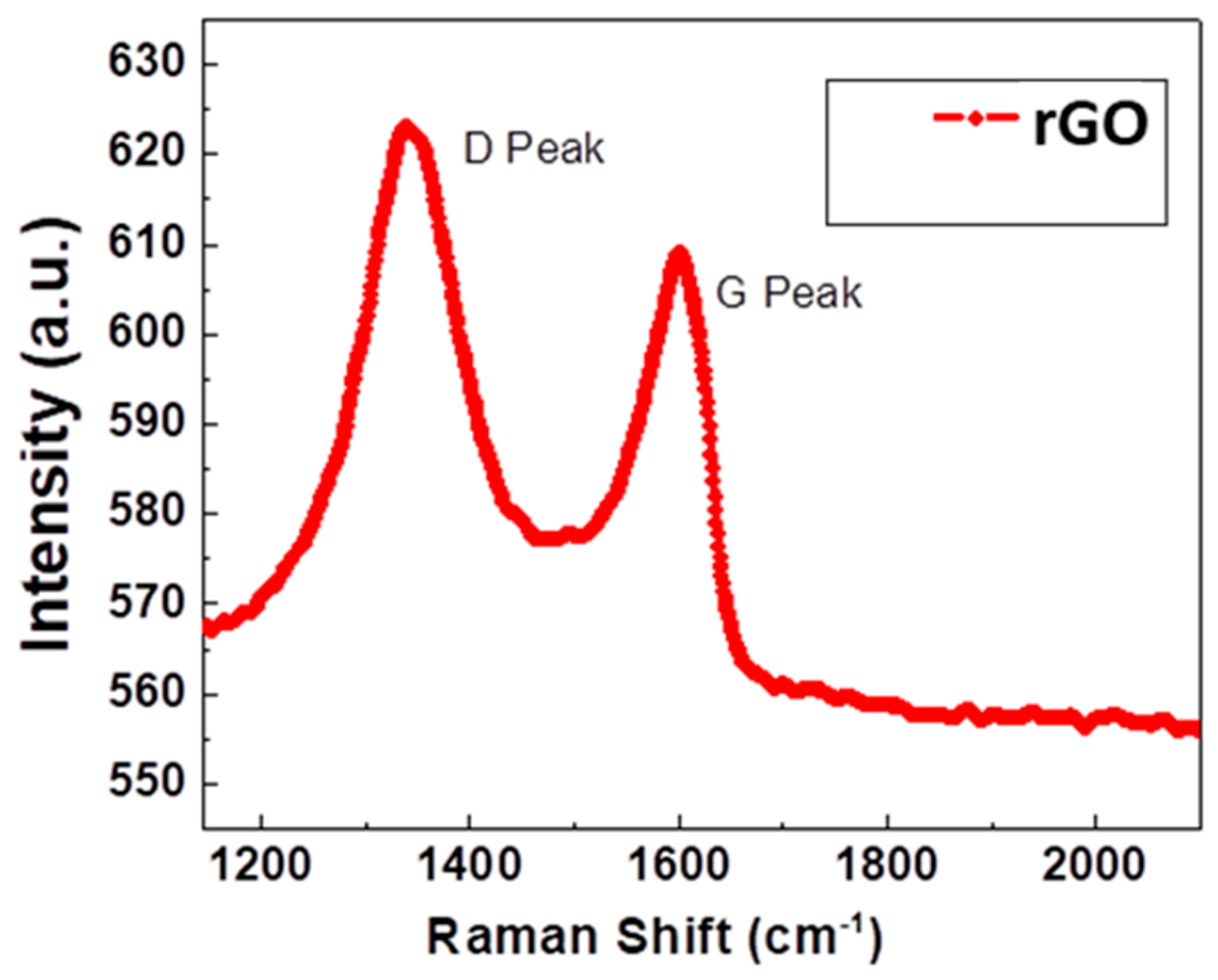
3.2. Raman Studies
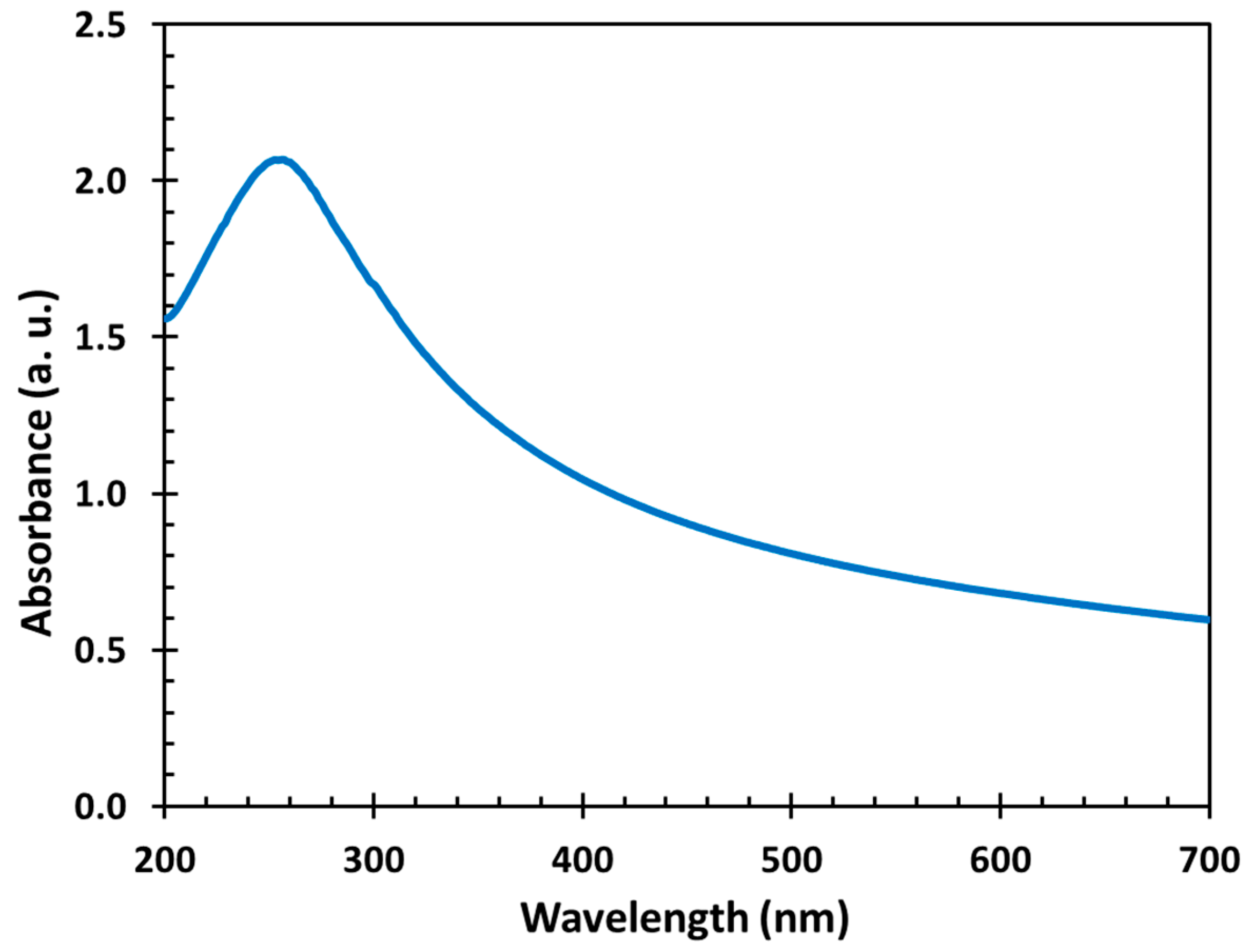
3.3. UV–vis Measurement
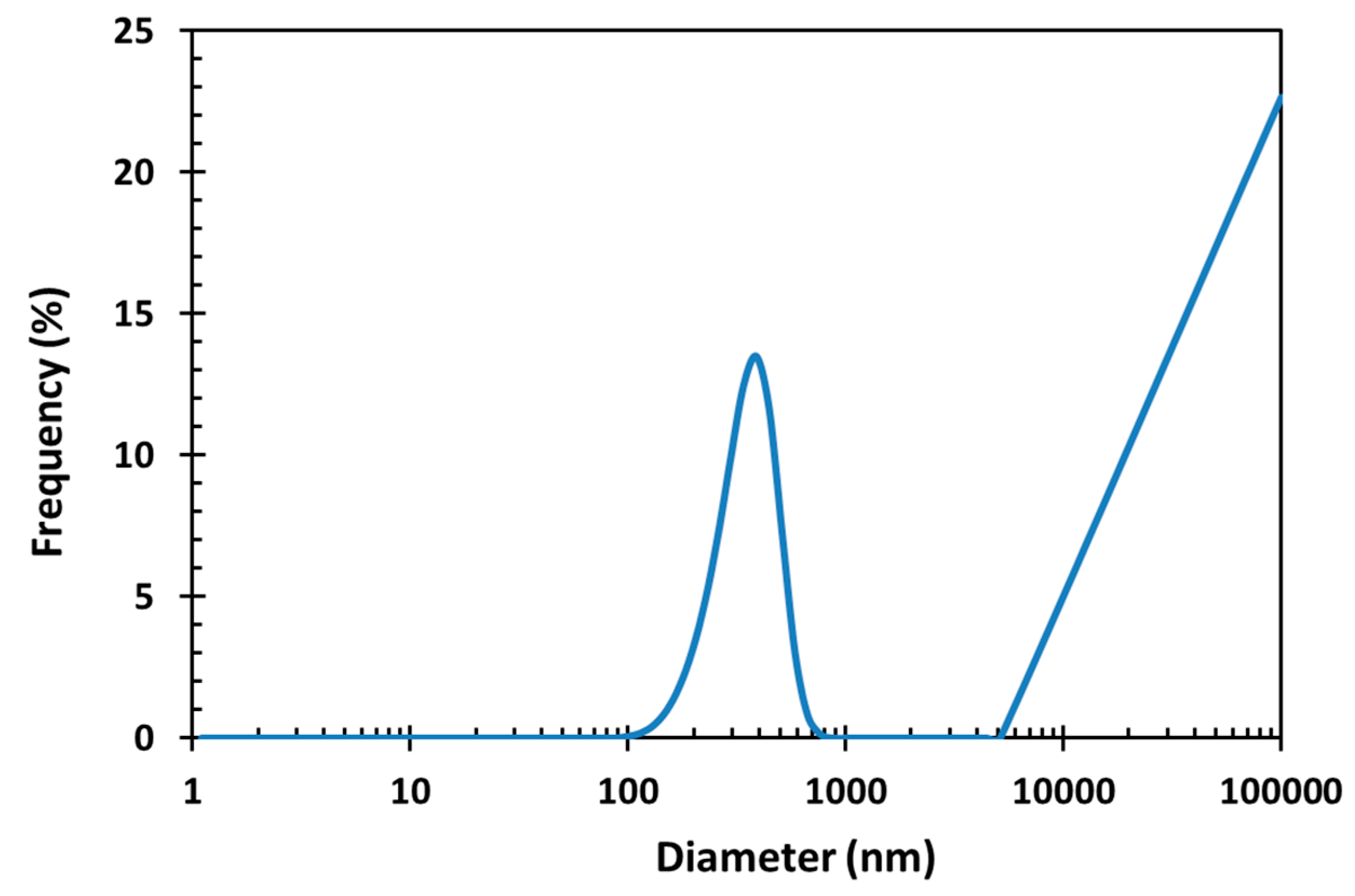
3.4. Dynamic Light Scattering
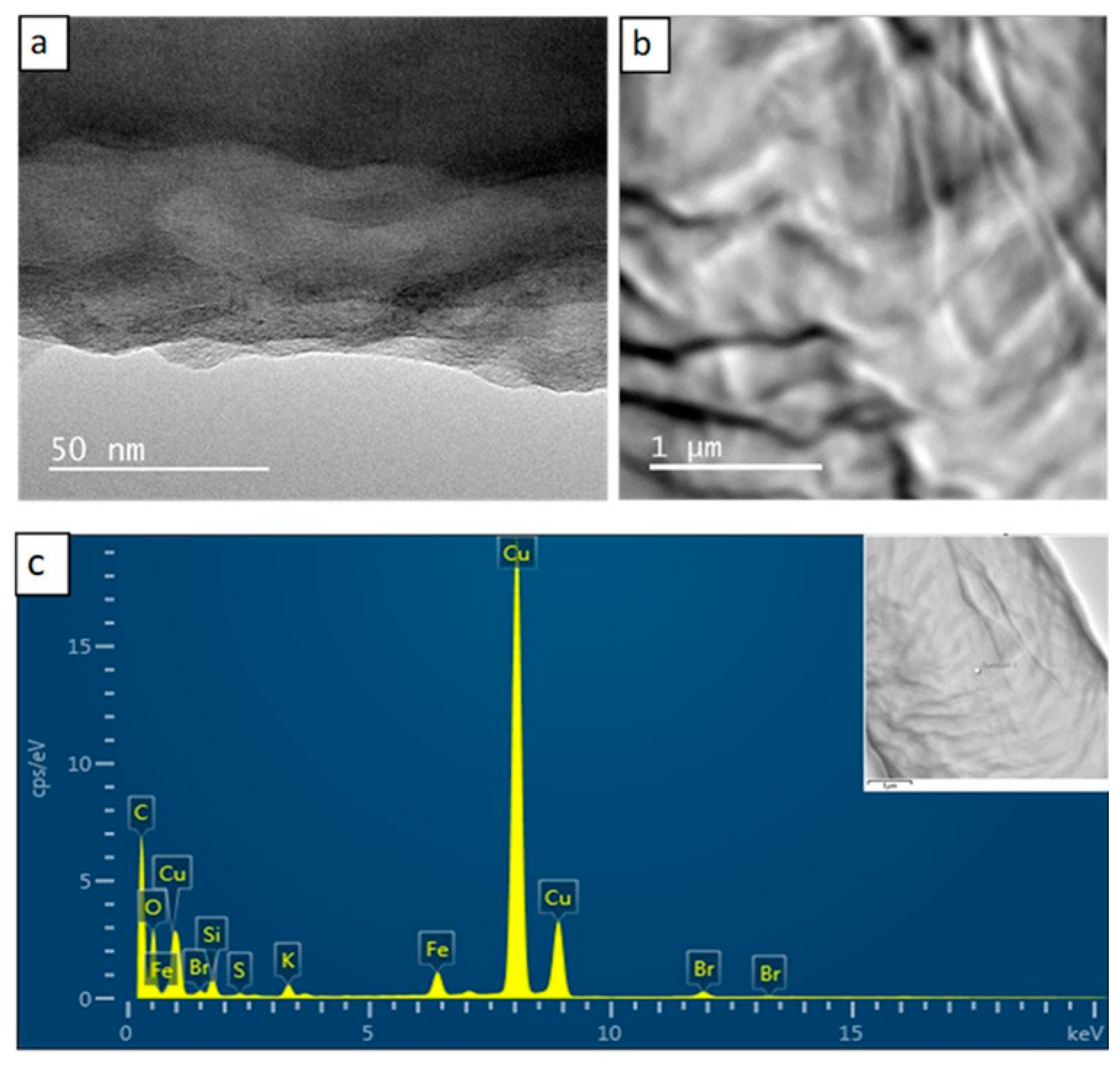
3.5. Transmission Electron Microscopy/Energy Dispersive X-ray Spectroscopy Studies
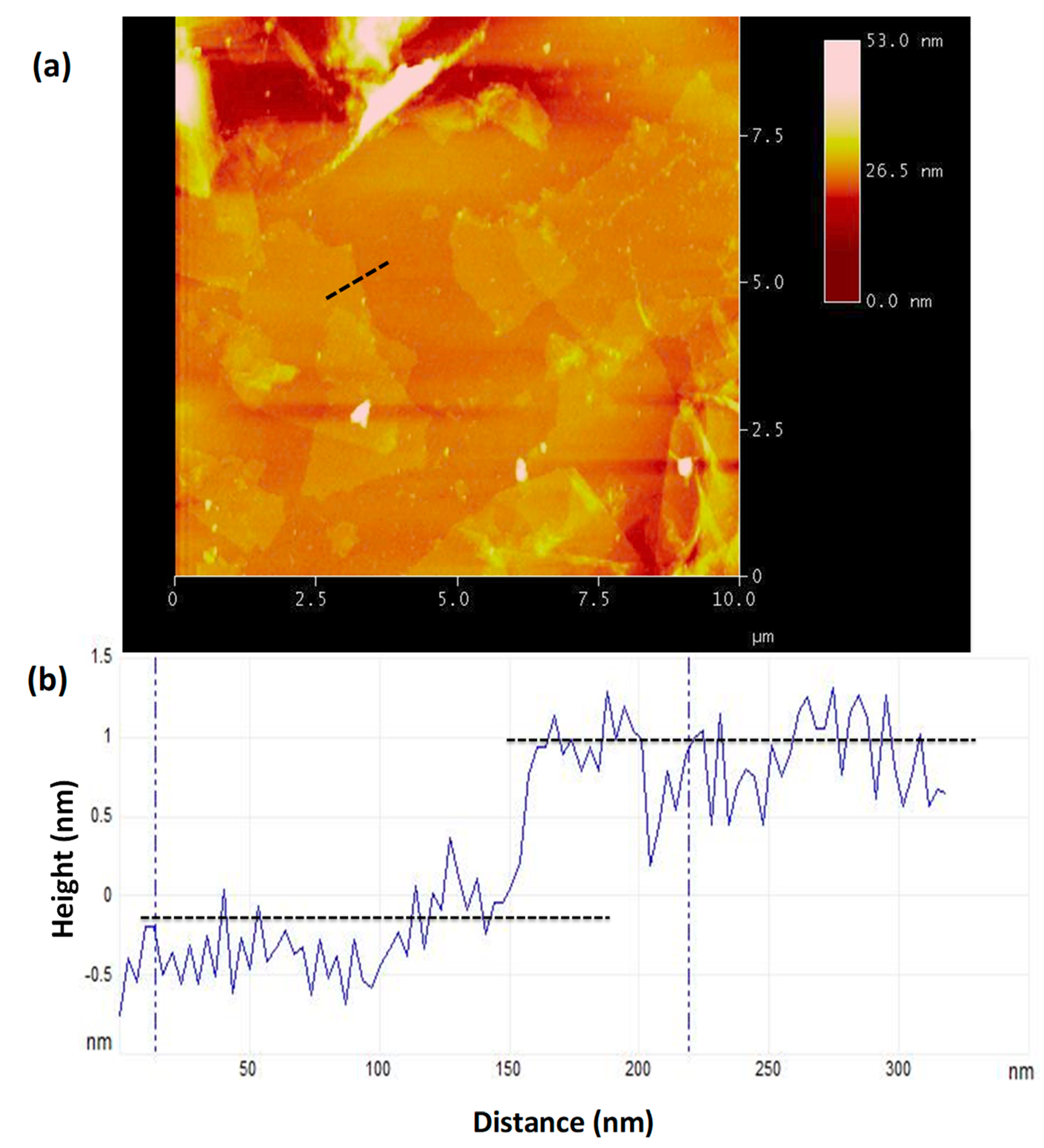
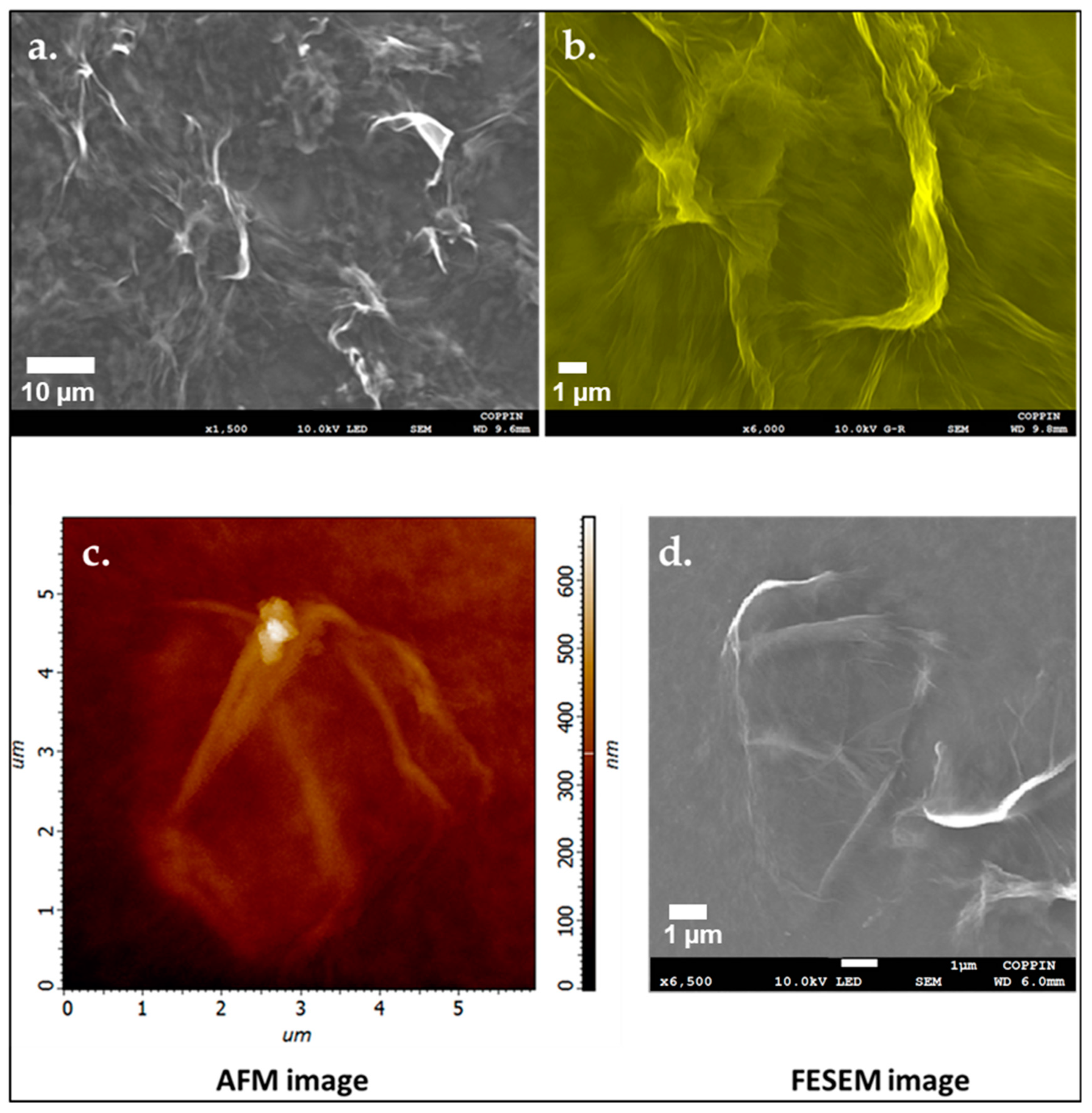
3.6. Atomic Force Microscopy Imaging
3.7. Field Emission Scanning Electron Microscopy Imaging
3.8. Current and Voltage Characteristics Measurements
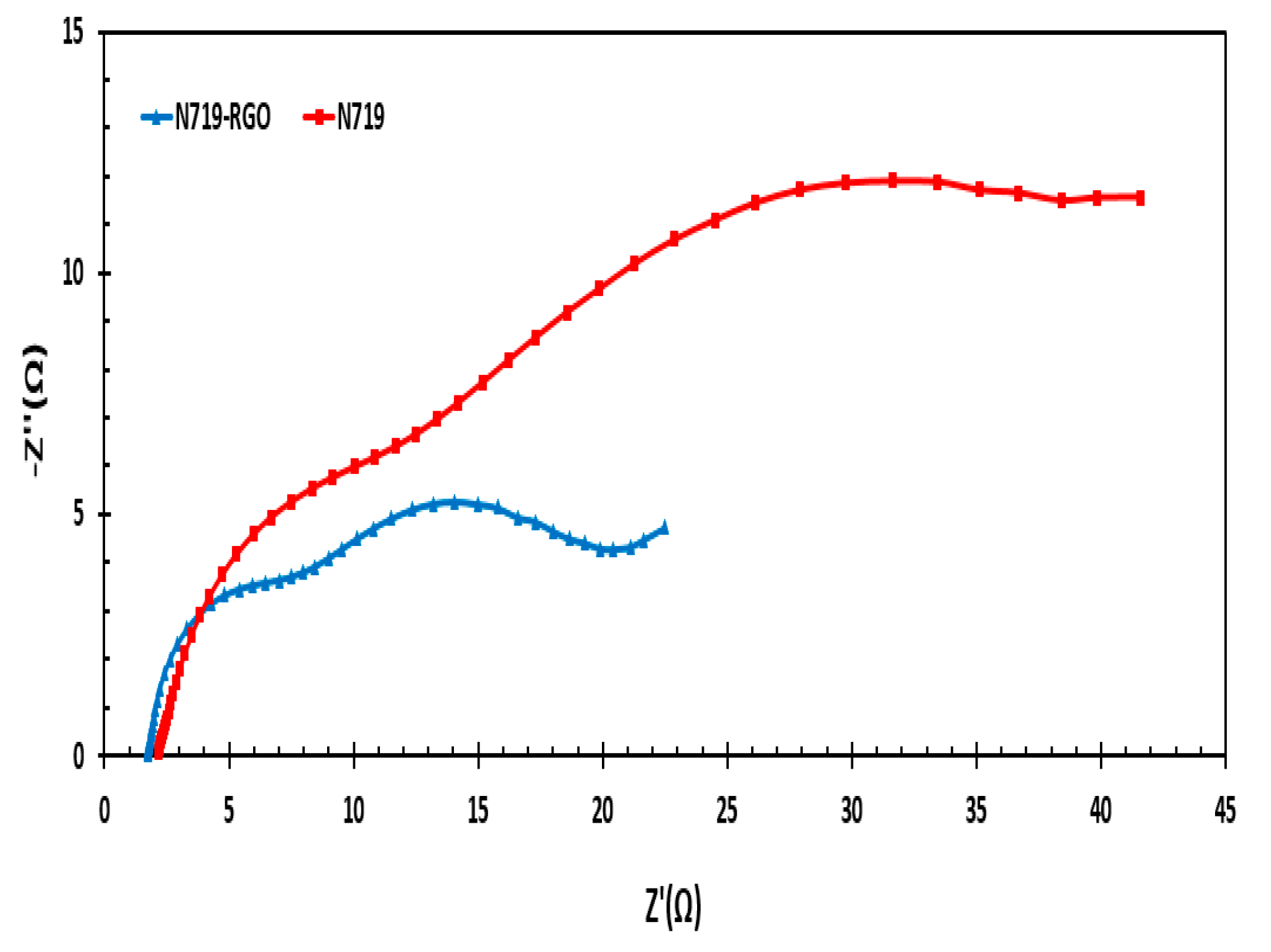
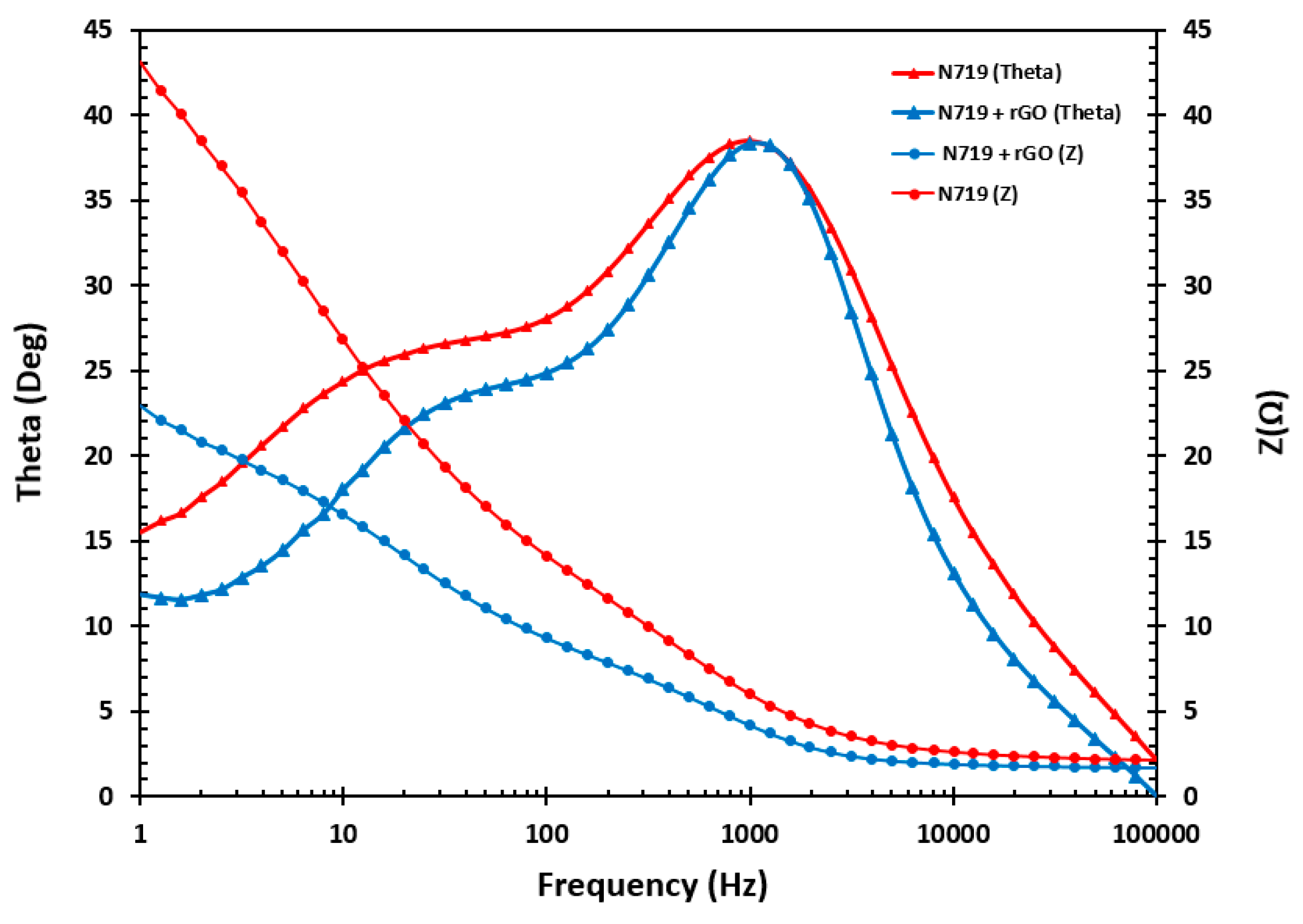
3.9. Impedance Measurement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Ghann, W.; Kang, H.; Emerson, E.; Oh, J.; Chavez-Gil, T.; Nesbitt, F.; Williams, R.; Uddin, J. Photophysical properties of near-IR cyanine dyes and their application as photosensitizers in dye sensitized solar cells. Inorg. Chim. Acta 2017, 467, 123–131. [Google Scholar] [CrossRef]
- Ghann, W.; Chavez-Gil, T.; Goede, C.I.; Kang, H.; Khan, S.; Sobhi, H.; Nesbitt, F.; Uddin, J. Photophysical, Electrochemical and Photovoltaic Properties of Porphyrin-Based Dye Sensitized Solar Cell. Adv. Mater. Phys. Chem. 2017, 7, 148–172. [Google Scholar] [CrossRef]
- Ghann, W.; Sobhi, H.; Kang, H.; Chavez-Gil, T.; Nesbitt, F.; Uddin, J. Synthesis and Characterization of Free and Copper (II) Complex of N, N′-Bis(Salicylidene)Ethylenediamine for Application in Dye Sensitized Solar Cells. J. Mater. Sci. Chem. Eng. 2017, 5, 46–66. [Google Scholar] [CrossRef]
- Ghann, W.; Kang, H.; Sheikh, T.; Yadav, S.; Chavez-Gil, T.; Nesbitt, F.; Uddin, J. Fabrication, Optimization and Characterization of Natural Dye Sensitized Solar Cell. Sci. Rep. 2017, 7, 41470. [Google Scholar] [CrossRef] [PubMed]
- Ghann, W.; Rahman, A.; Rahman, A.; Uddin, J. Interaction of Sensitizing Dyes with Nanostructured TiO2 Film in Dye-Sensitized Solar Cells Using Terahertz Spectroscopy. Sci. Rep. 2016, 6, 30140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, X.; Li, H.; Chen, S.; Sun, Z.; Yin, X.; Huang, S. Graphene-based counter electrode for dye-sensitized solar cells. Carbon 2011, 49, 5382–5388. [Google Scholar] [CrossRef]
- Fang, X.; Li, M.; Guo, K.; Zhu, Y.; Hu, Z.; Liu, X.; Chen, B.; Zhao, X. Improved properties of dye-sensitized solar cells by incorporation of graphene into the photoelectrodes. Electrochim. Acta 2012, 65, 174–178. [Google Scholar] [CrossRef]
- Chong, S.W.; Lai, C.W.; Hamid, S.B.A. Controllable Electrochemical Synthesis of Reduced Graphene Oxide Thin-Film Constructed as Efficient Photoanode in Dye-Sensitized Solar Cells. Materials (Basel) 2016, 9, 69. [Google Scholar] [CrossRef]
- Yeh, M.-H.; Lin, L.-Y.; Chang, L.-Y.; Leu, Y.-A.; Cheng, W.Y.; Lin, J.-J.; Ho, K.-C. Dye-Sensitized Solar Cells with Reduced Graphene Oxide as the Counter Electrode Prepared by a Green Photothermal Reduction Process. ChemPhysChem 2014, 15, 1175–1181. [Google Scholar] [CrossRef]
- Gupta, T.; Sakthivel, T.; Sakthivel, S. Recent development in 2D materials beyond graphene. Prog. Mater. Sci. 2015, 73, 44–126. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Ruoff, R.S.; Bielawski, C.W. From Conception to Realization: An Historical Account of Graphene and Some Perspectives for Its Future. Angew. Chem. Int. Ed. 2010, 49, 9336–9344. [Google Scholar] [CrossRef] [PubMed]
- Koppens, F.H.L.; Mueller, T.; Avouris, P.; Ferrari, A.C.; Vitiello, M.S.; Polini, M. Photodetectors based on graphene, other two-dimensional materials and hybrid systems. Nat. Nanotechnol. 2014, 9, 780–793. [Google Scholar] [CrossRef] [PubMed]
- Dubertret, B.; Heine, T.; Terrones, M. The Rise of Two-Dimensional Materials. Acc. Chem. Res. 2015, 48, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666. [Google Scholar] [CrossRef]
- Babak, A.; Maria, L.; Yury, G. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar]
- Fiori, G.; Bonaccorso, F.; Iannaccone, G.; Palacios, T.; Neumaier, D.; Seabaugh, A.; Banerjee, S.K.; Colombo, L. Electronics based on two-dimensional materials. Nat. Nanotechnol. 2014, 9, 768–779. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, S.; Lei, F.; Xiao, C.; Xie, Y. Ultrathin Two-Dimensional Inorganic Materials: New Opportunities for Solid State Nanochemistry. Acc. Chem. Res. 2015, 48, 3–12. [Google Scholar] [CrossRef]
- Zou, X.; Yakobson, B. An Open Canvas—2D Materials with Defects, Disorder, and Functionality. Acc. Chem. Res. 2015, 48, 73–80. [Google Scholar] [CrossRef]
- Khan, A.H.; Ghosh, S.; Pradhan, B.; Dalui, A.; Shrestha, L.K.; Acharya, S.; Ariga, K. Two-Dimensional (2D) Nanomaterials towards Electrochemical Nanoarchitectonics in Energy-Related Applications. Bull. Chem. Soc. Jpn. 2017, 90, 627–648. [Google Scholar] [CrossRef]
- Choi, H.; Kim, H.; Hwang, S.; Han, Y.; Jeon, M. Graphene counter electrodes for dye-sensitized solar cells prepared by electrophoretic deposition. J. Mater. Chem. 2011, 21, 7548–7551. [Google Scholar] [CrossRef]
- Roy-Mayhew, J.D.; Aksay, I.A. Graphene Materials and Their Use in Dye-Sensitized Solar Cells. Chem. Rev. 2014, 114, 6323–6348. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.B.; Lee, C.S.; Xu, J.; Liu, Z.T.; Chen, Z.H.; He, Z.; Cao, Y.L.; Yuan, G.; Song, H.; Chen, L.; et al. Incorporation of graphenes in nanostructured TiO2 films via molecular grafting for dye-sensitized solar cell application. ACS Nano 2010, 4, 3482–3488. [Google Scholar] [CrossRef] [PubMed]
- Muthoosamy, K.; Bai, R.G.; Abubakar, I.B.; Sudheer, S.M.; Lim, H.N.; Loh, H.S.; Huang, N.M.; Chia, C.H.; Manickam, S. Exceedingly Biocompatible and Thin-Layered Reduced Graphene Oxide Nanosheets Using an Eco-Friendly Mushroom Extract Strategy. Int. J. Nanomed. 2015, 10, 1505–1519. [Google Scholar]
- Robinson, J.T.; Perkins, F.K.; Snow, E.S.; Wei, Z.; Sheehan, P.E. Reduced Graphene Oxide Molecular Sensors. Nano Lett. 2008, 8, 3137–3140. [Google Scholar] [CrossRef] [PubMed]
- Gaidukevič, J.; Pauliukaitė, R.; Niaura, G.; Matulaitienė, I.; Opuchovič, O.; Radzevič, A.; Astromskas, G.; Bukauskas, V.; Barkauskas, J. Synthesis of Reduced Graphene Oxide with Adjustable Microstructure Using Regioselective Reduction in the Melt of Boric Acid: Relationship Between Structural Properties and Electrochemical Performance. Nanomaterials 2018, 8, 889. [Google Scholar] [CrossRef] [PubMed]
- Abdolhosseinzadeh, S.; Asgharzadeh, H.; Kim, H.S. Fast and fully-scalable synthesis of reduced graphene oxide. Sci. Rep. 2015, 5, 10160. [Google Scholar] [CrossRef] [PubMed]
- Chunder, A.; Pal, T.; Khondaker, S.I.; Zhai, L. Reduced Graphene Oxide/Copper Phthalocyanine Composite and Its Optoelectrical Properties. J. Phys. Chem. C 2010, 114, 15129–15135. [Google Scholar] [CrossRef]
- Rahman, M.Y.A.; Sulaiman, A.S.; Umar, A.A.; Salleh, M.M. Dye-sensitized solar cell (DSSC) utilizing reduced graphene oxide (RGO) films counter electrode: Effect of graphene oxide (GO) content. J. Mater. Sci. Mater. Electron. 2016, 28, 1674–1678. [Google Scholar] [CrossRef]
- Yang, B.; Zuo, X.; Chen, P.; Zhou, L.; Yang, X.; Zhang, H.; Li, G.; Wu, M.; Ma, Y.; Jin, S.; et al. Nanocomposite of Tin Sulfide nanoparticles with Reduced Graphene Oxide in High-Efficiency Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 137–143. [Google Scholar] [CrossRef]
- Marchezi, P.E.; Sonai, G.G.; Hirata, M.K.; Schiavon, M.A.; Nogueira, A.F. Understanding the Role of Reduced Graphene Oxide in the Electrolyte of Dye-Sensitized Solar Cells. J. Phys. Chem. C 2016, 120, 23368–23376. [Google Scholar] [CrossRef]
- Tang, B.; Ji, G.; Wang, Z.; Chen, H.; Li, X.; Yu, H.; Li, S.; Liu, H. Three-dimensional graphene networks and reduced graphene oxide nanosheets co-modified dye-sensitized solar cells. Power Sources 2012, 220, 95. [Google Scholar] [CrossRef]
- Zhu, G.; Xu, T.; Lv, T.; Pan, L.; Zhao, Q. Graphene-incorporated nanocrystalline TiO2 films for CdS quantum dot-sensitized solar cells. J. Electroanal. Chem. 2011, 650, 248. [Google Scholar] [CrossRef]
- He, Z.; Guai, G.; Liu, J.; Guo, C.; Loo, J.S.; Li, C.M.; Tan, T.T. Nanostructure control of graphene-composited TiO2 by a one-step solvothermal approach for high performance dye-sensitized solar cells. Nanoscale 2011, 3, 4613–4616. [Google Scholar] [CrossRef]
- Bell, N.J.; Ng, Y.H.; Du, A.; Smith, H.C.S.C.; Amal, R. Understanding the Enhancement in Photoelectrochemical Properties of Photocatalytically Prepared TiO2-Reduced Graphene Oxide Composite. J. Phys. Chem. C 2011, 115, 6004–6009. [Google Scholar] [CrossRef]
- Sun, S.; Gao, L.; Liu, Y. Enhanced de-sensitized solar cell using graphene-TiO2 photoanode prepared by heterogeneous coagulation. Appl. Phys. Lett. 2010, 96, 083113. [Google Scholar] [CrossRef]
- Park, J.H.; Seo, S.W.; Kim, J.H.; Choi, C.J.; Kim, H.; Lee, D.K.; Jung, W.S.; Ahn, K.S. Improved Efficiency of Dye-Sensitized Solar Cell Using Graphene-Coated Al2O3-TiO2 Nanocomposite Photoanode. Mol. Cryst. Liq. Cryst. 2011, 538, 285. [Google Scholar] [CrossRef]
- Tsai, T.-H.; Chiou, S.-C.; Chen, S.-M. Enhancement of Dye-Sensitized Solar Cells by using GrapheneTiO2 Composites as Photoelectrochemical Working Electrode. Int. J. Electrochem. Sci. 2011, 6, 3333. [Google Scholar]
- Joung, D.; Khondaker, S.I. Efros-Shklovskii variable range hopping in reduced graphene oxide sheets of varying carbon sp2 fraction. Phys. Rev. 2012, 86, 235423. [Google Scholar] [CrossRef]
- Joung, D.; Khondaker, S.I. Structural Evolution of Reduced Graphene Oxide of Varying Carbon sp2 Fractions Investigated via Coulomb Blockade Transport. J. Phys. Chem. 2013, 117, 26776–26782. [Google Scholar] [CrossRef]
- Kang, N.; Khondaker, S.I. The impact of carbon sp2 fraction of reduced graphene oxide on the performance of reduced graphene oxide contacted organic transistors. Phys. Lett. 2014, 105, 223301. [Google Scholar] [CrossRef]
- Joung, D.; Khondaker, S.I. Two- to one-dimensional crossover in graphene quantum dot arrays observed in reduced graphene oxide nanoribbons. Phys. Rev. B 2014, 89, 245411–2454117. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Grimm, S.; Schweiger, M.; Eigler, S.; Zaumseil, J. High-Quality Reduced Graphene Oxide by CVD-Assisted Annealing. J. Phys. Chem. C 2016, 120, 3036–3041. [Google Scholar] [CrossRef]
- Çiplak, Z.; Yildiz, N.; Çalimli, A. Investigation of Graphene/Ag Nanocomposites Synthesis Parameters for Two Different Synthesis Methods. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 361–370. [Google Scholar] [CrossRef]
- King, A.A.K.; Davies, B.R.; Noorbehesht, N.; Newman, P.; Church, T.L.; Harris, A.T.; Raza, J.M.; Minett, A.I. A New Raman Metric for the Characterization of Graphene oxide and its Derivatives. Sci. Rep. 2016, 6, 19491. [Google Scholar] [CrossRef]
- G´omez-Navarro, C.; Weitz, R.T.; Bittner, A.M.; Scolari, M.; Mews, A.; Burghard, M.; Kern, K. Electronic transport properties of individual chemically reduced graphene oxide sheets. Nano Lett. 2007, 7, 3499–3503. [Google Scholar] [CrossRef]
- Jung, I.; Dikin, D.A.; Piner, D.R.; Ruoff, R.S. Tunable electrical conductivity of individual graphene oxide sheets reduced at “low” temperatures. Nano Lett. 2008, 8, 4283. [Google Scholar] [CrossRef]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270. [Google Scholar] [CrossRef]
- Kotchey, G.P.; Allen, B.L.; Vedala, H.; Yanamala, N.; Kapralov, A.A.; Tyurina, Y.Y.; Klein-Seetharaman, J.; Kagan, V.E.; Star, A. The Enzymatic Oxidation of Graphene Oxide. The enzymatic oxidation of graphene oxide. ACS Nano 2011, 5, 2098–2108. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S.; Kim, S.O. Three-Dimensional Self-Assembly of Graphene Oxide Platelets into Mechanically Flexible Macroporous Carbon Film. Angew. Chem. 2010, 122, 10282–10286. [Google Scholar]
- Chowdhury, F.A.; Mochida, T.; Otsuki, J.; Alam, M.S. Thermally reduced solution-processed graphene oxide thin film: An efficient infrared photodetector. Chem. Phys. Lett. 2014, 593, 198–203. [Google Scholar] [CrossRef]
- Kumar, P.V.; Bernardi, M.; Grossman, J.C. The impact of functionalization on the stability, work function, and photoluminescence of reduced graphene oxide. ACS Nano 2013, 7, 1638. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Dikin, R.S.R.D.A.; Stankovich, S.; Zimney, E.J.; et al. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457. [Google Scholar] [CrossRef]
- Chowdhury, F.A.; Hossain, M.A.; Uchida, K.; Tamura, T.; Sugawa, K.; Mochida, T.; Otsuki, J.; Mohiuddin, T.; Boby, M.A.; Alam, M.S. Graphene oxide/carbon nanoparticle thin film based IR detector: Surface properties and device characterization. AIP Adv. 2015, 5, 107228. [Google Scholar] [CrossRef]
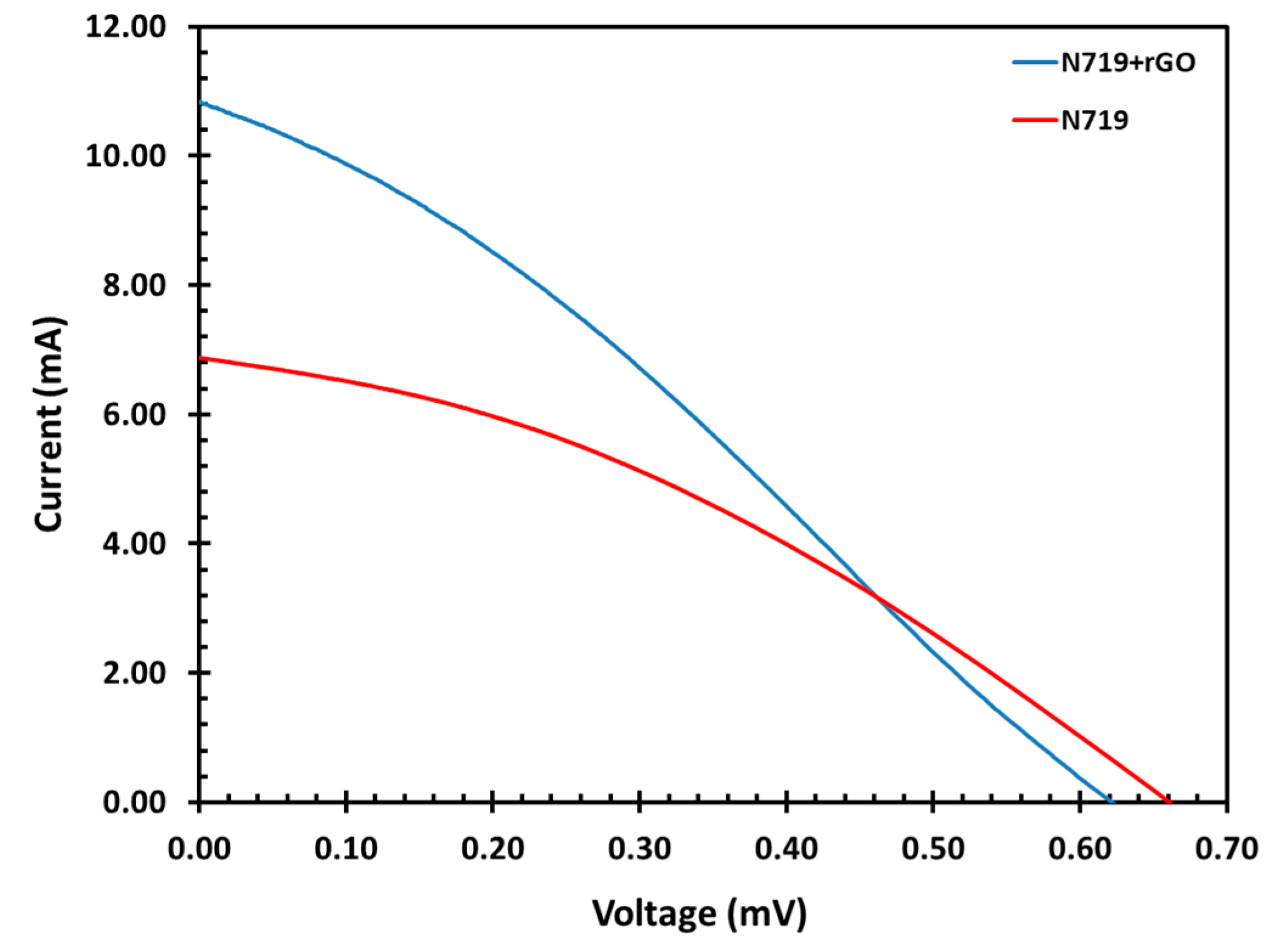
| Sample | Vmp | Imp | Voc | Isc | FF | Efficiency (%) |
|---|---|---|---|---|---|---|
| N719 only | 0.37 | 4.33 | 0.66 | 6.87 | 0.35 | 1.61 |
| N719+rGO | 0.31 | 6.46 | 6.24 | 10.80 | 0.30 | 2.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghann, W.E.; Kang, H.; Uddin, J.; Chowdhury, F.A.; Khondaker, S.I.; Moniruzzaman, M.; Kabir, M.H.; Rahman, M.M. Synthesis and Characterization of Reduced Graphene Oxide and Their Application in Dye-Sensitized Solar Cells. ChemEngineering 2019, 3, 7. https://doi.org/10.3390/chemengineering3010007
Ghann WE, Kang H, Uddin J, Chowdhury FA, Khondaker SI, Moniruzzaman M, Kabir MH, Rahman MM. Synthesis and Characterization of Reduced Graphene Oxide and Their Application in Dye-Sensitized Solar Cells. ChemEngineering. 2019; 3(1):7. https://doi.org/10.3390/chemengineering3010007
Chicago/Turabian StyleGhann, William E., Hyeonggon Kang, Jamal Uddin, Farzana Aktar Chowdhury, Saiful I. Khondaker, Mohammed Moniruzzaman, Md Humayun Kabir, and Mohammed M. Rahman. 2019. "Synthesis and Characterization of Reduced Graphene Oxide and Their Application in Dye-Sensitized Solar Cells" ChemEngineering 3, no. 1: 7. https://doi.org/10.3390/chemengineering3010007
APA StyleGhann, W. E., Kang, H., Uddin, J., Chowdhury, F. A., Khondaker, S. I., Moniruzzaman, M., Kabir, M. H., & Rahman, M. M. (2019). Synthesis and Characterization of Reduced Graphene Oxide and Their Application in Dye-Sensitized Solar Cells. ChemEngineering, 3(1), 7. https://doi.org/10.3390/chemengineering3010007






