Euler–Lagrange Modeling of Bubbles Formation in Supersaturated Water
Abstract
1. Introduction
2. Model Description
2.1. Liquid Dynamics
2.2. Bubble Dynamics
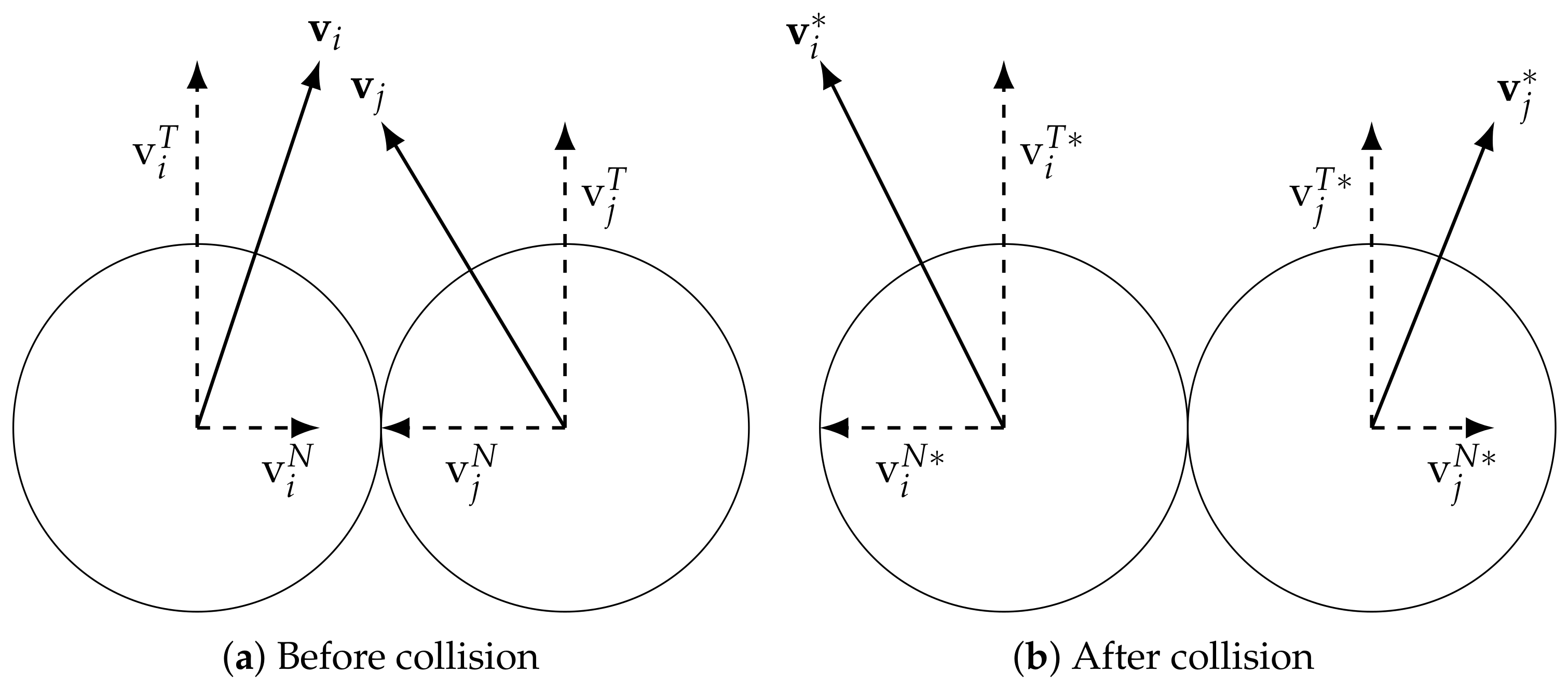
2.2.1. Bubble Interactions
2.3. Species Transport and Mass Transfer
2.4. Verification
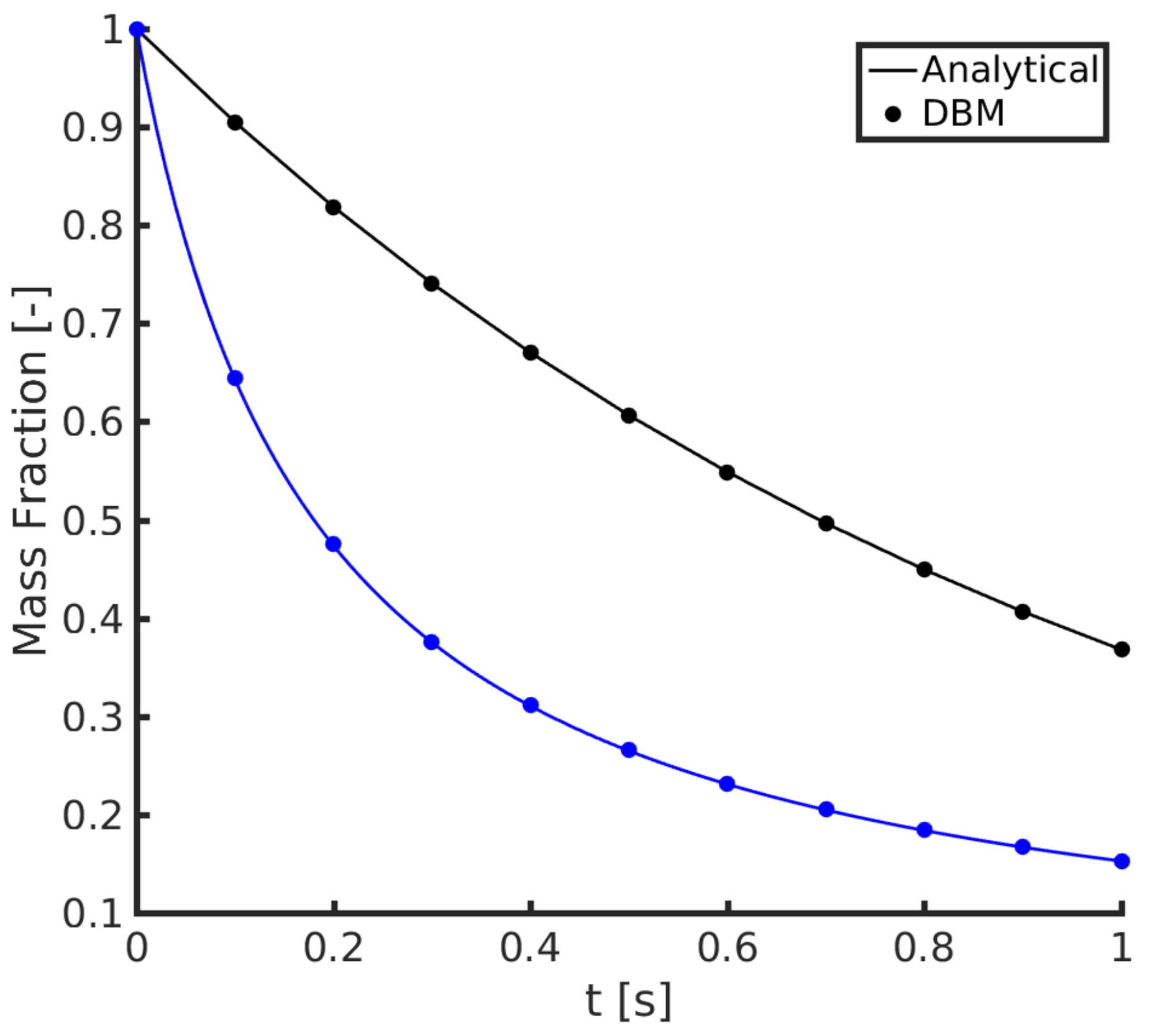
2.4.1. 1D Convection-Diffusion
2.4.2. Batch Reaction
2.5. Phase Transition
2.5.1. Theoretical Overview
- Type I:
- The classical homogeneous nucleation, i.e., the spontaneous creation of a bubble in the liquid bulk. This mechanisms is characterized by the high energy requirements to form a new gas–liquid interface and the necessity to have enough gas molecules in close proximity to each other. For these reasons, only a system which exceeds a supersaturation ratio of 100 is able to form bubbles, which is extremely large. For example, for common drinks such as soda and champagne, typically ranges from 2 to 5, which would mean it is impossible to have Type I nucleations for such systems [6,46].
- Type II:
- The classical heterogeneous nucleation. This mechanism assumes that the new interface is created in small cavities or imperfections at a solid surface (such as a wall or suspended impurities). However, a new interface still has to be created, so that supersaturation levels comparable with the homogeneous nucleation are required.
- Type III/IV:
- Pseudo-classical and non-classical nucleation, respectively. These two types represent the vast majority of the nucleation events in liquids with low supersaturation. Both mechanisms account for a pre-existing gas pocket, for instance as a consequence of another type of nucleation event (e.g., Type II) or simply because of the inability of the liquid to fully fill cavities on a surface. The difference between these two types lies in the geometry of the cavity. It is worth mentioning that the energy requirement for both types is respectively low and zero, meaning that Type IV is the one responsible for the long term cycle of bubble production in carbonated drinks. In this case, the production of bubbles will continue until the critical radius, increasing as a consequence of the depletion of gas in the liquid, reaches a value equal to the radius of the cavity meniscus and thus nucleation stops.
2.5.2. Initialization
2.5.3. Nucleation
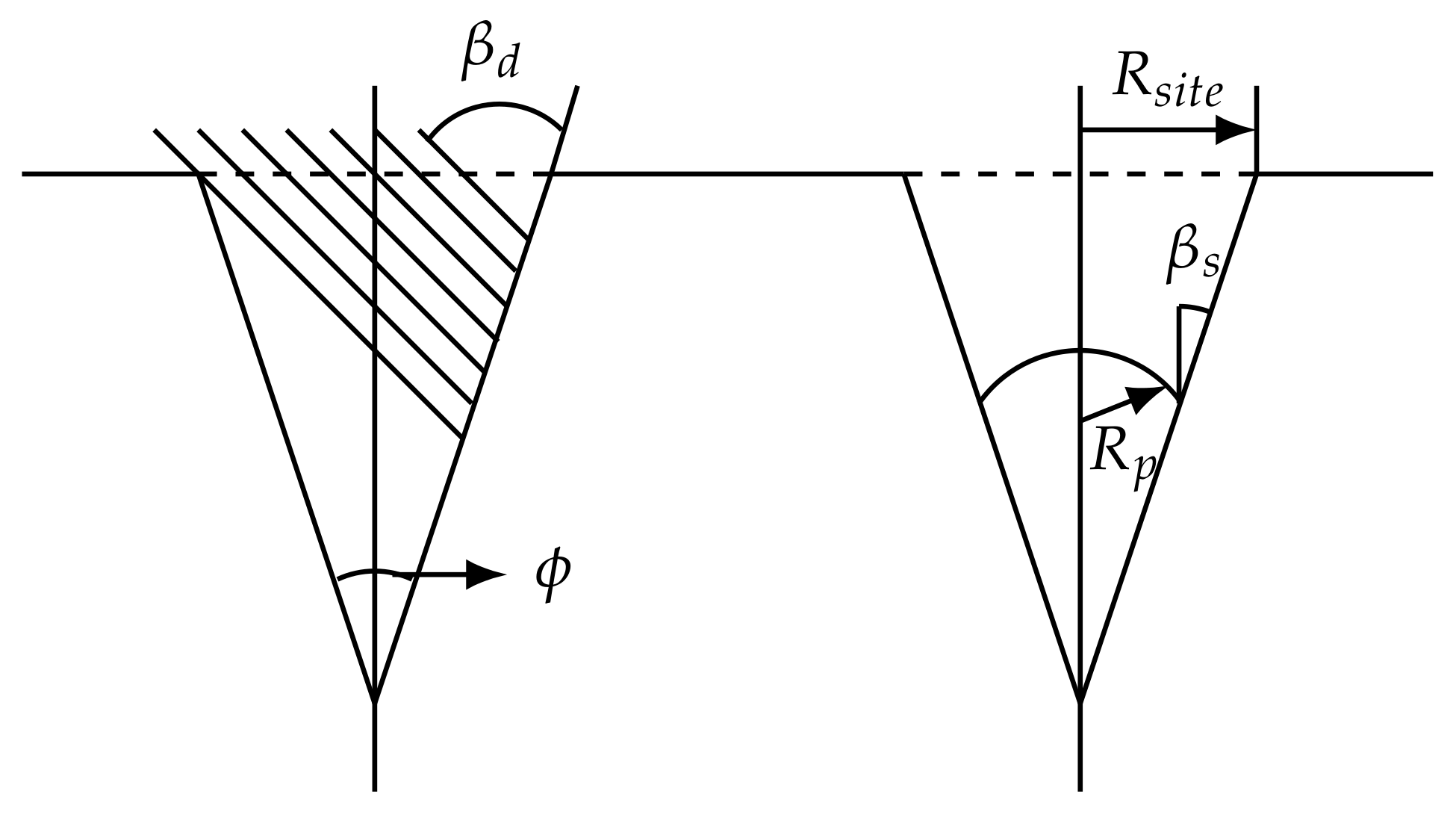
2.5.4. Detachment
2.5.5. Limitations and Assumptions
2.5.6. Summary of the Implementation
3. Results
3.1. Numerical Setup
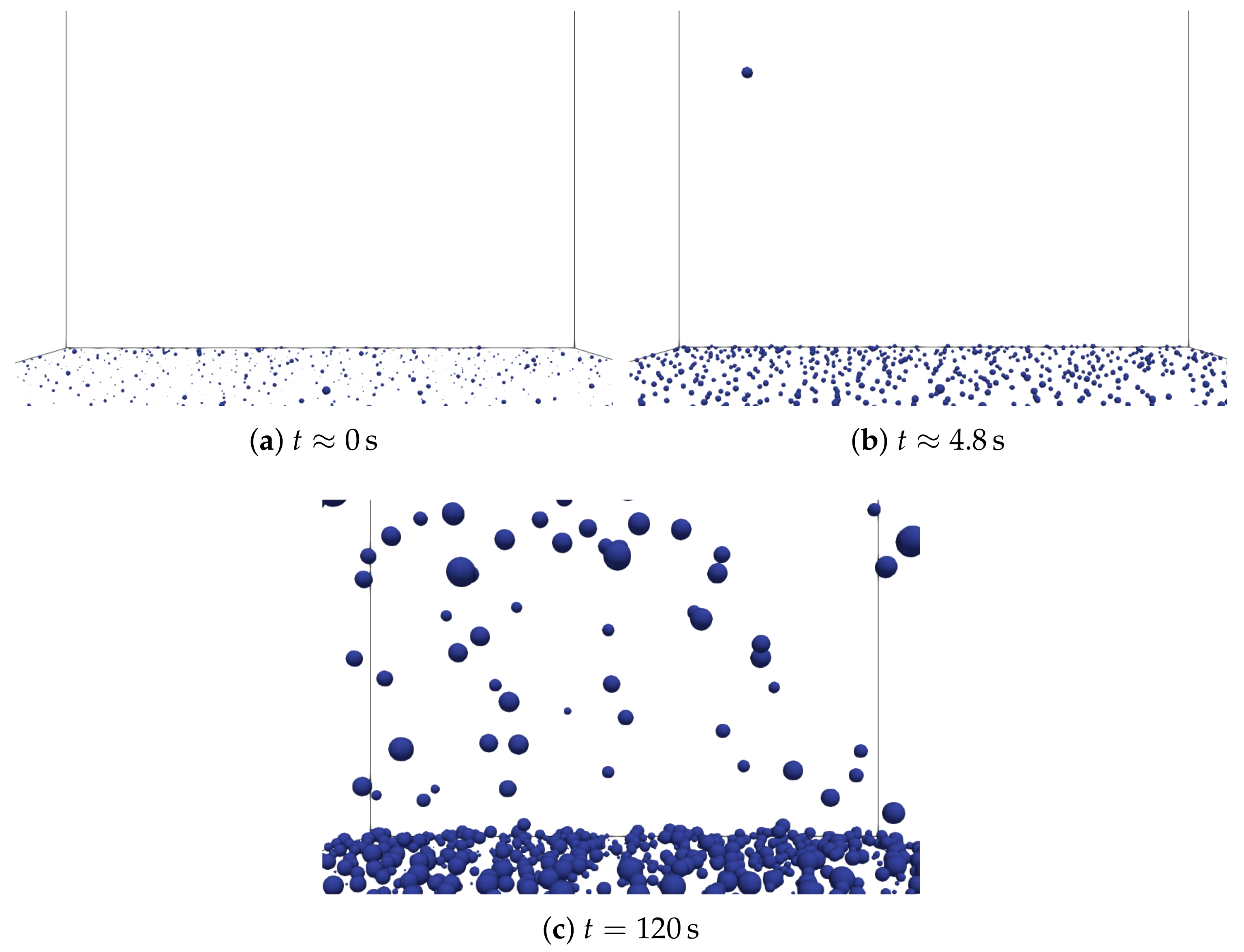
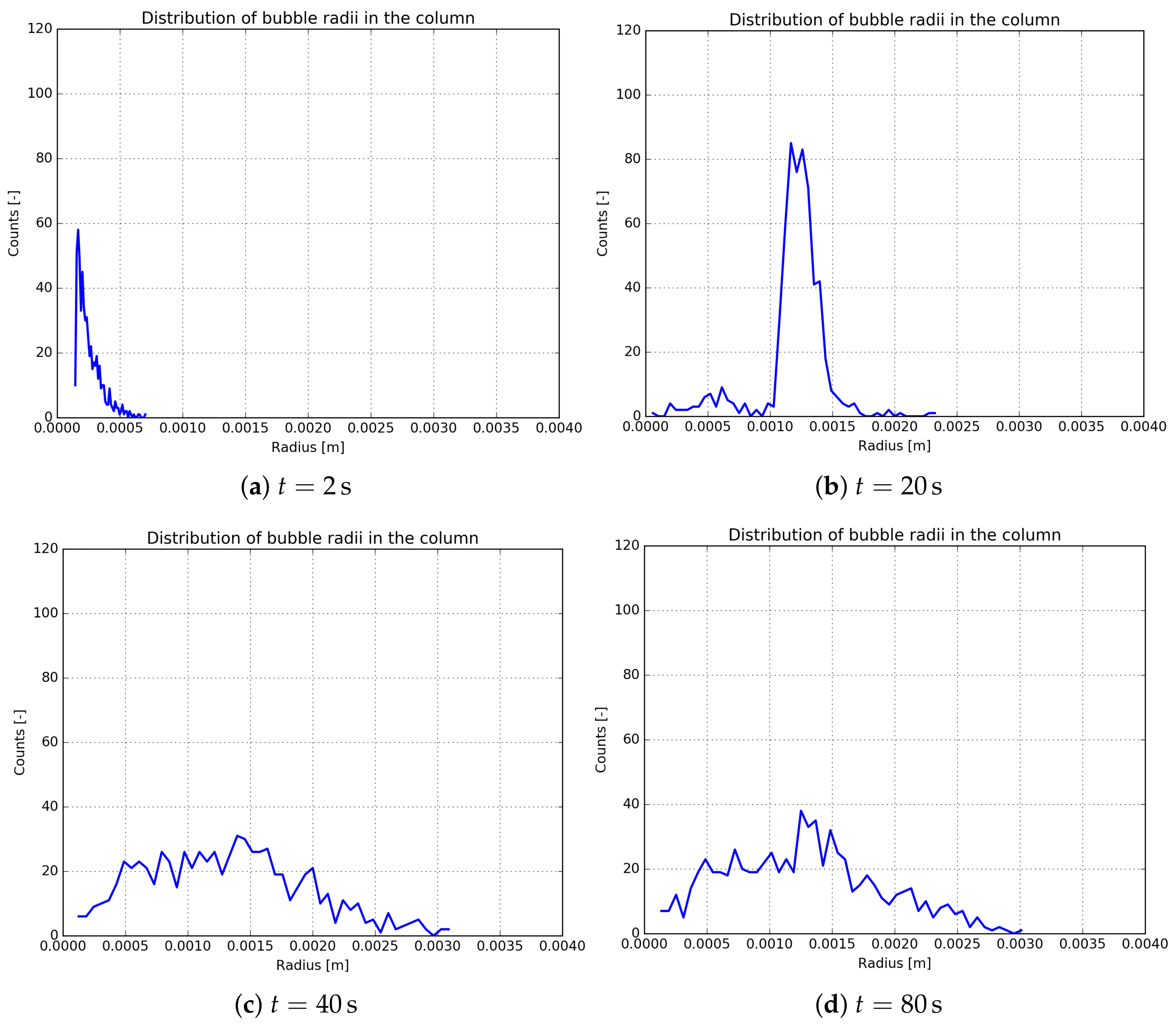
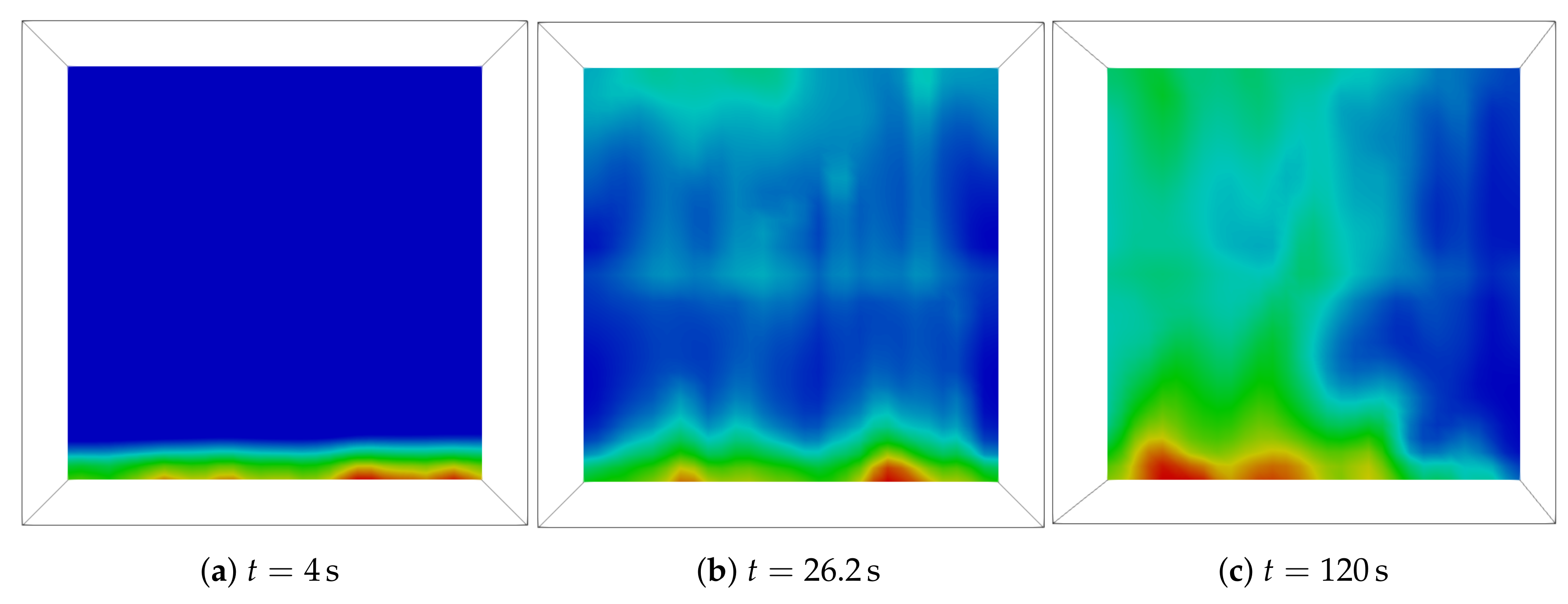
3.2. Analysis of the Nucleation Process
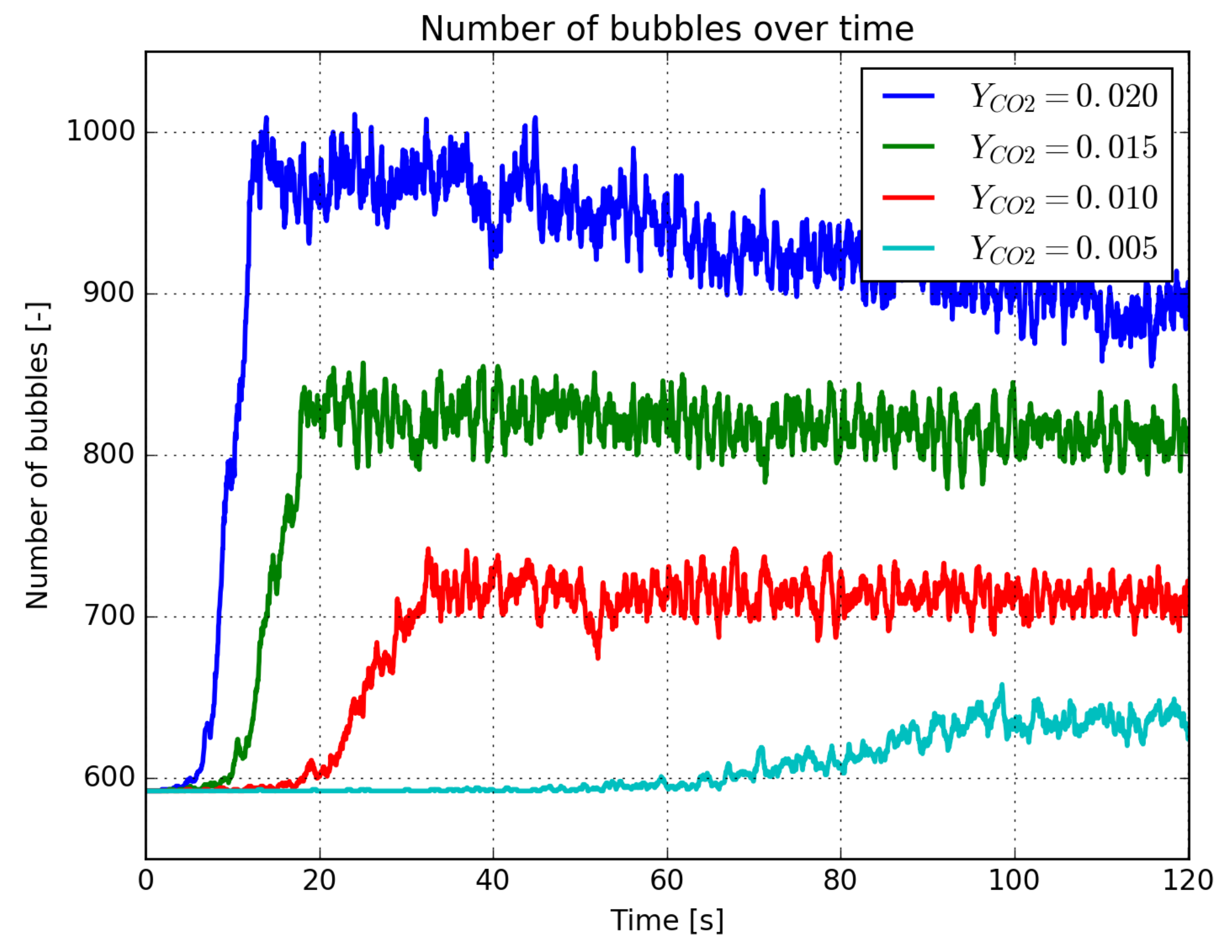
3.3. Effect of the Supersaturation Ratio
3.4. Effect of the Surface Properties
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CFD | Computational Fluid Dynamics |
| DNS | Direct Numerical Simulation |
| PBM | Population Balance Model |
| VOF | Volume of Fluid |
| MD | Molecular Dynamics |
| DBM | Discrete Bubble Model |
| LES | Large Eddy Simulation |
Nomenclature
| Greek Symbols | |
| Volume fraction, | |
| Transport coefficient, | |
| Supersaturation ratio, | |
| Film thickness, | |
| Dynamic viscosity, | |
| Mass density, | |
| Surface tension, | |
| Stress tensor, | |
| Interphase momentum transfer, | |
| Latin Symbols | |
| A | Surface area, |
| C | Model coefficients, |
| c | Concentration, |
| Equivalent diameter , | |
| Bubble diameter, | |
| Diffusion coefficient, | |
| Eötvös number , | |
| Bubble aspect ratio, | |
| Force, | |
| Gravitational acceleration, | |
| Mass transfer coefficient, | |
| H | Henry constant, |
| Volume averaged interfacial mass transfer, | |
| Individual bubble mass transfer, | |
| m | Mass, |
| N | Number of nucleation sites, |
| p | Pressure, |
| Reynolds number , | |
| Radius, | |
| s | Standard deviation, |
| S | Source term (reaction), |
| Schmidt number , | |
| Sherwood number , | |
| t | Time, |
| Liquid velocity, | |
| Gas velocity, | |
| V | Volume, |
| Weber number , | |
| Position, | |
| Mass fraction of component j, | |
| Sub/superscripts | |
| b | Bubble |
| c | Critical |
| d | Distorted |
| D | Drag |
| Equivalent | |
| f | Fritz |
| G | Gravity |
| Indexes | |
| L | Lift |
| Effective | |
| l | Liquid |
| N | Normal |
| P | Pressure |
| Relative | |
| s | Saturation |
| T | Tangential |
| Virtual mass | |
| W | Wall-interactions |
References
- Darmana, D.; Deen, N.G.; Kuipers, J.A.M. Detailed modeling of hydrodynamics, mass transfer and chemical reactions in a bubble column using a discrete bubble model. Chem. Eng. Sci. 2005, 60, 3383–3404. [Google Scholar] [CrossRef]
- Besagni, G.; Inzoli, F.; Ziegenhein, T. Two-Phase Bubble Columns: A Comprehensive Review. ChemEngineering 2018, 2, 13. [Google Scholar] [CrossRef]
- Van Sint Annaland, M.; Deen, N.G.; Kuipers, J.A.M. Multi-Level Modeling of Dispersed Gas-Liquid Two-Phase Flows; Springer: Berlin, Germany, 2003. [Google Scholar]
- Volanschi, A.; Oudejans, D.; Olthuis, W.; Bergveld, P. Gas phase nucleation core electrodes for the electrolytical method of measuring the dynamic surface tension in aqueous solutions. Sens. Actuators B 1996, 35–36, 73–79. [Google Scholar] [CrossRef]
- Verhaart, H.; de Jonge, R.; van Stralen, S. Growth rate of a gas bubble during electrolysis in supersaturated liquid. Int. J. Heat Mass Transf. 1980, 23, 293–299. [Google Scholar] [CrossRef]
- Liger-Belair, G.; Vignes-Adler, M.; Voisin, C.; Bertrand, R.; Jeandet, P. Kinetics of Gas Discharging in a Glass of Champagne: The Role of Nucleation Sites. Langmuir 2002, 18, 1294–1301. [Google Scholar] [CrossRef]
- Amon, M.; Denson, C.D. A study of the dynamics of foam growth: Analysis of the growth of closely spaced spherical bubbles. Polym. Eng. Sci. 1984, 24, 1026–1034. [Google Scholar] [CrossRef]
- Pooladi-Darvish, M.; Firoozabadi, A. Solution-gas drive in heavy oil reservoirs. J. Can. Pet. Technol. 1999, 38, 54–61. [Google Scholar] [CrossRef]
- Askari, E.; Proulx, P.; Passalacqua, A. Modelling of Bubbly Flow Using CFD-PBM Solver in OpenFOAM: Study of Local Population Balance Models and Extended Quadrature Method of Moments Applications. ChemEngineering 2018, 2, 8. [Google Scholar] [CrossRef]
- Liu, L.; Cai, W.; Chen, Y.; Wang, Y. Fluid Dynamics and Mass Transfer Study of Electrochemical Oxidation by CFD Prediction and Experimental Validation. Ind. Eng. Chem. Res. 2018, 57, 6493–6504. [Google Scholar] [CrossRef]
- El-Askary, W.; Sakr, I.; Ibrahim, K.; Balabel, A. Hydrodynamics characteristics of hydrogen evolution process through electrolysis: Numerical and experimental studies. Energy 2015, 90, 722–737. [Google Scholar] [CrossRef]
- Liu, H.; Pan, L.M.; Wen, J. Numerical simulation of hydrogen bubble growth at an electrode surface. Can. J. Chem. Eng. 2015, 94, 192–199. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, X.; Ye, P. Numerical and experimental investigation of two phase flow during boiling in a coiled tube. Int. J. Heat Mass Transf. 2008, 51, 1003–1016. [Google Scholar] [CrossRef]
- Zhuan, R.; Wang, W. Simulation on nucleate boiling in micro-channel. Int. J. Heat Mass Transf. 2010, 53, 502–512. [Google Scholar] [CrossRef]
- Yang, Z.L.; Dinh, T.; Nourgaliev, R.; Sehgal, B. Numerical investigation of bubble coalescence characteristics under nucleate boiling condition by a lattice-Boltzmann model. Int. J. Therm. Sci. 2000, 39, 1–17. [Google Scholar] [CrossRef]
- Weijs, J.H.; Snoeijer, J.H.; Lohse, D. Formation of Surface Nanobubbles and the Universality of Their Contact Angles: A Molecular Dynamics Approach. Phys. Rev. Lett. 2012, 108, 104501. [Google Scholar] [CrossRef] [PubMed]
- van der Linde, P.; Moreno Soto, Á.; Peñas-López, P.; Rodríguez-Rodríguez, J.; Lohse, D.; Gardeniers, H.; van der Meer, D.; Fernández Rivas, D. Electrolysis-Driven and Pressure-Controlled Diffusive Growth of Successive Bubbles on Microstructured Surfaces. Langmuir 2017, 33, 12873–12886. [Google Scholar] [CrossRef] [PubMed]
- Mandin, P.; Hamburger, J.; Bessou, S.; Picard, G. Modelling and calculation of the current density distribution evolution at vertical gas-evolving electrodes. Electrochim. Acta 2005, 51, 1140–1156. [Google Scholar] [CrossRef]
- Nierhaus, T.; van Parys, H.; Dehaeck, S.; van Beeck, J.; Deconinck, H.; Deconinck, J.; Hubin, A. Simulation of the Two-Phase Flow Hydrodynamics in an IRDE Reactor. J. Electrochem. Soc. 2009, 156, P139–P148. [Google Scholar] [CrossRef]
- Van Parys, H.; van Damme, S.; Maciel, P.; Nierhaus, T.; Tomasoni, F.; Hubin, A.; Deconinck, H.; Deconinck, J. Eulerian-Lagrangian Model for gas-evolving processes based on supersaturation. WIT Trans. Eng. Sci. 2009, 65, 109–118. [Google Scholar] [CrossRef]
- Van Damme, S.; Maciel, P.; van Parys, H.; Deconinck, J.; Hubin, A.; Deconinck, H. Bubble nucleation algorithm for the simulation of gas evolving electrodes. Electrochem. Commun. 2010, 12, 664–667. [Google Scholar] [CrossRef]
- Hreiz, R.; Abdelouahed, L.; Fünfschilling, D.; Lapicque, F. Electrogenerated bubbles induced convection in narrow vertical cells: PIV measurements and Euler–Lagrange CFD simulation. Chem. Eng. Sci. 2015, 134, 138–152. [Google Scholar] [CrossRef]
- Delnoij, E.; Kuipers, J.A.M.; van Swaaij, W.P.M. A three-dimensional CFD model for gas-liquid bubble columns. Chem. Eng. Sci. 1999, 54, 2217–2226. [Google Scholar] [CrossRef]
- Lau, Y.M.; Bai, W.; Deen, N.G.; Kuipers, J.A.M. Numerical study of bubble break-up in bubbly flows using a deterministic Euler-Lagrange framework. Chem. Eng. Sci. 2014, 108, 9–22. [Google Scholar] [CrossRef]
- Vreman, A.W. An eddy-viscosity subgrid-scale model for turbulent shear flow: Algebraic theory and applications. Phys. Fluids 2004, 16, 3670–3681. [Google Scholar] [CrossRef]
- Dijkhuizen, W.; Roghair, I.; Van Sint Annaland, M.; Kuipers, J.A.M. DNS of gas bubbles behaviour using an improved 3D front tracking model–Drag force on isolated bubbles and comparison with experiments. Chem. Eng. Sci. 2010, 65, 1415–1426. [Google Scholar] [CrossRef]
- Roghair, I.; Lau, Y.M.; Deen, N.G.; Slagter, H.M.; Baltussen, M.W.; Van Sint Annaland, M.; Kuipers, J.A.M. On the drag force of bubbles in bubble swarms at intermediate and high Reynolds numbers. Chem. Eng. Sci. 2011, 66, 3204–3211. [Google Scholar] [CrossRef]
- Tomiyama, A.; Tamai, H.; Zun, I.; Hosokawa, S. Transverse migration of single bubbles in simple shear flows. Chem. Eng. Sci. 2002, 57, 1849–1858. [Google Scholar] [CrossRef]
- Auton, T.R. The lift force on a spherical rotational flow. J. Fluid Mech. 1987, 183, 199–218. [Google Scholar] [CrossRef]
- Tomiyama, A.; Matsuoka, T.; Fukuda, T.; Sakaguchi, T. A Simple Numerical Method for Solving an Incompressible Two-Fluid Model in a General Curvilinear Coordinate System. In Multiphase Flow 1995; Kyoto University: Kyoto, Japan, 1995; pp. 241–252. [Google Scholar] [CrossRef]
- Jain, D.; Deen, N.G.; Kuipers, J.A.M. Numerical modeling of carbon dioxide chemisorption in sodium hydroxide solution in a micro-structured bubble column. Chem. Eng. Sci. 2015, 137, 685–696. [Google Scholar] [CrossRef]
- Legendre, D.; Zevenhoven, R. A numerical Euler–Lagrange method for bubble tower CO2 dissolution modeling. Chem. Eng. Res. Des. 2016, 111, 49–62. [Google Scholar] [CrossRef]
- Asad, A.; Kratzsch, C.; Schwarze, R. Influence of drag closures and inlet conditions on bubble dynamics and flow behavior inside a bubble column. Eng. Appl. Comput. Fluid Mech. 2017, 11, 127–141. [Google Scholar] [CrossRef][Green Version]
- Mer, S.; Praud, O.; Neau, H.; Merigoux, N.; Magnaudet, J.; Roig, V. The emptying of a bottle as a test case for assessing interfacial momentum exchange models for Euler–Euler simulations of multi-scale gas-liquid flows. Int. J. Multiph. Flow 2018, 106, 109–124. [Google Scholar] [CrossRef]
- Deen, N.G.; Van Sint Annaland, M.; Kuipers, J.A.M. Multi-scale modeling of dispersed gas-liquid two-phase flow. Chem. Eng. Sci. 2004, 59, 1853–1861. [Google Scholar] [CrossRef]
- Magnaudet, J.; Eames, I. The Motion of High-Reynolds-Number Bubbles in Inhomogeneous Flows. Annu. Rev. Fluid Mech. 2000, 32, 659–708. [Google Scholar] [CrossRef]
- Hoomans, B.P.B.; Kuipers, J.A.M.; Briels, W.J.; van Swaaij, W.P.M. Discrete particle simulation of bubble and slug formation in a two-dimensional gas-fluidised bed: A hard-sphere approach. Chem. Eng. Sci. 1996, 51, 99–118. [Google Scholar] [CrossRef]
- Prince, M.J.; Blanch, H.W. Bubble coalescence and break-up in air-sparged bubble columns. AIChE J. 1990, 36, 1485–1499. [Google Scholar] [CrossRef]
- Sommerfeld, M.; Bourloutski, E.; Bröder, D. Euler/Lagrange Calculations of Bubbly Flows with Consideration of Bubble Coalescence. Can. J. Chem. Eng. 2003, 81, 508–518. [Google Scholar] [CrossRef]
- Liao, Y.; Lucas, D. A literature review of theoretical models for drop and bubble breakup in turbulent dispersions. Chem. Eng. Sci. 2009, 64, 3389–3406. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2007; pp. 240–241. [Google Scholar]
- Balay, S.; Abhyankar, S.; Adams, M.F.; Brown, J.; Brune, P.; Buschelman, K.; Dalcin, L.; Eijkhout, V.; Gropp, W.D.; Kaushik, D.; et al. PETSc Users Manual; Technical Report ANL-95/11, Revision 3.7; Argonne National Laboratory: Lemont, IL, USA, 2016.
- Balay, S.; Gropp, W.D.; McInnes, L.C.; Smith, B.F. Efficient Management of Parallelism in Object Oriented Numerical Software Libraries. In Modern Software Tools in Scientific Computing; Arge, E., Bruaset, A.M., Langtangen, H.P., Eds.; Birkhäuser Press: Basel, Switzerland, 1997; pp. 163–202. [Google Scholar]
- Deen, N.G.; Solberg, T.; Hjertager, B.H. Large eddy simulation of the Gas-Liquid flow in a square cross-sectioned bubble column. Chem. Eng. Sci. 2001, 56, 6341–6349. [Google Scholar] [CrossRef]
- Ogata, A.; Banks, R.B. A Solution of the Differential Equation of Longitudinal Dispersion in Porous Media; Technical Report; US Government Printing Office: Washington, DC, USA, 1961.
- Enríquez, O.R.; Hummelink, C.; Bruggert, G.W.; Lohse, D.; Prosperetti, A.; van der Meer, D.; Sun, C. Growing bubbles in a slightly supersaturated liquid solution. Rev. Sci. Instrum. 2013, 84, 065111. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.F.; Evans, G.M.; Galvin, K.P. Bubble nucleation from gas cavities—A review. Adv. Colloid Interface Sci. 1999, 80, 27–50. [Google Scholar] [CrossRef]
- Bankoff, S. Entrapment of Gas in the Spreading of a Liquid Over a Rough Surface. AIChE J. 1958, 4, 24–26. [Google Scholar] [CrossRef]
- Tong, W.; Bar-Cohen, A.; Simons, T.; You, S. Contact angle effects on boiling incipience of highly-wetting liquids. Int. J. Heat Mass Transf. 1990, 33, 91–103. [Google Scholar] [CrossRef]
- Fritz, W. Maximum Volume of Vapor Bubbles. Phys. Z. 1935, 36, 354–379. [Google Scholar]
- Moreno Soto, A.; Prosperetti, A.; Lohse, D.; van der Meer, D. Gas depletion through single gas bubble diffusive growth and its effect on subsequent bubbles. J. Fluid Mech. 2017, 831, 474–490. [Google Scholar] [CrossRef]
- Enríquez, O.R.; Sun, C.; Lohse, D.; Prosperetti, A.; van der Meer, D. The quasi-static growth of CO2 bubbles. J. Fluid Mech. 2014, 741. [Google Scholar] [CrossRef]

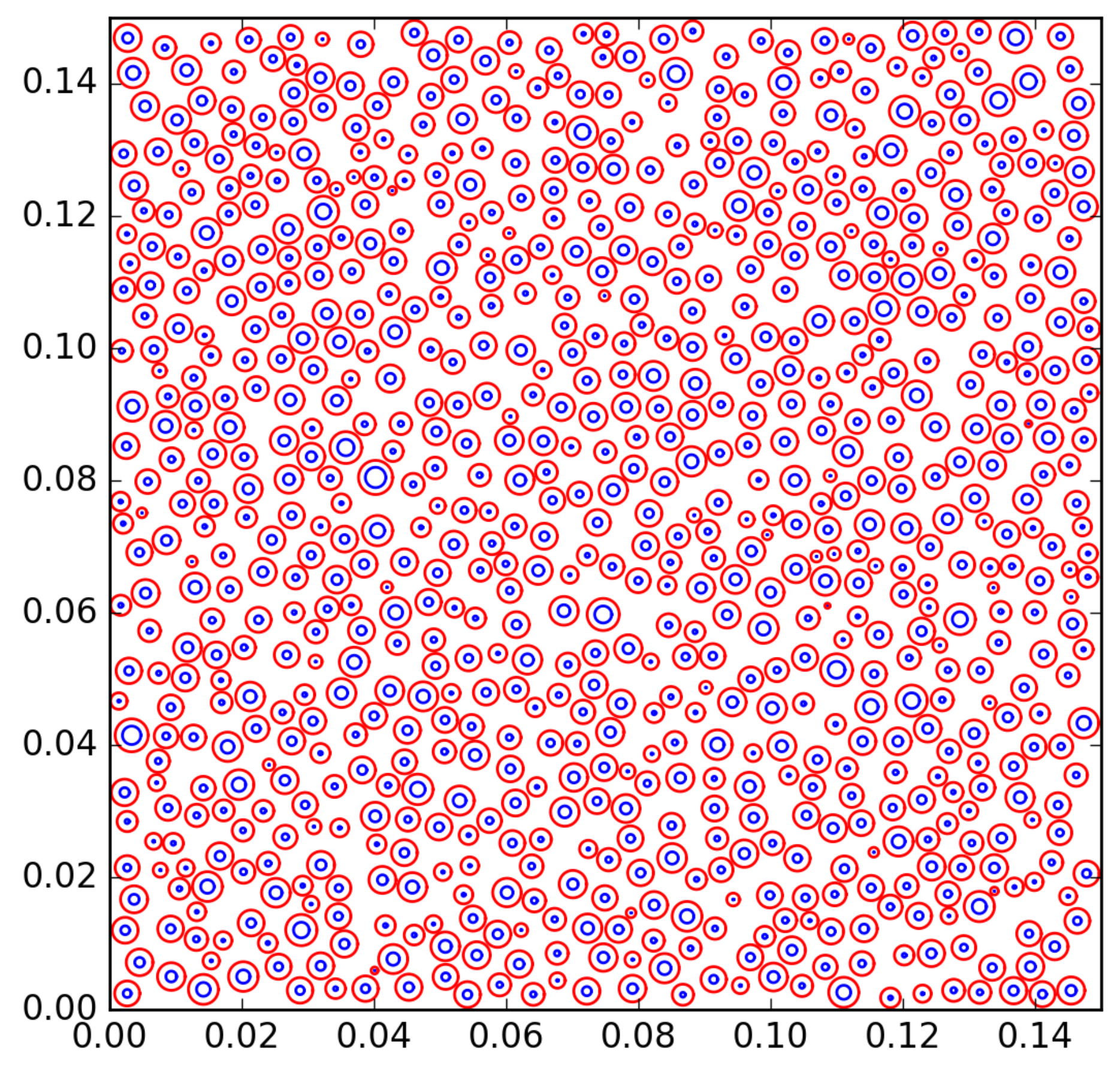

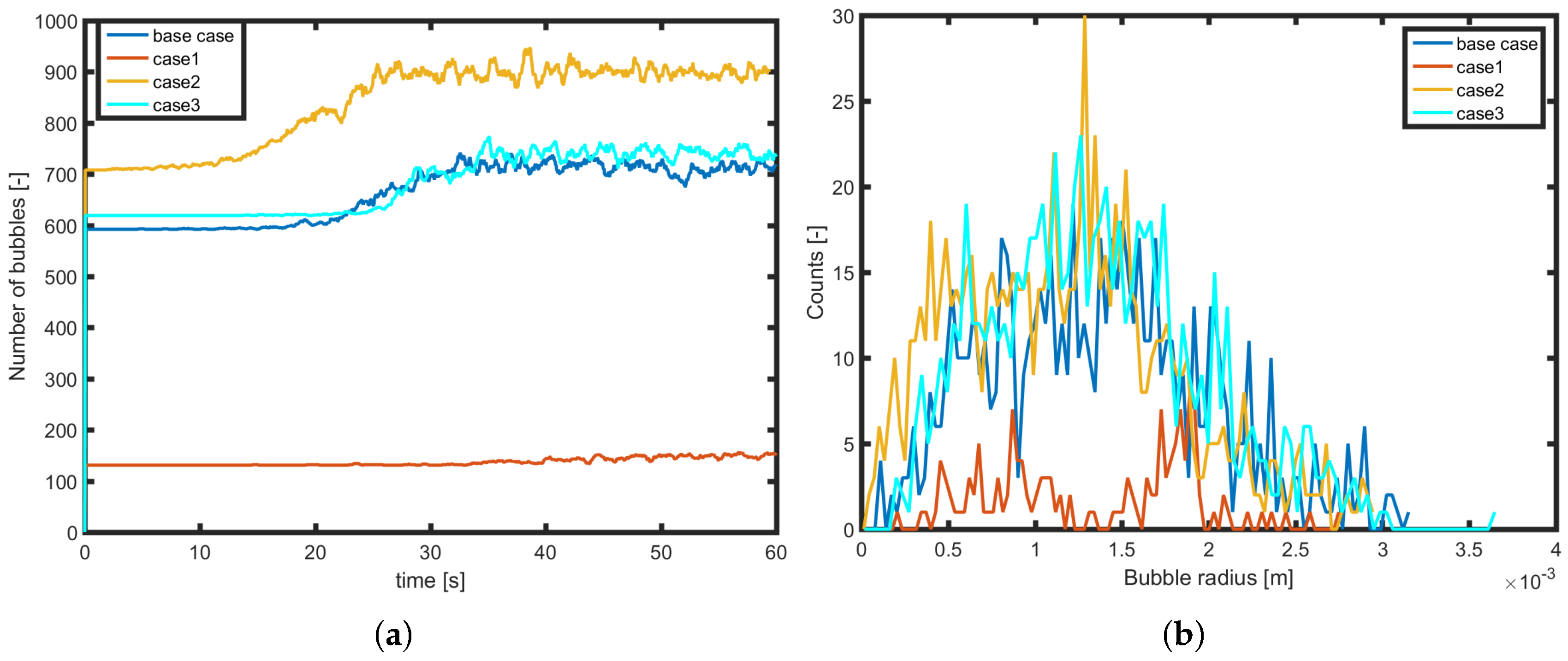
| Case | Description | Parameters |
|---|---|---|
| base case | , case described in Section 3.1 | N = 1300 |
| R = 0.5 | ||
| s = 0.3 | ||
| Case 1 | Decreased number of nucleation sites | N = 200 |
| Case 2 | Decreased mean radius of radii distribution | R = 0.25 |
| Case 3 | Decreased standard deviation of radii distribution | s = 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battistella, A.; Aelen, S.S.C.; Roghair, I.; Van Sint Annaland, M. Euler–Lagrange Modeling of Bubbles Formation in Supersaturated Water. ChemEngineering 2018, 2, 39. https://doi.org/10.3390/chemengineering2030039
Battistella A, Aelen SSC, Roghair I, Van Sint Annaland M. Euler–Lagrange Modeling of Bubbles Formation in Supersaturated Water. ChemEngineering. 2018; 2(3):39. https://doi.org/10.3390/chemengineering2030039
Chicago/Turabian StyleBattistella, Alessandro, Sander S. C. Aelen, Ivo Roghair, and Martin Van Sint Annaland. 2018. "Euler–Lagrange Modeling of Bubbles Formation in Supersaturated Water" ChemEngineering 2, no. 3: 39. https://doi.org/10.3390/chemengineering2030039
APA StyleBattistella, A., Aelen, S. S. C., Roghair, I., & Van Sint Annaland, M. (2018). Euler–Lagrange Modeling of Bubbles Formation in Supersaturated Water. ChemEngineering, 2(3), 39. https://doi.org/10.3390/chemengineering2030039