A Comparative Study of Ultrasound Biodiesel Production Using Different Homogeneous Catalysts
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Catalyst Preparation
2.3. Ultrasonic Irradiation Unit
2.4. Transesterification Reaction
2.5. Methyl Ester Analysis
3. Results and Discussion
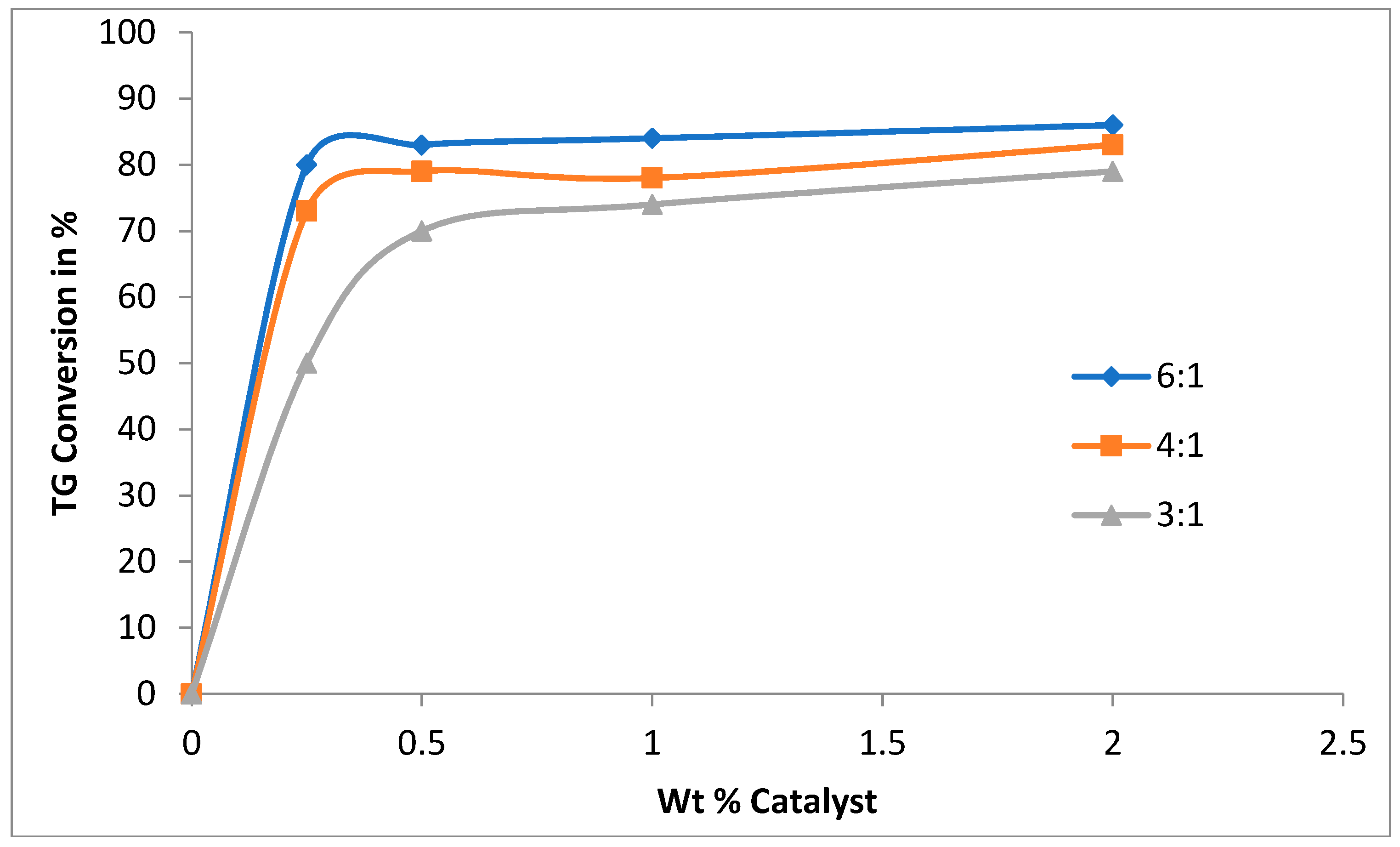
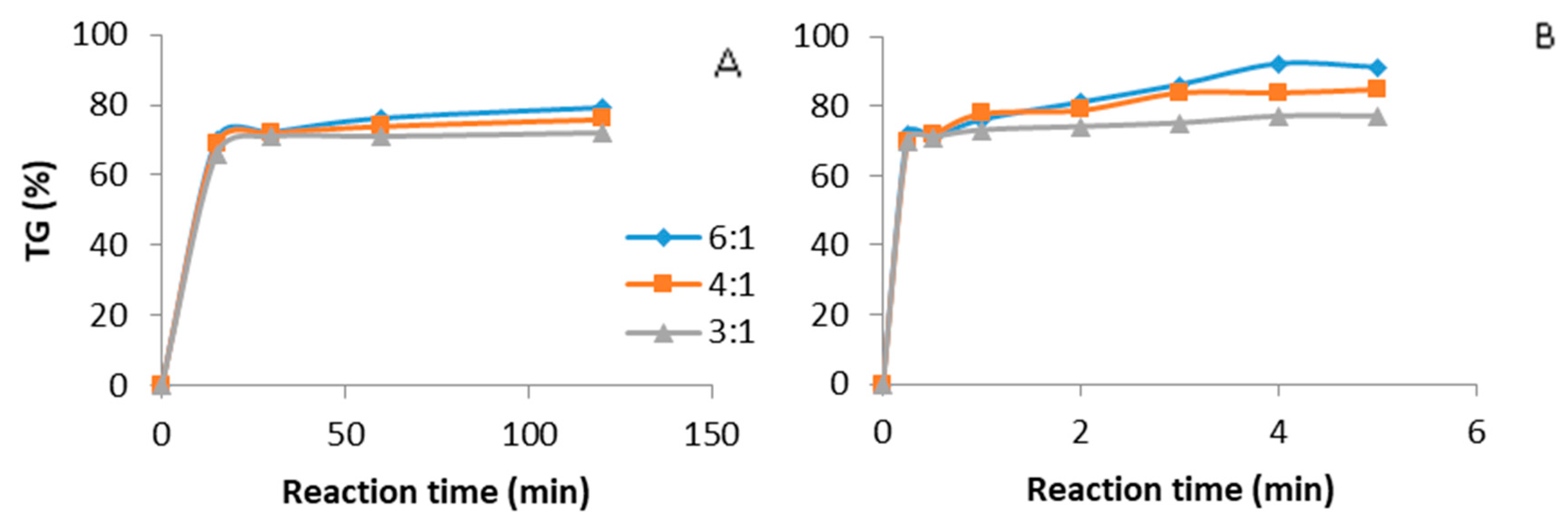
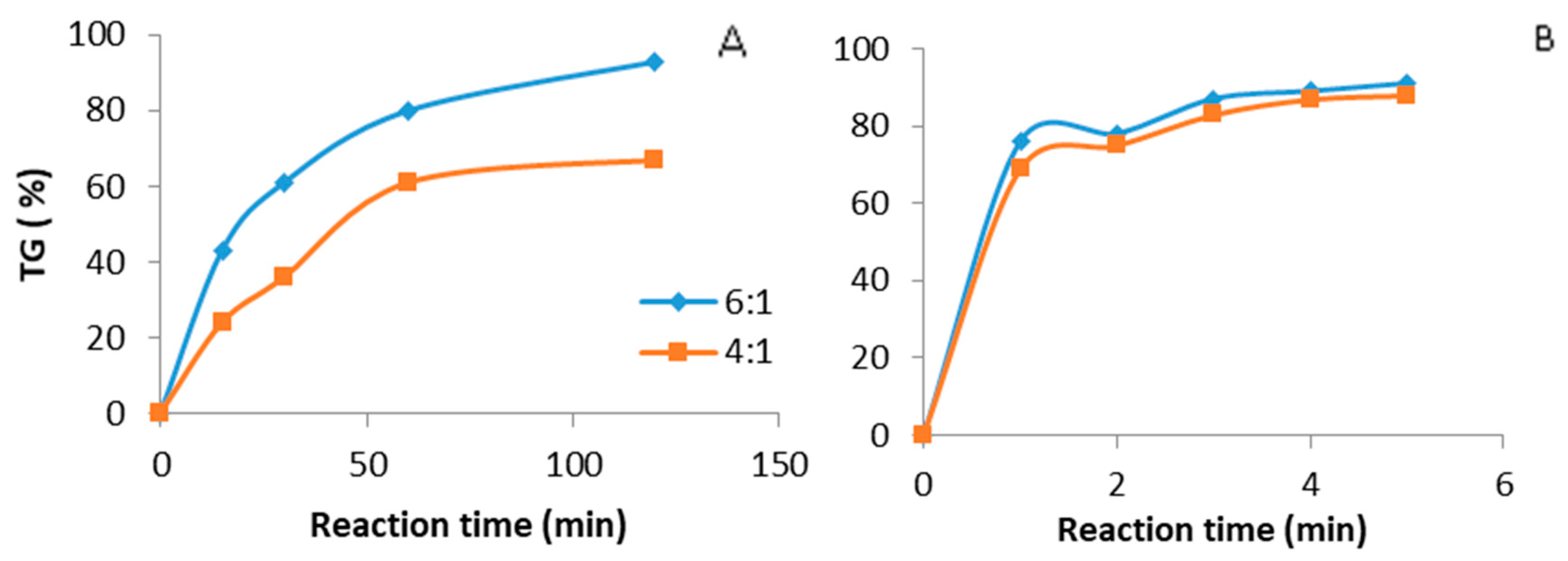
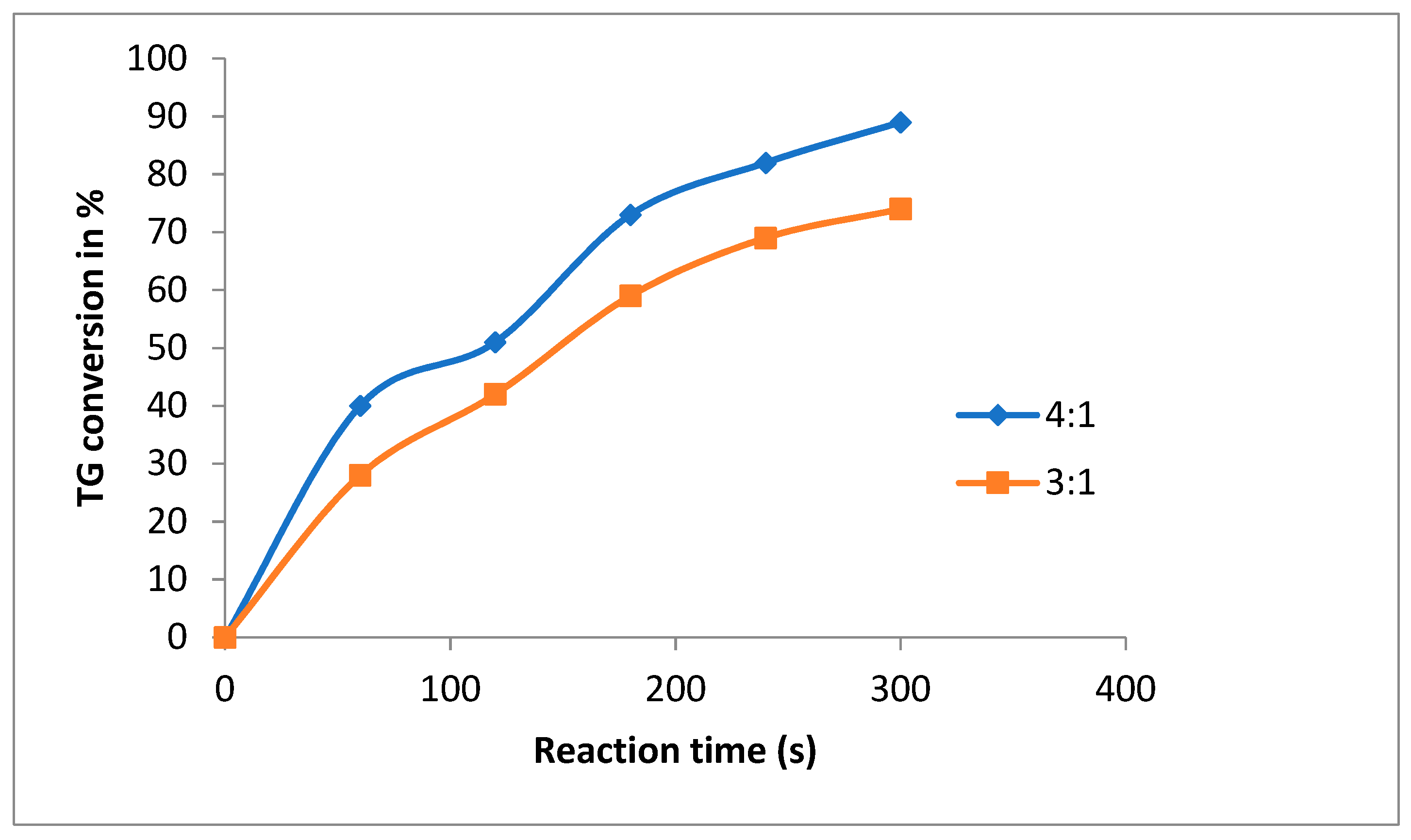
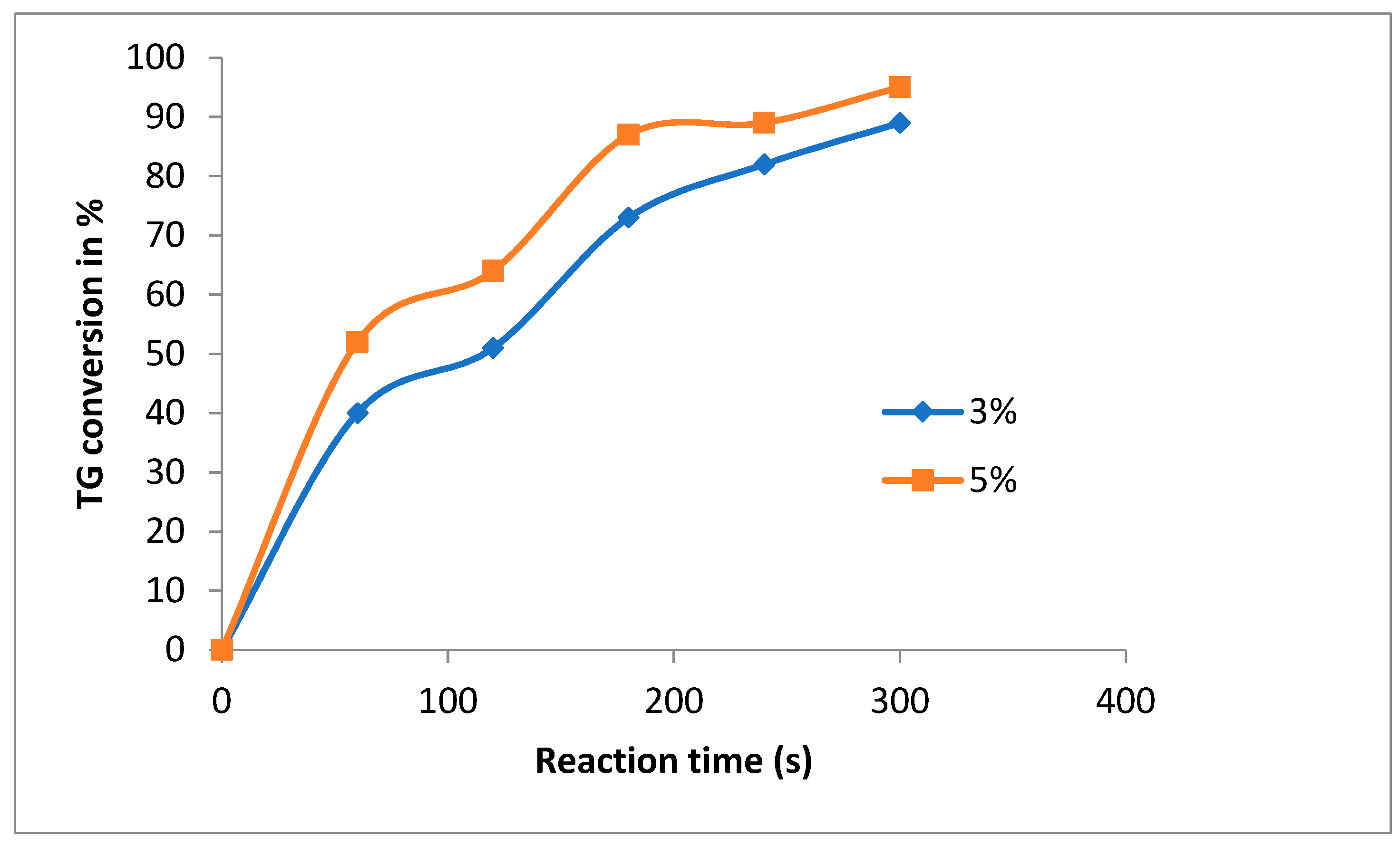
3.1. Catalyst Concentration and the Effect of Methanol to Oil Ratio
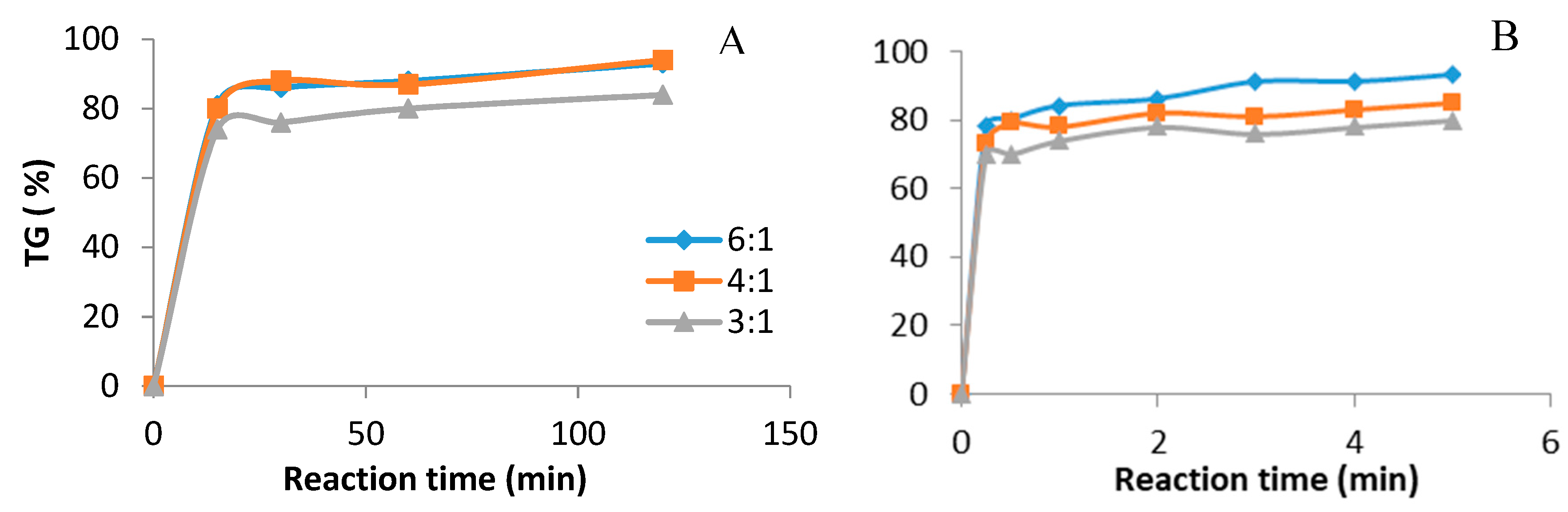
3.2. Comparison between Ultrasound and Mechanical Stirring in the Presence of CH3ONa
3.3. Comparison between Ultrasound and Mechanical Stirring in the Presence of KOH
3.4. Comparison between Ultrasound and Mechanical Stirring in the Presence of NaOH
3.5. Comparison between Ultrasound and Mechanical Stirring in the Presence of Tetramethyl Ammonium Hydroxide
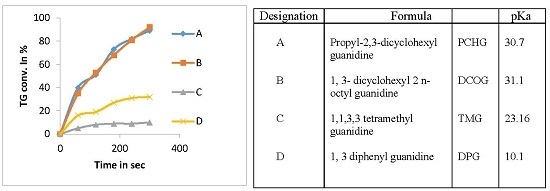
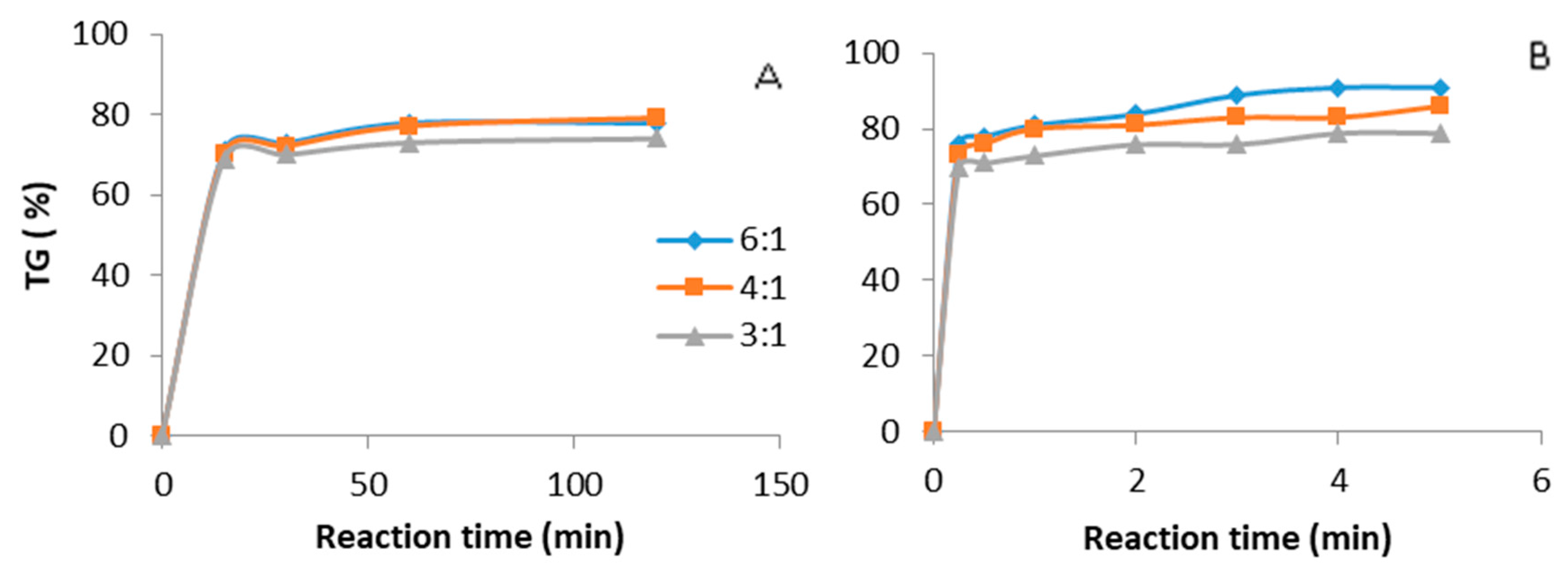
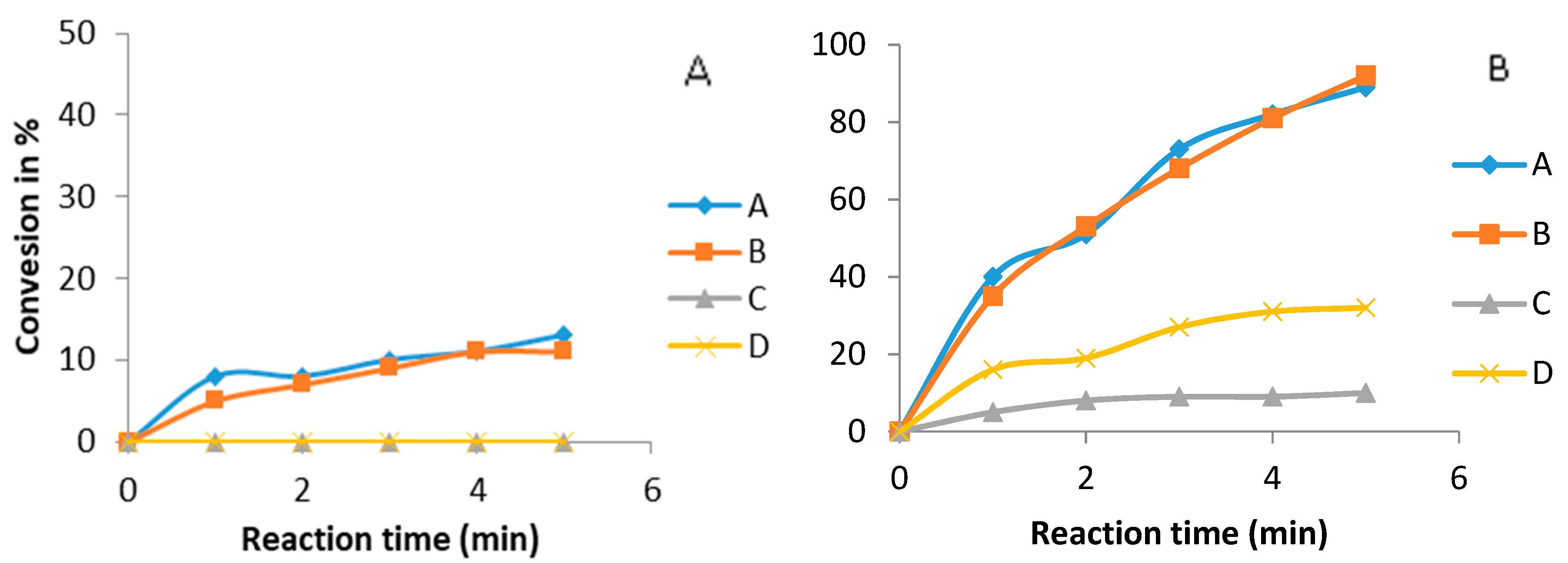
3.6. Comparison between Ultrasound and Mechanical Stirring Using Guanidines as Catalysts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- The Royal Society. Sustainable Biofuel: Prospects and Challenges; The Royal Society: London, UK, 2008. [Google Scholar]
- Talebian-Kiakalaieh, A.; Saidina Amin, N.A.; Mazaheri, H. A review on novel process of biodiesel production from waste cooking oil. Appl. Energy 2013, 104, 683–710. [Google Scholar] [CrossRef]
- Schuchardt, U.; Sercheli, R.; Vargas, R.M. Transesterification of Vegetable Oils: A Review. J. Braz. Chem. Soc. 1998, 9, 199–210. [Google Scholar] [CrossRef]
- Canakci, M.; Gerpen, J.V. A pilot plant to produce biodiesel from high free fatty acid feedstocks. Trans. ASAE 2003, 46, 945–954. [Google Scholar] [CrossRef]
- Gerpen, J.V. Biodiesel processing and production. Fuel Process Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Jeong, G.T.; Park, D.H. Batch (one- and two-stage) production of biodiesel fuel from rapeseed oil. Biotechnol. Appl. Biochem. 1996, 131, 668–679. [Google Scholar] [CrossRef]
- Bournay, L.; Cassanave, D.; Delfort, B.; Hillion, G.; Chadorge, J.A. New heterogeneous process for biodiesel production: A way to improve the quality and the value of the crude glycerin produced by biodiesel plants. Catal. Today 2005, 106, 190–192. [Google Scholar] [CrossRef]
- Muhammad, N.; Elsheikh, Y.A.; Mutalib, M.I.A.; Bazmi, A.A.; Khan, R.A.; Khan, H.; Rafiq, S.; Man, Z.; Khan, I. An overview of the role of ionic liquids in biodiesel reactions. J. Ind. Eng. Chem. 2015, 21, 1–10. [Google Scholar] [CrossRef]
- Dossin, T.F.; Reyniers, M.F.; Berger, R.J.; Marin, G.B. Simulation of heterogeneously MgO-catalyzed transesterification for fine-chemical and biodiesel industrial production. Appl. Catal. B Environ. 2006, 67, 136–148. [Google Scholar] [CrossRef]
- Freedman, B.; Pryde, E.H.; Mounts, T.L. Variables affecting the yield of fatty esters from transesterified vegetable oils. J. Am. Oil Chem. Soc. 1984, 61, 1638–1643. [Google Scholar] [CrossRef]
- Cerce, T.; Peter, S.; Weidner, E. Biodiesel-transesterification of biological oils with liquid catalysts: Thermodynamic properties of oil-methanol-amine mixtures. Ind. Eng. Chem. Res. 2005, 44, 9535–9541. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, L.; Yang, J. Transesterification of rapeseed oil catalyzed by liquid organic amine in supercritical methanol in a continuous tubular-flow reactor. Eur. J. Lipid Sci. Technol. 2008, 110, 747–753. [Google Scholar] [CrossRef]
- Stavarache, C.; Vinatoru, M.; Maeda, Y. Aspect of ultrasonically assisted transesterification of various vegetable oils with methanol. Ultrason. Sonochem. 2007, 14, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Omotola, B.; Leslie, P.; Bamikole, A.; Farouk, A. Low-Cost feedstock conversion to biodiesel via ultrasound technology. Energies 2009, 3, 1691–1703. [Google Scholar]
- Kumar, D.; Kumar, G.; Poonam Singh, C.P. Ultrasonic-assisted transeterification of jatropha oil using solid catalyst, Na/SiO2. Ultrason. Sonochem. 2010, 17, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Stavarache, C.; Vinatoru, M.; Nishimura, R.; Maeda, Y. Conversion of vegetable oil to Biodiesel using ultrasonic irradiation. Chem. Lett. 2003, 32, 716–717. [Google Scholar] [CrossRef]
- Taleyarkhan, R.P.; Cho, J.S.; West, C.D.; Nigmatulin, R.I.; Block, R.C. Additional evidence of nuclear emissions during acoustic cavitation. Phys. Rev. 2004, 69, 361–369. [Google Scholar]
- Encinar, J.M.; González, J.F.; Rodríguez, J.J.; Tejedor, A. Biodiesel fuels from vegetable oils: Transesterification of Cynara cardunculus L. Oils with Ethanol. Energy Fuels 2002, 16, 443–450. [Google Scholar] [CrossRef]
- Encinar, J.M.; Juan, F.; Gonzalez, J.F.; Rodriguez, J.R. Biodiesel from Used Frying oil: Variables Affecting the Yields and Characteristics of the Biodiesel. Ind. Eng. Chem. Res. 2005, 44, 5491–5499. [Google Scholar] [CrossRef]
- Hanh, H.D.; Dong, N.T.; Okitsu, K.; Nishimura, R.; Maeda, Y. Biodiesel production through transesterification of triolein with various alcohols in an ultrasonic field. Renew. Energy 2009, 34, 766–768. [Google Scholar] [CrossRef]
- Kotrba, R. Ultrasonic biodiesel processing. Biodiesel Magazine, 19 May 2010. [Google Scholar]
- Shinde, K.; Nohair, B.; Kaliaguine, S. A Parametric Study of Biodiesel Production Under Ultrasounds. Int. J. Chem. React. Eng. 2017, 15, 117–125. [Google Scholar] [CrossRef]
- Singh, A.K.; Fernando, S.D.; Hernandez, R. Base-catalyzed fast transesterification of soybean oil using ultrasonication. Energy Fuels 2007, 21, 1161–1164. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, G.; Poonam; Singh, C.P. Fast, easy ethanolysis of coconut oil for biodiesel production assisted by ultrasonication. Ultrason. Sonochem. 2010, 17, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Boffito, D.C.; Galli, F.; Martinez, P.R.; Pirola, C.; Bianchi, C.L.; Patience, G.S. Transesterification of Triglycerides in a New Ultrasonic-Assisted Mixing Device. Chem. Eng. Trans. 2015, 43. [Google Scholar] [CrossRef]
- Martinez-Guerra, E.; Gude, V.G. Determining optimum pulse mode for ultrasound enhanced biodiesel production. J. Ind. Eng. Chem. 2016, 35, 14–19. [Google Scholar] [CrossRef]
- Shinde, K.; Kaliaguine, S. Triglycerides Transesterification Reactions under Ultrasounds. ChemistrySelect 2016, 1, 6008–6010. [Google Scholar] [CrossRef]
- Reyman, D.; Saiz Bermejo, A.; Ramirez Uceda, I.; Rodriguez Gamero, M. A new FTIR method to monitor transesterification in biodiesel production by ultrasonication. Environ. Chem. Lett. 2014, 12, 235–240. [Google Scholar] [CrossRef]
- Maliverney, C.; Sur Bibost, T.; Ireland, S.J. Method for Sealing and Assembling Components of a Drive Train. U.S. Patent 8,470,950 B2, 25 June 2013. [Google Scholar]
- Schuchardt, U.; Vargas, R.M.; Gelbard, G. Alkylguanidines as catalysts for the transesterification of rapeseed oil. J. Mol. Catal. A Chem. 1995, 99, 65–70. [Google Scholar] [CrossRef]
- Bautista, L.F.; Vicente, G.; Rodrıguez, R.; Pacheco, M. Optimisation of FAME production from waste cooking oil for biodiesel use. Biomass Bioenergy 2009, 33, 862–872. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Singh, B.; Upadhyay, S.N. Advancements in development and characterization of biodiesel: A review. Fuel 2008, 87, 2355–2373. [Google Scholar] [CrossRef]
| Designation | Formula | Designation | pKa |
|---|---|---|---|
| A | Propyl-2,3-dicyclohexyl guanidine | PCHG | 30.7 |
| B | 1,3-dicyclohexyl 2 n-octyl guanidine | DCOG | 31.1 |
| C | 1,1,3,3 tetramethyl guanidine | TMG | 23.16 |
| D | 1,3 diphenyl guanidine | DPG | 10.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinde, K.; Kaliaguine, S. A Comparative Study of Ultrasound Biodiesel Production Using Different Homogeneous Catalysts. ChemEngineering 2019, 3, 18. https://doi.org/10.3390/chemengineering3010018
Shinde K, Kaliaguine S. A Comparative Study of Ultrasound Biodiesel Production Using Different Homogeneous Catalysts. ChemEngineering. 2019; 3(1):18. https://doi.org/10.3390/chemengineering3010018
Chicago/Turabian StyleShinde, Kiran, and Serge Kaliaguine. 2019. "A Comparative Study of Ultrasound Biodiesel Production Using Different Homogeneous Catalysts" ChemEngineering 3, no. 1: 18. https://doi.org/10.3390/chemengineering3010018
APA StyleShinde, K., & Kaliaguine, S. (2019). A Comparative Study of Ultrasound Biodiesel Production Using Different Homogeneous Catalysts. ChemEngineering, 3(1), 18. https://doi.org/10.3390/chemengineering3010018