Photodegradation of Stearic Acid Adsorbed on Copper Oxide Heterojunction Thin Films Prepared by Magnetron Sputtering
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
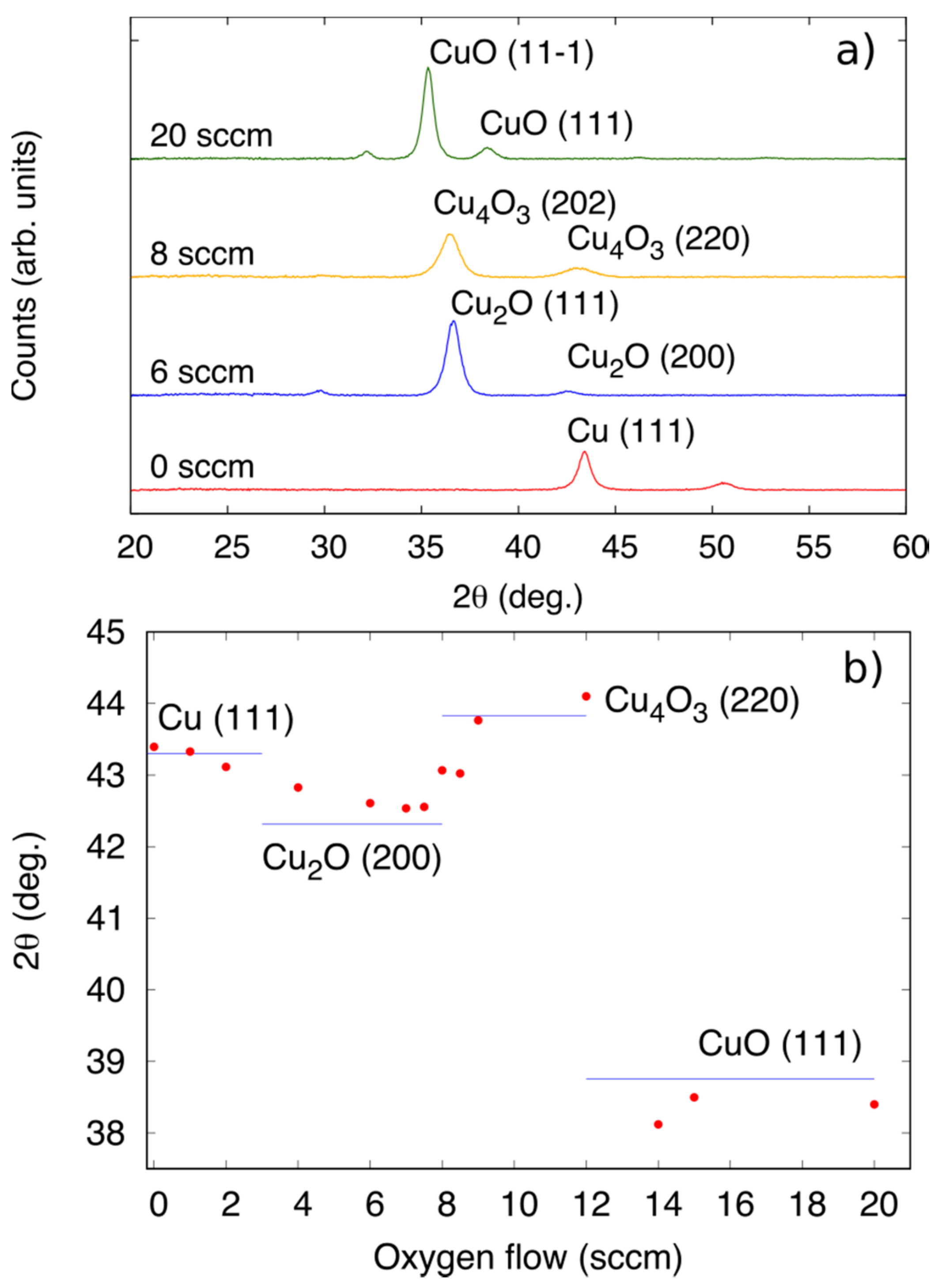
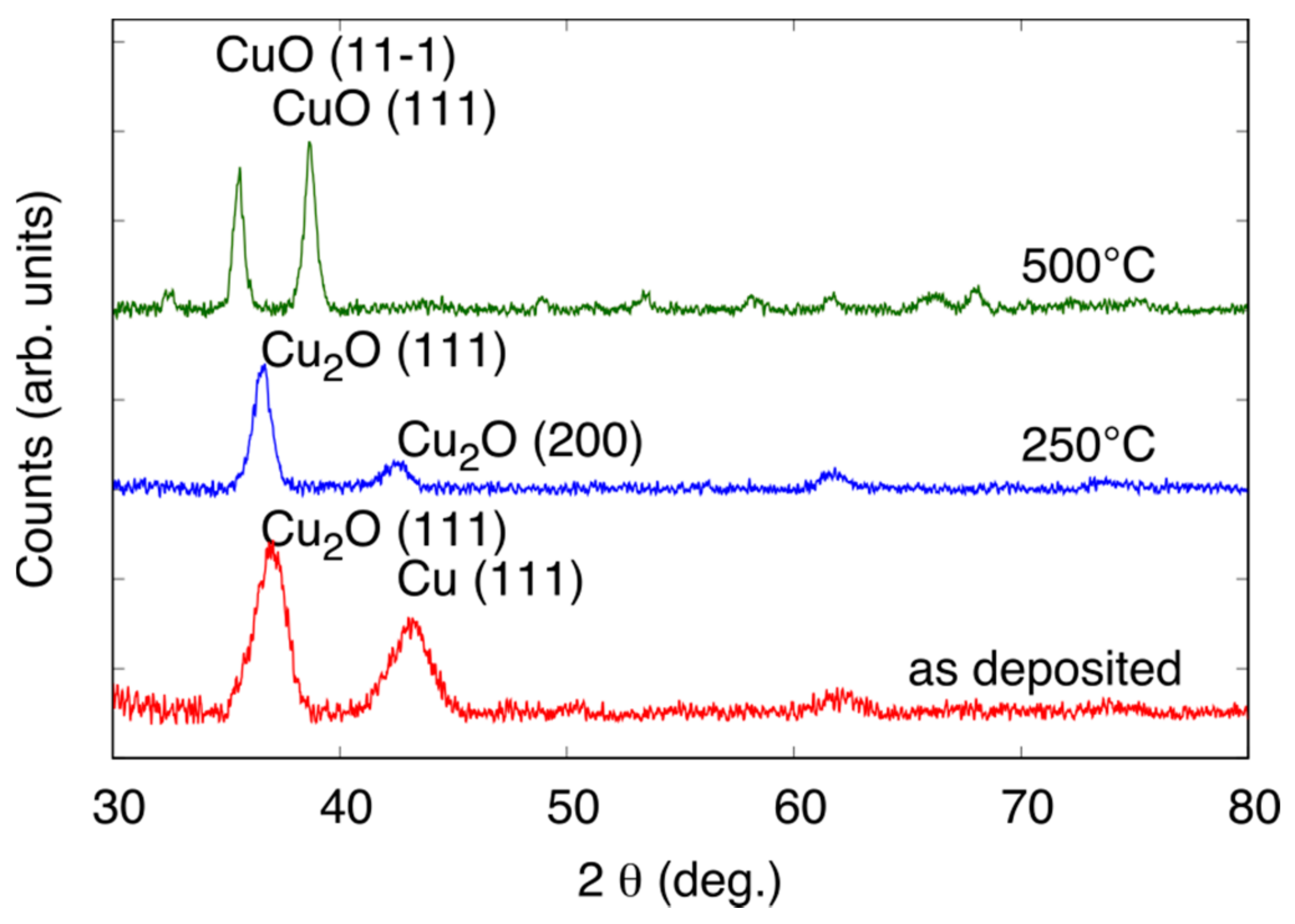
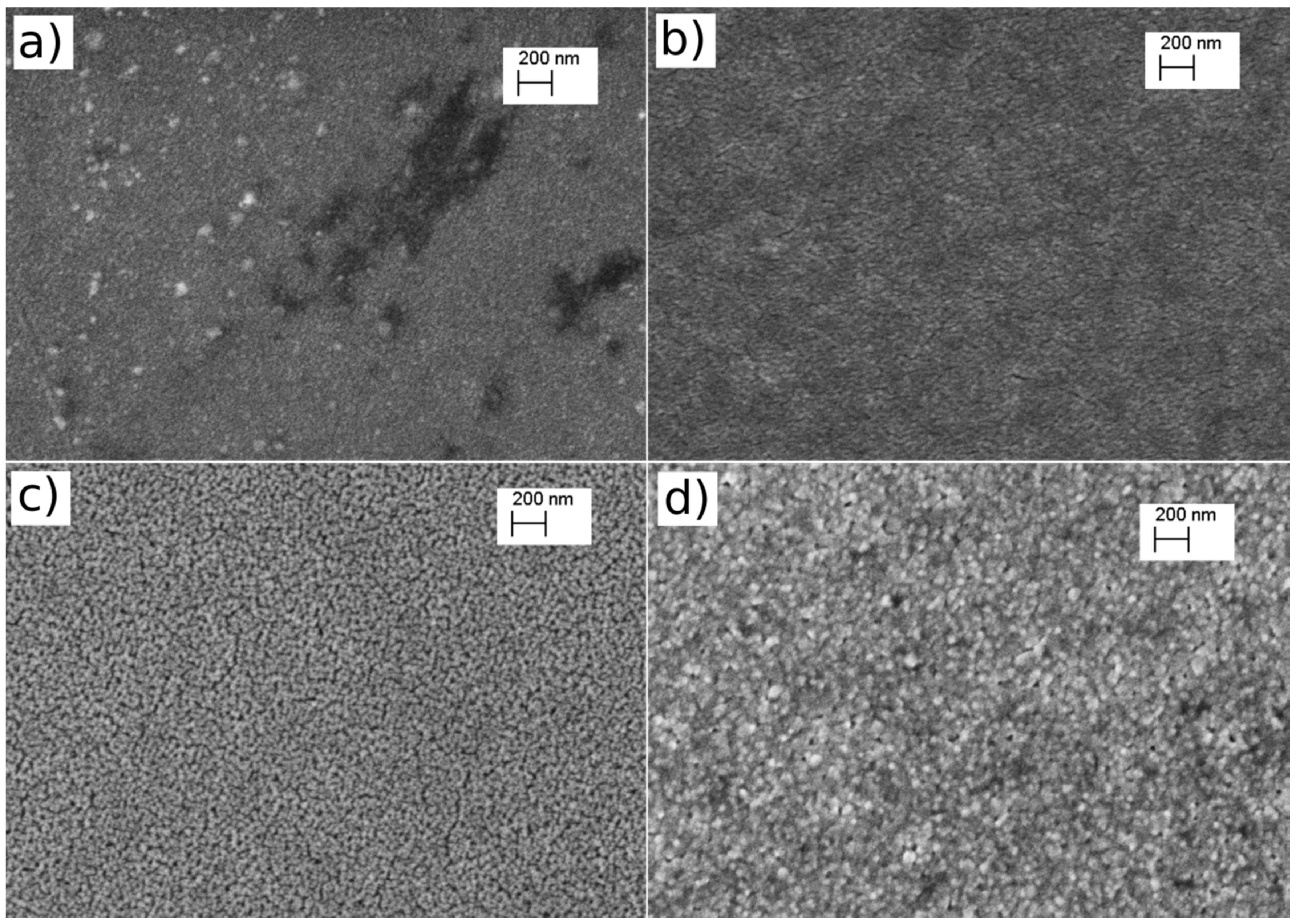
3.1. Deposition of CuxOy by Magnetron Sputtering
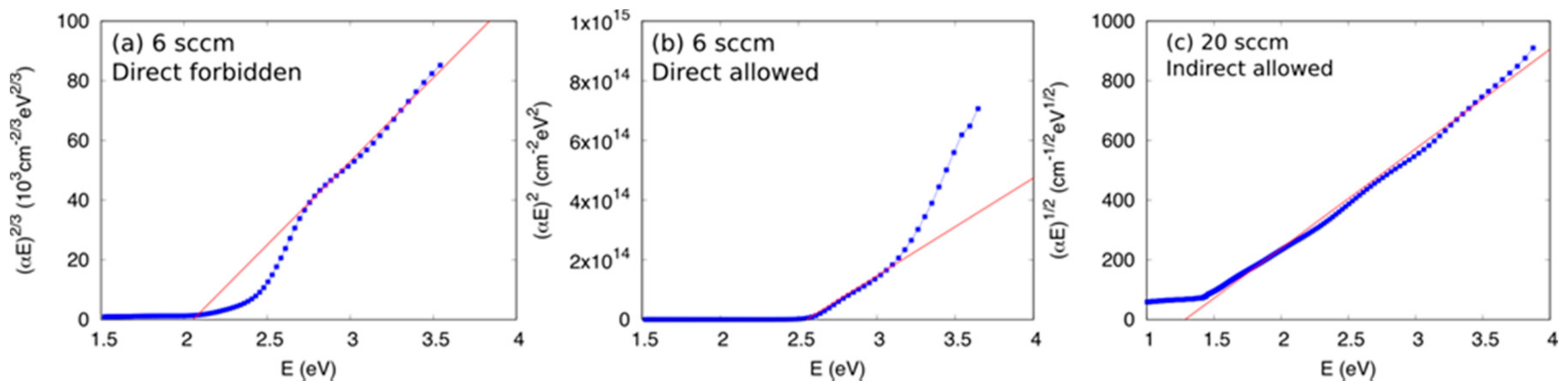
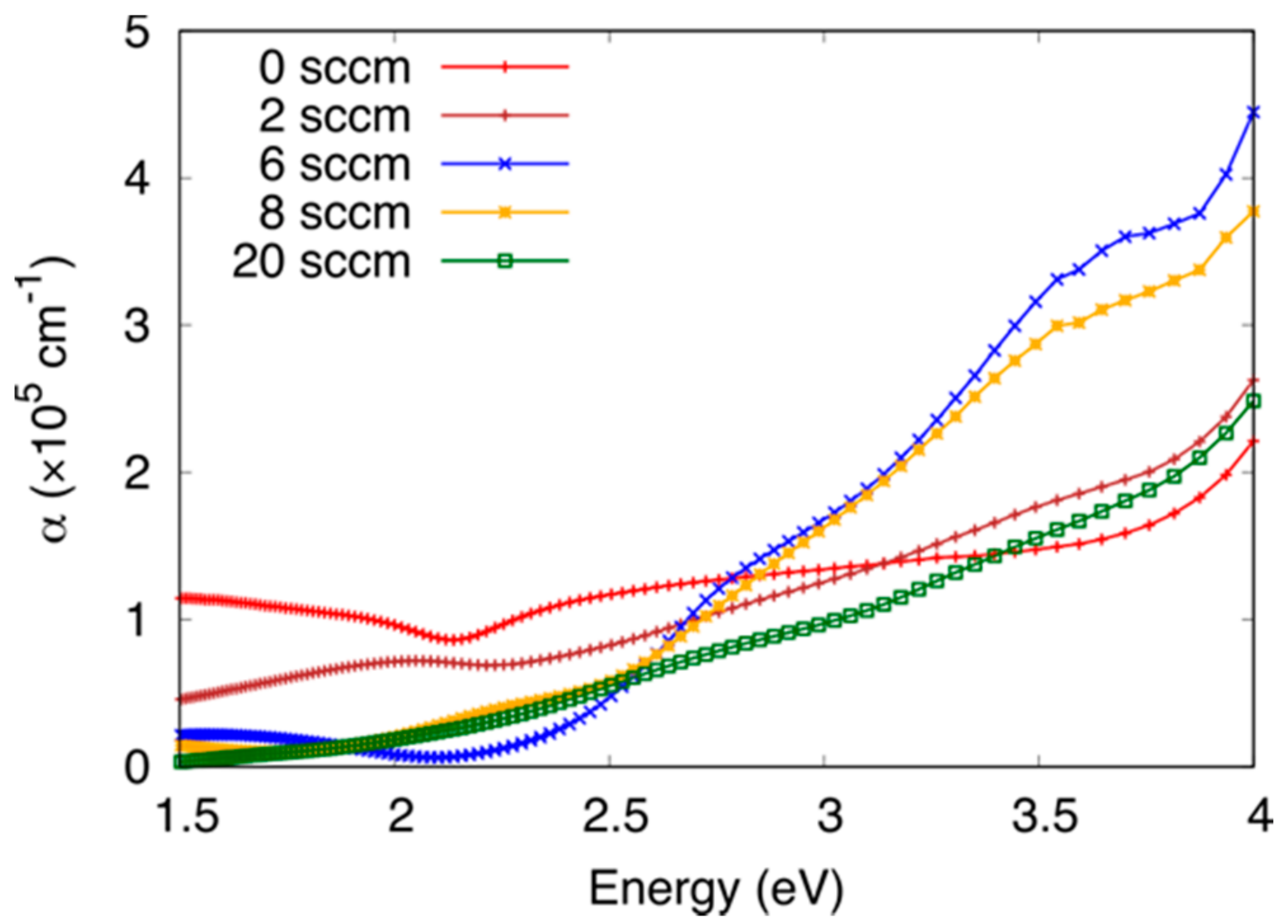
3.2. Optical Properties
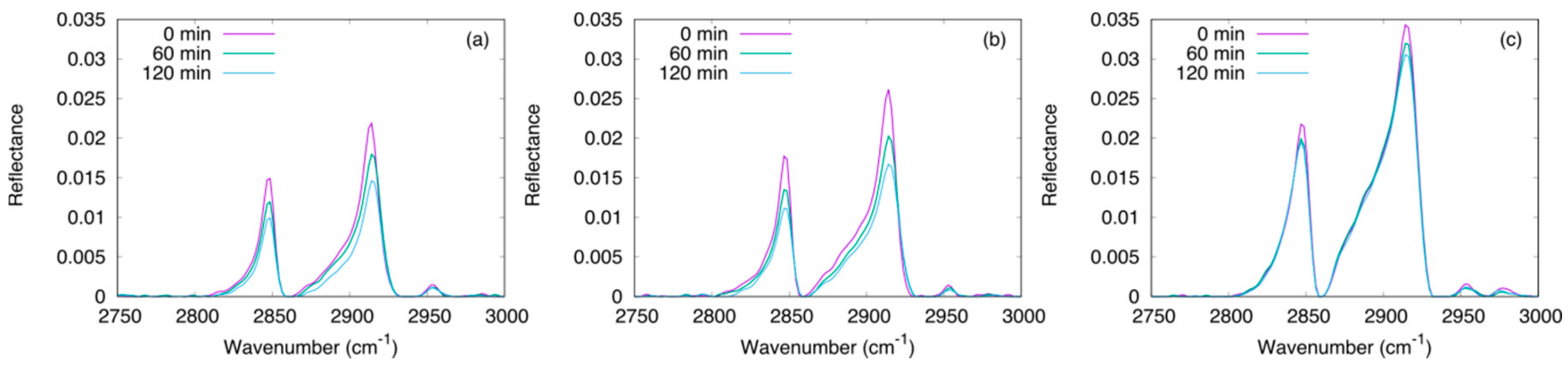
3.3. Photodegradation of Stearic Acid
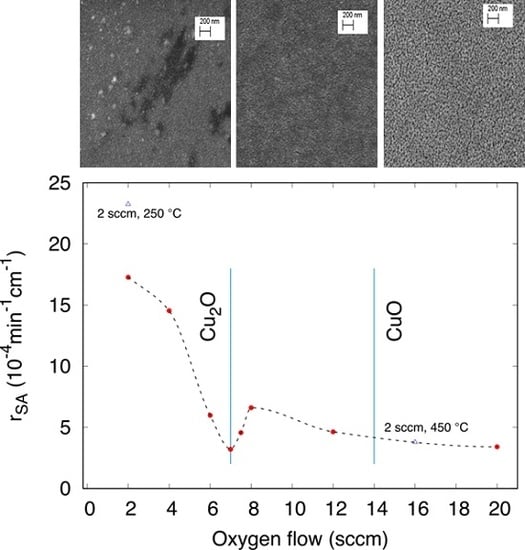
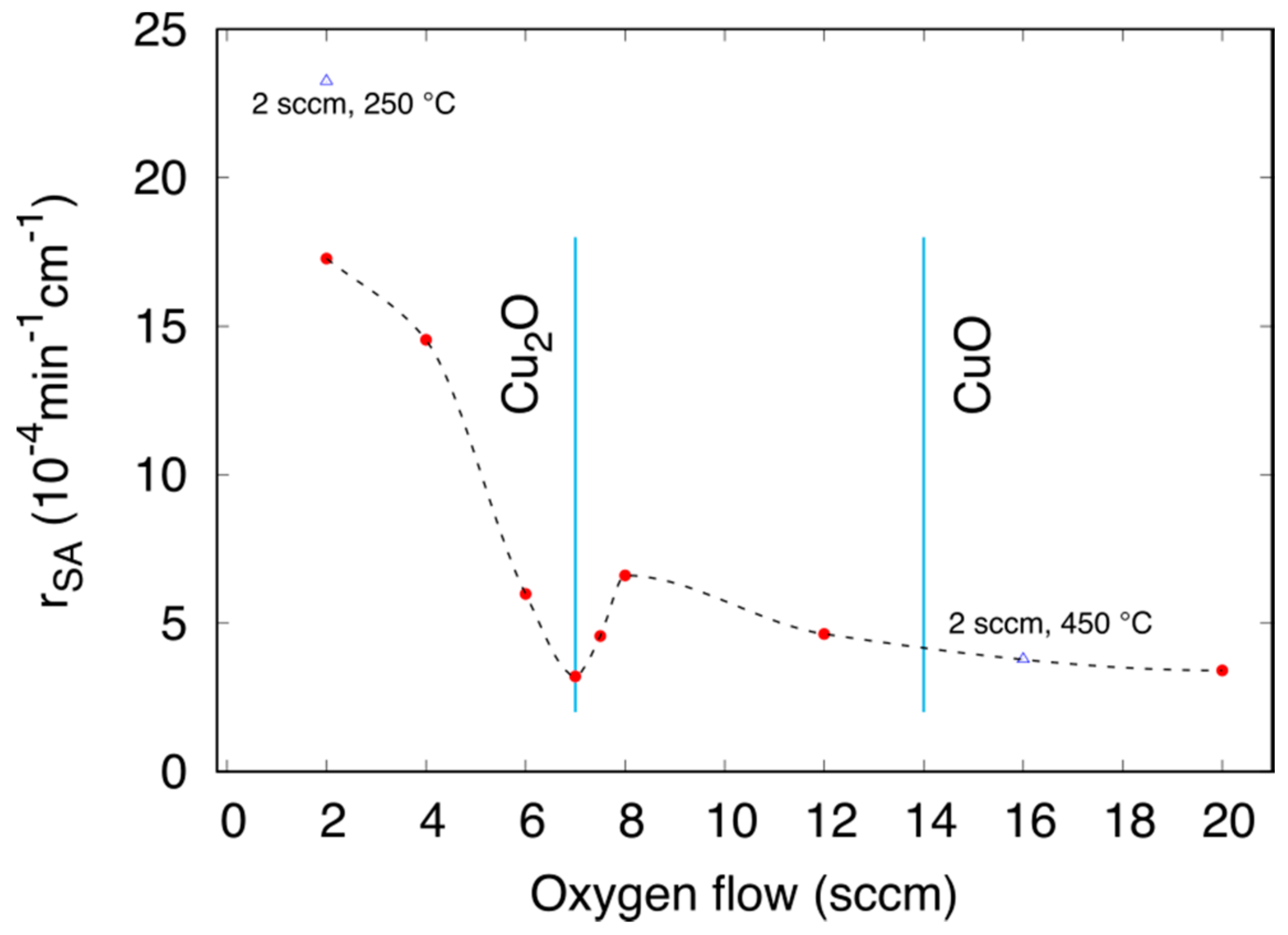
3.3.1. Effect of the Oxygen Flux
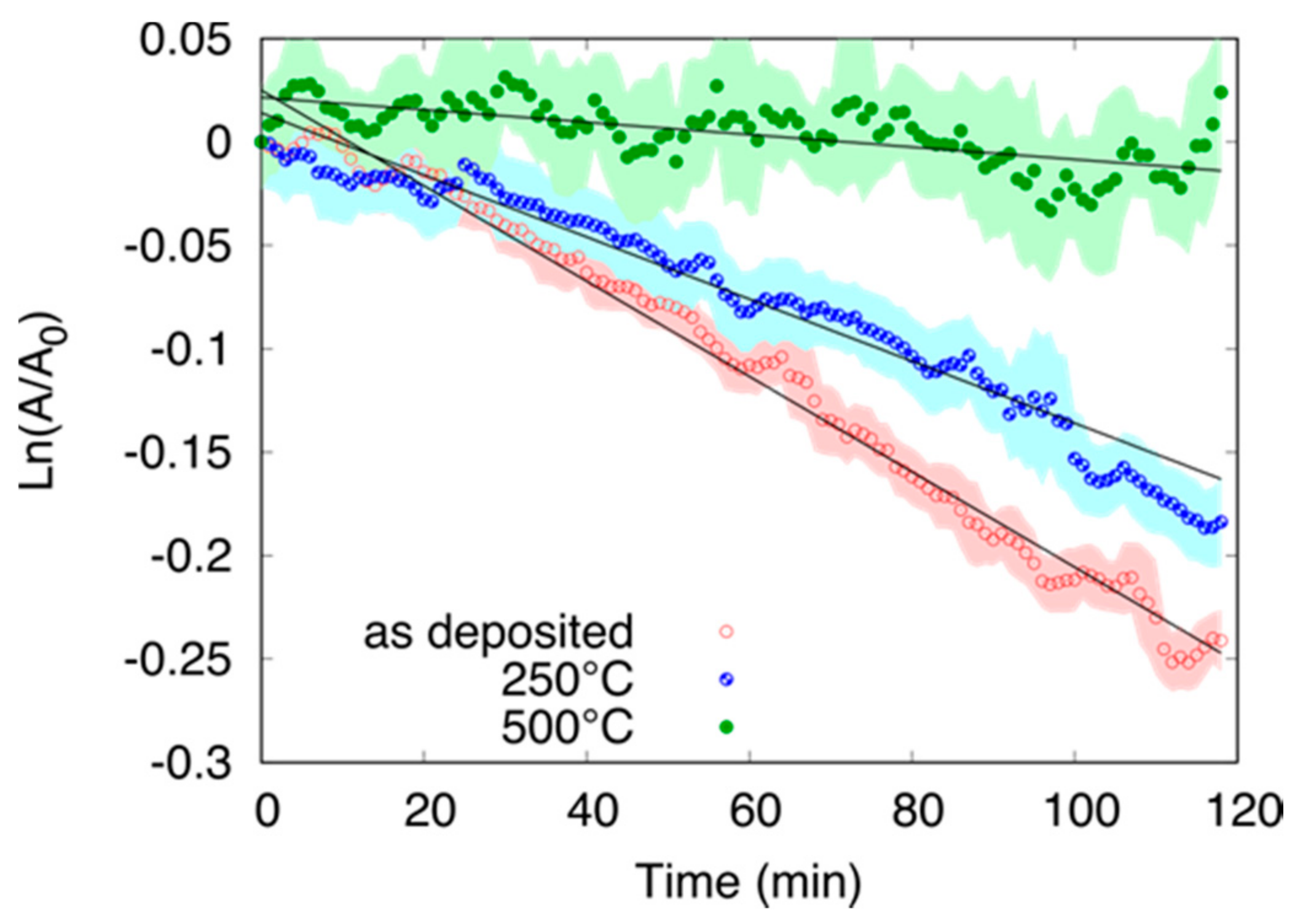
3.3.2. Effect of the Annealing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hisatomi, T.; Kubota, J.; Domen, K. Recent Advances in Semiconductors for Photocatalytic and Photoelectrochemical Water Splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Serpone, N.; Emeline, A.V. Suggested Terms and Definitions in Photocatalysis and Radiocatalysis. Int. J. Photoenergy 2002, 4, 91–131. [Google Scholar] [CrossRef]
- Basov, L.L.; Kuzmin, G.N.; Prudnikov, I.M.; Solonitsyn, Y.P. Photoadsorption Processes on Metal Oxides; Vilesov, T.H.I., Ed.; Leningrad State University: Leningrad, Russia, 1976. [Google Scholar]
- Serpone, N.; Emeline, A.V. Modelling Heterogeneous Photocatalysis by Metal-Oxide Nanostructured Semiconductor and Insulator Materials: Factors That Affect the Activity and Selectivity of Photocatalysts. Res. Chem. Intermed. 2005, 31, 391–432. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous Photocatalyst Materials for Water Splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.S.; Hu, J.S.; Liang, H.P.; Cao, A.M.; Song, W.G.; Wan, L.J. Self-Assembled 3D Flowerlike Iron Oxide Nanostructures and Their Application in Water Treatment. Adv. Mater. 2006, 18, 2426–2431. [Google Scholar] [CrossRef]
- Vayssieres, L.; Sathe, C.; Butorin, S.M.; Shuh, D.K.; Nordgren, J.; Guo, J. One-Dimensional Quantum-Confinement Effect in α-Fe2O3 Ultrafine Nanorod Arrays. Adv. Mater. 2005, 17, 2320–2323. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical Photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Kondo, T.; Komoda, M.; Ikeda, S.; Shinohara, K.; Tanaka, A.; Kondo, J.N.; Domen, K.; Hara, M.; Shinohara, K.; et al. Cu2O as a Photocatalyst for Overall Water Splitting under Visible Light Irradiation. Chem. Commun. 1998, 2, 357–358. [Google Scholar] [CrossRef]
- Huang, W.C.; Lyu, L.M.; Yang, Y.C.; Huang, M.H. Synthesis of Cu2O Nanocrystals from Cubic to Rhombic Dodecahedral Structures and Their Comparative Photocatalytic Activity. J. Am. Chem. Soc. 2012, 134, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, B.; Zhang, T.; Gao, D.; Xu, A. Shape Effects of Cu2O Polyhedral Microcrystals on Photocatalytic Activity. J. Phys. Chem. C 2010, 114, 5073–5079. [Google Scholar] [CrossRef]
- Lim, Y.F.; Chua, C.S.; Lee, C.J.J.; Chi, D. Sol-Gel Deposited Cu2O and CuO Thin Films for Photocatalytic Water Splitting. Phys. Chem. Chem. Phys. 2014, 16, 25928–25934. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Fornasiero, P.; Gasparotto, A.; Gombac, V.; Maccato, C.; Montini, T.; Tondello, E. The Potential of Supported Cu2O and CuO Nanosystems in Photocatalytic H2 Production. ChemSusChem 2009, 2, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Sadollahkhani, A.; Hussain Ibupoto, Z.; Elhag, S.; Nur, O.; Willander, M. Photocatalytic Properties of Different Morphologies of CuO for the Degradation of Congo Red Organic Dye. Ceram. Int. 2014, 40, 11311–11317. [Google Scholar] [CrossRef]
- Wijesundera, R.P. Fabrication of the CuO/Cu2O Heterojunction Using an Electrodeposition Technique for Solar Cell Applications. Semicond. Sci. Technol. 2010, 25, 045015. [Google Scholar] [CrossRef]
- Li, H.; Su, Z.; Hu, S.; Yan, Y. Free-Standing and Flexible Cu/Cu2O/CuO Heterojunction Net: A Novel Material as Cost-Effective and Easily Recycled Visible-Light Photocatalyst. Appl. Catal. B Environ. 2017, 207, 134–142. [Google Scholar] [CrossRef]
- Jiang, D.; Xue, J.; Wu, L.; Zhou, W.; Zhang, Y.; Li, X. Photocatalytic Performance Enhancement of CuO/Cu2O Heterostructures for Photodegradation of Organic Dyes: Effects of CuO Morphology. Appl. Catal. B Environ. 2017, 211, 199–204. [Google Scholar] [CrossRef]
- Thornton, J.A. Structure and Topography of Sputtering Coatings. Ann. Rev. Mater. Sci. 1977, 7, 239–260. [Google Scholar] [CrossRef]
- Le Bellac, D.; Niklasson, G.A.; Granqvist, C.G. Angular-Selective Optical Transmittance of Anisotropic Inhomogeneous Cr-Based Films Made by Sputtering. J. Appl. Phys. 1995, 77, 6145–6151. [Google Scholar] [CrossRef]
- Safi, I. Recent Aspects Concerning DC Reactive Magnetron Sputtering of Thin Films: A Review. Surf. Coatings Technol. 2000, 127, 203–218. [Google Scholar] [CrossRef]
- Kadlec, S.; Musil, J.; Vyskocil, H. Hysteresis Effect in Reactive Sputtering: A Problem of System Stability. J. Phys. D. Appl. Phys. 1986, 19. [Google Scholar] [CrossRef]
- Hong, W.Q. Extraction of Extinction Coefficient of Weak Absorbing Thin Films from Special Absorption. J. Phys. D. Appl. Phys. 1989, 22, 1384–1385. [Google Scholar] [CrossRef]
- Roos, A. Use of an Integrating Sphere in Solar Energy Research. Sol. Energy Mater. Sol. Cells 1993, 30, 77–94. [Google Scholar] [CrossRef]
- Wang, Y.; Lany, S.; Ghanbaja, J.; Fagot-Revurat, Y.; Chen, Y.P.; Soldera, F.; Horwat, D.; Mücklich, F.; Pierson, J.F. Electronic Structures of Cu2O, Cu4O3, and CuO: A Joint Experimental and Theoretical Study. Phys. Rev. B 2016, 94, 245418. [Google Scholar] [CrossRef]
- Meyer, B.K.; Polity, A.; Reppin, D.; Becker, M.; Hering, P.; Klar, P.J.; Sander, T.; Reindl, C.; Benz, J.; Eickhoff, M.; et al. Binary Copper Oxide Semiconductors: From Materials towards Devices. Phys. Status Solidi 2012, 249, 1487–1509. [Google Scholar] [CrossRef]
- Meyer, B.K.; Polity, A.; Reppin, D.; Becker, M.; Hering, P.; Kramm, B.; Klar, P.J.; Sander, T.; Reindl, C.; Heiliger, C.; et al. The Physics of Copper Oxide (Cu2O). Semicond. Semimet. 2013, 88, 201–226. [Google Scholar]
- Shalimova, V.K. Fisica de Los Semiconductores; MIR: Moscow, Russia, 1975. [Google Scholar]
- Roberts, S. Optical Properties of Copper. Phys. Rev. 1960, 118, 1509. [Google Scholar] [CrossRef]
- Sawunyama, P.; Jiang, L.; Fujishima, A.; Hashimoto, K. Photodecomposition of a Langmuir-Blodgett Film of Stearic Acid on TiO2 Film Observed by in Situ Atomic Force Microscopy and FT-IR. J. Phys. Chem. B 1997, 101, 11000–11003. [Google Scholar] [CrossRef]
- Querry, M.R. Optical Constants, Contractor Report; US Army Chemical Research, Development and Engineering Center (CRDC): Aberdeen Proving Ground, MD, USA, 1985.
- Mattsson, A.; Hu, S.; Hermansson, K.; Österlund, L. Adsorption of Formic Acid on Rutile TiO2 (110) Revisited: An Infrared Reflection-Absorption Spectroscopy and Density Functional Theory Study. J. Chem. Phys. 2014, 140, 034705. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Lepre, A.; Elliott, N.; Bhopal, S.; Parkin, I.P.; O’Neill, S.A. Characterisation of the Photocatalyst Pilkington ActivTM: A Reference Film Photocatalyst? J. Photochem. Photobiol. A Chem. 2003, 160, 213–224. [Google Scholar] [CrossRef]
- Johansson, M.B.; Niklasson, G.A.; Österlund, L. Structural and Optical Properties of Visible Active Photocatalytic WO3 Thin Films Prepared by Reactive Dc Magnetron Sputtering. J. Mater. Res. 2012, 27, 3130–3140. [Google Scholar] [CrossRef]
- Mills, A.; Wang, J. Simultaneous Monitoring of the Destruction of Stearic Acid and Generation of Carbon Dioxide by Self-Cleaning Semiconductor Photocatalytic Films. J. Photochem. Photobiol. A Chem. 2006, 182, 181–186. [Google Scholar] [CrossRef]
- Balamurugan, B.; Mehta, B.R. Optical and Structural Properties of Nanocrystalline Copper Oxide Thin Films Prepared by Activated Reactive Evaporation. Thin Solid Films 2001, 396, 90–96. [Google Scholar] [CrossRef]

| Oxygen Flow (sccm) | PO2 (×10−3 mbar) | Growth Rate (nm/min) | Thickness (nm) | Discharge Current (mA) | Grain Size (Å) |
|---|---|---|---|---|---|
| 0 | 0.0 | 80 | 200 | 569 | 118.4 |
| 2 | 0.0 | 36.4 | 100 | 475 | 53.6 |
| 4 | 1.6 | 23.4 | 215 | 465 | 90.2 |
| 6 | 2.3 | 25.7 | 180 | 444 | 97.4 |
| 7 | 2.1 | 24.1 | 193 | 458 | 100.4 |
| 7.5 | 3.1 | 25.9 | 207 | 459 | 104.9 |
| 8 | 3.3 | 22.2 | 200 | 441 | 74.4 |
| 8.5 | 4.0 | 23.2 | 209 | 460 | 78.5 |
| 12 | 4.2 | 28.4 | 199 | 439 | 104.3 |
| 15 | 5.5 | 23.5 | 190 | 435 | 124.5 |
| 20 | 8.3 | 22.9 | 218 | 420 | 129.5 |
| O2 (sccm) | k (×10−3 min−1) | Molecules (×1015 cm−2) | Absorbed Photons (×1016 cm−2 s−1) | Φ (×10−6) |
|---|---|---|---|---|
| 2 | 2.30 | 7.29 | 7.96 | 3.51 |
| 2 (250 °C) | 1.50 | 15.04 | 4.30 | 8.31 |
| 2 (450 °C) | 0.30 | 12.20 | 6.10 | 1.00 |
| 4 | 1.70 | 8.30 | 6.46 | 3.64 |
| 6 | 0.70 | 8.30 | 5.34 | 1.81 |
| 7 | 0.50 | 6.22 | 5.56 | 0.93 |
| 7.5 | 0.80 | 5.54 | 5.60 | 1.32 |
| 8 | 1.90 | 3.37 | 6.40 | 1.67 |
| 9 | 0.40 | 4.48 | 6.78 | 0.44 |
| 12 | 0.60 | 7.50 | 6.47 | 1.16 |
| 20 | 0.20 | 16.50 | 6.63 | 0.83 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero, J.; Österlund, L. Photodegradation of Stearic Acid Adsorbed on Copper Oxide Heterojunction Thin Films Prepared by Magnetron Sputtering. ChemEngineering 2018, 2, 40. https://doi.org/10.3390/chemengineering2030040
Montero J, Österlund L. Photodegradation of Stearic Acid Adsorbed on Copper Oxide Heterojunction Thin Films Prepared by Magnetron Sputtering. ChemEngineering. 2018; 2(3):40. https://doi.org/10.3390/chemengineering2030040
Chicago/Turabian StyleMontero, José, and Lars Österlund. 2018. "Photodegradation of Stearic Acid Adsorbed on Copper Oxide Heterojunction Thin Films Prepared by Magnetron Sputtering" ChemEngineering 2, no. 3: 40. https://doi.org/10.3390/chemengineering2030040
APA StyleMontero, J., & Österlund, L. (2018). Photodegradation of Stearic Acid Adsorbed on Copper Oxide Heterojunction Thin Films Prepared by Magnetron Sputtering. ChemEngineering, 2(3), 40. https://doi.org/10.3390/chemengineering2030040