Abstract
The wastewater of the industrial production of artificial sweetener sucralose contained an average 1100 mg/L of total organic carbon (TOC) with 2100 mg/L of chemical oxygen demand and 10 mg/L of biological oxygen demand. Biodegradability of the wastewater components was low due to chlorinated organic substances. The combined chemical and biological treatment of this wastewater in the bioreactors with hematite iron ore removed up to 70% of TOC. About 20% of TOC was removed quickly by adsorption on iron ore particles, but adsorption/precipitation of others up to 50% of TOC was due to ferrous/ferric ions and hydroxides produced during microbial reduction and dissolution of iron ore. The calculated dosage of iron ore with 150 regeneration cycles could be 46.7 g/L of wastewater. Thus, the treatment of wastewater with iron ore and iron-reducing bacteria diminished the quantity of granulated activated carbon that is used in the treatment of sucralose production wastewater by up to 70%.
1. Introduction
The sequential bioreduction of Fe3+ and the (bio)oxidation of Fe2+ can be used for the numerous environmental and geotechnical engineering applications [1,2]. The potential applications of these processes have been described in the US patent 7393452 “Compositions and methods for the treatment of wastewater and other waste” [3]. The major steps of the patented technology are the sequential anaerobic bioreduction of ferric (hydr)oxides and aerobic (bio)oxidation of Fe2+ ions. Both Fe2+ and Fe3+ ions could be hydrolyzed and precipitated as ferrous/ferric hydroxide or react with organic and inorganic substances forming insoluble compounds. These reactions can be widely used for the removal of phosphate from water and wastewater [4,5,6,7], the biodegradation of estrogens in wastewater [8], the enhancement of anaerobic treatment of fat-containing wastewater [9,10] and wastewater containing sulphate [11,12,13,14], and to decrease the hydraulic conductivity of soil or sand using biogrout containing iron [15,16]. A priori, this technology could be used also to remove the polarized recalcitrant halogenated compounds from water and wastewater due to their precipitation by positively charged ferric and ferrous ions, as well as due to adsorption on both positively and negatively charged ferric and ferrous (hydr)oxides that are produced as shown in the following equations:
where CH2O is the empirical formula for carbohydrates. The aim of this study was to demonstrate a possibility of how to remove chlorinated recalcitrant compounds from the wastewater of sucralose production.
2Fe2O3 + CH2O + 8H+ → 4Fe2+ + CO2 + 5H2O
Fe2+ + x1H2O → x2Fe(OH)+ + x3Fe(OH)2 + x4Fe(OH)3−
4Fe2+ + O2 + 4H+ → 4Fe3+ + 2H2O
Fe3+ + x5H2O → x6Fe(OH)2+ + x7Fe(OH)3 + x8Fe(OH)4−
The object of the study was wastewater from the production of sucralose (other names include trichlorosucrose; Splenda™; Altern™; 4, 1’, 6’–trichlorogalactosucrose; trichlorogalactosucrose, 4,1’,6’-trichloro-4,1’,6’-trideoxygalactosucrose; 4-chloro-4-deoxy-α, D-galactopyranosyl-1,6-dichloro-1,6-dideoxy-β, D-fructofuranoside), which is one of the most popular zero-calorie artificial sweeteners for personal consumption and in the food industry. Sucralose is used as an artificial sweetener in food and beverages in over 80 countries. The sweetener, which is 600 times sweeter than sugar, is used in over 4000 products including drinks, breakfast bars and ice creams (www.foodprocessing-technology.com/projects/tateandlyle/). Sucralose is now produced by Tate & Lyle (McIntosh, Alabama, USA); by HYET Sweet B.V. (Breda, The Netherlands); by JK Sucralose Inc. (Jiangsu, China); Nantong Changhai Food Additive Co. Ltd. (Nantong, China); Shandong Kanbo Biochemical Technology Co. (Dongying City, China), and Anhui Jinhe Industry (Chuzhou, China). It is possible that production of sucralose around the world will further increase after 2020 when the patent protection for Tate & Lyle (London, UK) expires.
An estimated daily intake of sucralose in the USA ranges from 0.1 to 2.0 mg/kg body weight [17]. The substance passes through the human organism unchanged; it does not biodegrade on the municipal wastewater treatment plant (MWWTP), so its concentration in natural water is considered as a tracer of anthropogenic pollution [18]. It is considered as an emerging water contaminant due to its resistance to wastewater biotreatment and persistence in nature. However, the ecotoxicological contribution of sucralose remains unknown, but there could possibly be toxicity of the photodegradation products [19]. The concentration of sucralose in effluent of five Beijing MWWTPs were on the levels of 2.7–3.5 μg/L [20]. The sucralose is also not removed during septic treatment, therefore its concentration in septic effluent can reach 7.6 μg/L [21]. The analysis of river water samples from 27 European countries showed that the concentration of sucralose can be up to 1 μg/L [22]. Detectable levels of sucralose have even been found in North American coastal and open ocean waters [23].
Sucralose has very low degradability levels in nature [24], but there is a known patent on isolated microbial consortia which is capable of degrading chlorinated carbohydrates [25]. The wastewater after sucralose production contains not only sucralose, but also such recalcitrant chlorinated compounds as 4,l’-dichlorogalactosucrose; 4,l’-dichloro-3’,6’-anhydrogalactosucrose; 4,6’-dichlorogalactosucrose; l’,6’-dichlorosucrose; 6,6’-dichlorosucrose; 4,l’,6’-trichlorosucrose; 4,1’,6’-trichlorogalactosucrose; 4,6,6’-trichlorogalactosucrose; 6,l’,6’-trichlorosucrose; 4,1’,4’,6’-tetrachlorotagatogalactosucrose; 4,6,l’,6’-tetrachlorogalactosucrose; and different penta-chlorinated carbohydrates [25].
One possible (albeit very expensive) way for removing these recalcitrant compounds from industrial wastewater is adsorption on granulated activated carbon (GAC). The aim of this study was to test cheap commodity, hematite iron ore, and products of its bioreduction as adsorbent to diminish the quantity of GAC for the treatment of sucralose production wastewater.
2. Materials and Methods
2.1. Enrichment Culture
Enrichment culture (EC) for Fe(III) bioreduction must be able to grow in medium with salinity up to 40 g/L and use sucrose directly, or after acidogenic fermentation, in order to reduce Fe(III). The samples, which can serve as potential sources of enrichment cultures of halotolerant iron-reducing and fermenting bacteria, were as follows: (a) the mangrove swamp sediment samples were used as it is known that halotolerant iron-reducing bacteria are the common inhabitants of marine and mangrove swamp sediments; (b) the sediment from the bottom of small embayment of the Dead Sea in Jordan. Salinity in the Dead Sea varies from 26 to 35%. The site for sampling was selected due to the presence of the brown film of iron hydroxides on the surface of water, which is a sign of natural anaerobic Fe(III) bioreduction, diffusion of soluble Fe(II) towards surface, and aerobic oxidation of dissolved Fe(II) at the surface of water.
To produce the enrichment culture, 50 g of a mixture of these samples had initially been used to inoculate medium, and then three anaerobic transfers and cultivations had been made in the medium of the following composition, g/L: iron ore, 10; sucrose, 0.5; sucralose, 0.2, sea salt, 50; NH4Cl, 0.5; KH2PO4, 0.4; NaHCO3, 1.5; MgSO4, 0.2; trace elements stock solution, 5 mL/L, tap water to 1 L; pH was adjusted to 7. The trace elements stock solution consists of the following components, g/L: ZnSO4·7H2O, 0.1; MnSO4·H2O, 0.085; H3BO3, 0.06; CoCl2·6H2O, 0.02; CuCl2, 0.004; NiSO4·6H2O, 0.028; Na2MoO4·2H2O, 0.04, and FeCl2, 0.3, pH was adjusted to 2.0.
One liter of this medium was placed in 1.5 L plastic bottles, inoculated with 100 mL of the previous culture, flushed with nitrogen gas and placed on the shaker at 150 rpm at 30 °C for 20 days. Cultivation of each enrichment culture was done in triplicates and the average means were presented. When necessary, abiotic control was performed by the addition of 15% (v/v) of ethanol.
2.2. Wastewater Treatment
The samples of wastewater (WW) after industrial thermophilic membrane bioreactor were delivered from a sucralose production plant in Singapore. The variations of wastewater content were very significant during the two years of study, but the typical characteristics of WW were as follows (average value ± s.d. are shown): mg/L: 1100 ± 500 mg/L of total organic carbon (TOC), 2100 ± 300 mg/L of chemical oxygen demand (COD), and 10 ± 5 mg/L of biological oxygen demand (BOD). For example, the characteristics of WW in one set of the experiments were as follows (average value ± s.d. are shown): mg/L: total dissolved solids (TDS), 42,300 ± 1200; total organic carbon (TOC), 710 ± 140; chemical oxygen demand (COD), 2192 ± 139; biological oxygen demand (BOD), 7.4 ± 1.6; SO4−, 8250 ± 1500; N-NH4+, 236 ± 26; N-NO3−, 27± 6; P-PO43−, 2.9 ± 0.3 mg/L; alkalinity, 625 ± 50; pH 8.1± 0.3; color, 3300 ± 250 units. The variations of TOC measurements in one sample were lower than 5% of the average value, but there were significant variations of TOC, up to 50% of the average value, in the samples of industrial wastewater taken during the two years of study. For this case, to diminish the variability of the experimental data, one sample of wastewater was used for a set of experiments.
The following components have been added in 1 L of WW: sucrose, 0.5; NH4Cl, 0.5; KH2PO4, 0.4; MgSO4, 0.2; trace elements stock solution, 5 mL/L. The WW with additions was called “influent”. The biodegradability of organic components of wastewater measured by BOD/COD ratio was 0.34%.
The major reagent for the treatment was hematite (Fe2O3) of iron ore with the iron content 60% (w/w), supplied from China. The iron ore was crushed and sieved to produce a fraction containing iron ore particles, sized from 1 to 3 mm. The porosity of the iron ore was 46 ± 0.5% (v/v, average value ± s.d.). The laboratory facility to treat wastewater consists of five column reactors (Figure 1). Three anaerobic columns were filled in with iron ore, and two anaerobic columns were not. The columns with iron ore were filled up with influent for 14 days batch treatment, then the continuous process with the hydraulic retention time (HRT) in anaerobic columns of five days was started. The filling of the laboratory facility was done in several steps: (a) the connection between the columns with iron ore and the last two columns without iron ore was closed; (b) the anaerobic part of the facility was flushed with nitrogen gas; (c) influent was flushed with nitrogen gas, inoculum was added.

Figure 1.
The laboratory facility for the treatment of sucralose production wastewater with recalcitrant organic compounds.
2.3. Analysis
All analyses were standard, using “Standard Methods for Water and Wastewater Examination” outlined by the American Public Health Association (APHA). The samples were taken under the flow of nitrogen gas and the total Fe(II) concentration was measured using the phenanthroline method (Method 3500-FeD, APHA 2005) using the spectrophotometer UV-1201V (Shimadsu, Kyoto, Japan). For the measurement of total Fe(II) concentration, the samples were acidified immediately after collection by the addition of 2 mL concentrated HCl per 100 mL sample. TOC in solution was measured by Standard Method 5310C (APHA, 2005) after filtration of the 5 mL sample through a 0.45 µm nylon membrane using a TOC analyser C-VCSH (Shimadzu, Kyoto, Japan). The concentrations of COD were determined in the well-mixed samples through standard method (APHA, 2005). The excess of chlorine was removed before COD measurement, using a solution of silver. The concentration of nitrite and nitrate were measured according to the standard methods APHA 4500-NO2-B and APHA 4500-NO3-I (APHA 2005) after the sample had been centrifuged and filtered through a 0.45 μm nylon membrane. The spectrophotometer UV-1201V (Shimadsu, Kyoto, Japan) was used to measure the concentrations of nitrite and nitrate. Ammonium concentration and total nitrogen were determined, respectively, by the 4500-NH3 H Flow Injection Analysis method and 4500-NO3-F Automated cadmium reduction method APHA using Flow Injection Analyzer, QuickChem 8000 (LaChat Instruments, Loveland, CO, USA). The concentration of total suspended solids (TSS) was determined by standard method 2540D APHA. Total phosphorus was determined using 4500-PE Ascorbic acid method APHA using the spectrophotometer UV-1201V (Shimadsu, Kyoto, Japan). Color was determined using Multiprobe YSI556 (Pine, Yellow Springs, OH, USA). pH and oxidation-reduction potential were measured by the Cyberscan PCD 6500 (Thermo Fisher Scientific Inc., Waltham, MA, USA). The statistical and correlation analysis of the results were performed with Microsoft Excel programs. The analyses were done in triplicates and means ± standard deviations are shown in the Results.
3. Results
3.1. Batch Treatment of Wastewater
The following results have been obtained during wastewater treatment in the columns filled with iron ore, wastewater, and inoculated with enrichment culture. The oxidation-reduction potential (ORP) changed from the initial value of −34 mV to the final value of −225 mV. The pH increased during treatment from 7.2 to 7.8. The removals of TOC and COD were 70% and 68%, respectively, after 21 days of batch treatment (Table 1).

Table 1.
Batch anaerobic treatment of wastewater in the columns.
A role in TOC removal plays adsorption of organic compounds on initial iron ore. In abiotic control, decrease of TOC after 2 h of contact between wastewater and iron ore was in the range 17–21%. The batch process was performed in triplicates with very similar results, which are not shown to avoid oversizing. The duration of the batch process depends on the growth of iron-reducing bacteria biomass in batch culture, which is a slow process typically continuing over 15–21 days [6,8,12,16], while an adsorption of organic substances on produced ferrous hydroxide(s) could take just a few hours.
3.2. Continuous Treatment of Wastewater
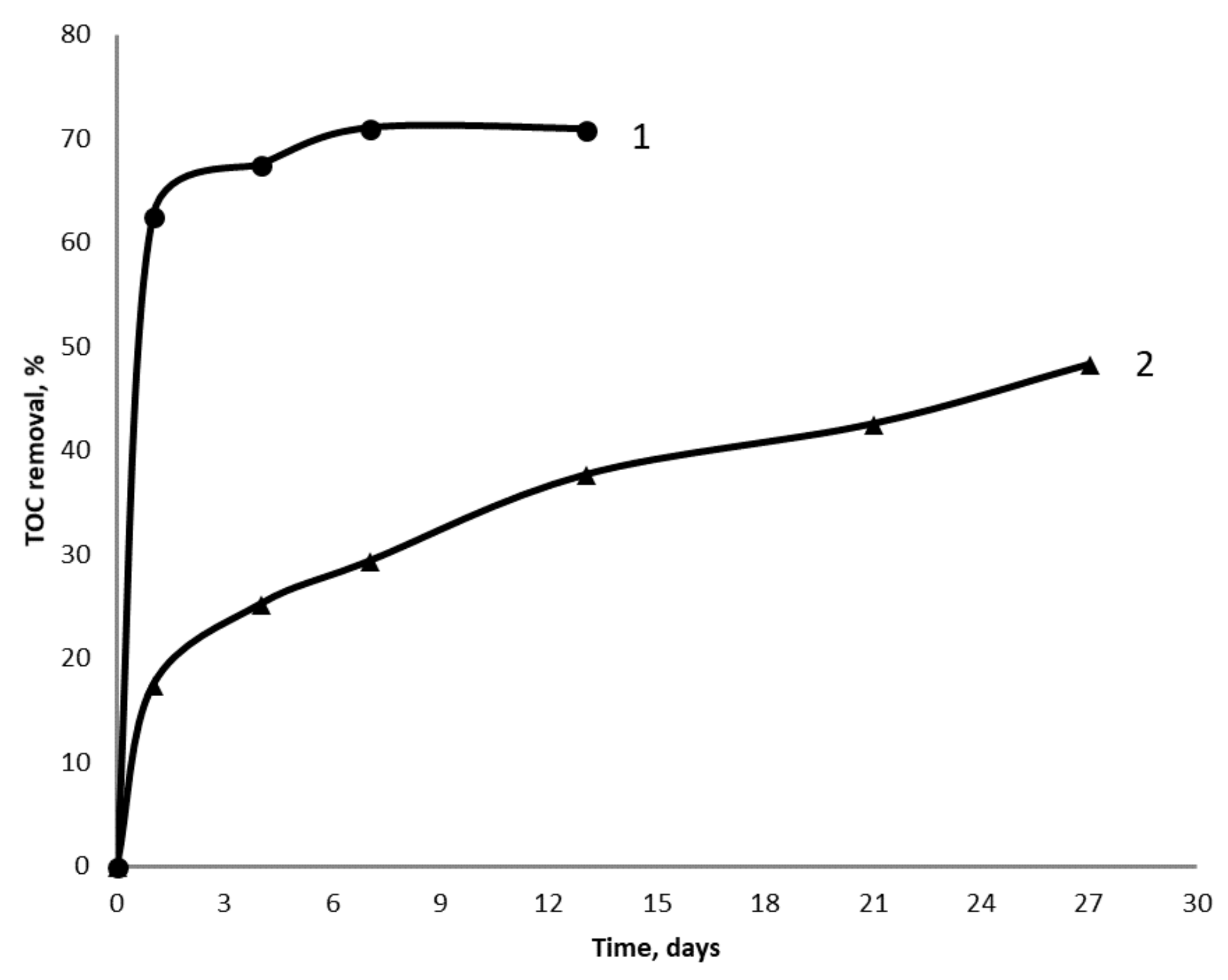
The continuous treatment of wastewater is going with the grown biofilm of iron-reducing bacteria, therefore hydraulic retention time (HRT) could be reduced from 21 days in batch culture to 5 days in continuous culture. However, efficiency of the continuous treatment of wastewater in the columns permanently decreased during the 25 days, from 70% to 23% in the first experiment, and to 20% in the second experiment. It was believed that this permanent decrease was due to the changes of the iron ore surface. To examine this hypothesis, iron ore that that was used for 21 days in batch treatment and 25 days in continuous treatment was extracted from the columns and washed out with water. Then, new sets of batch and continuous experiments had been started in the columns filled with either fresh or used iron ore. TOC removal was 71% and 38% after 14 days of wastewater treatment with either fresh or used iron ore (Figure 2).
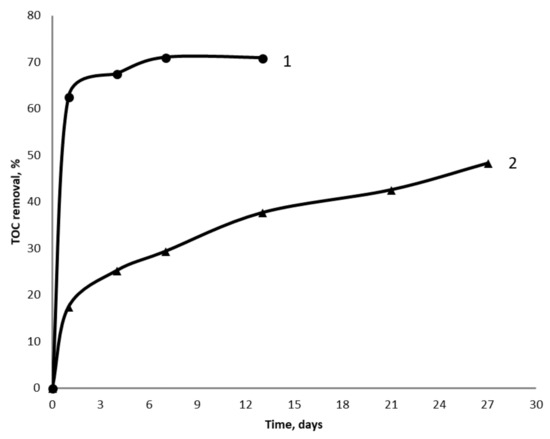
Figure 2.
The removal of TOC in the laboratory facility operated in batch mode with either fresh iron ore (curve 1) or used iron ore (curve 2).
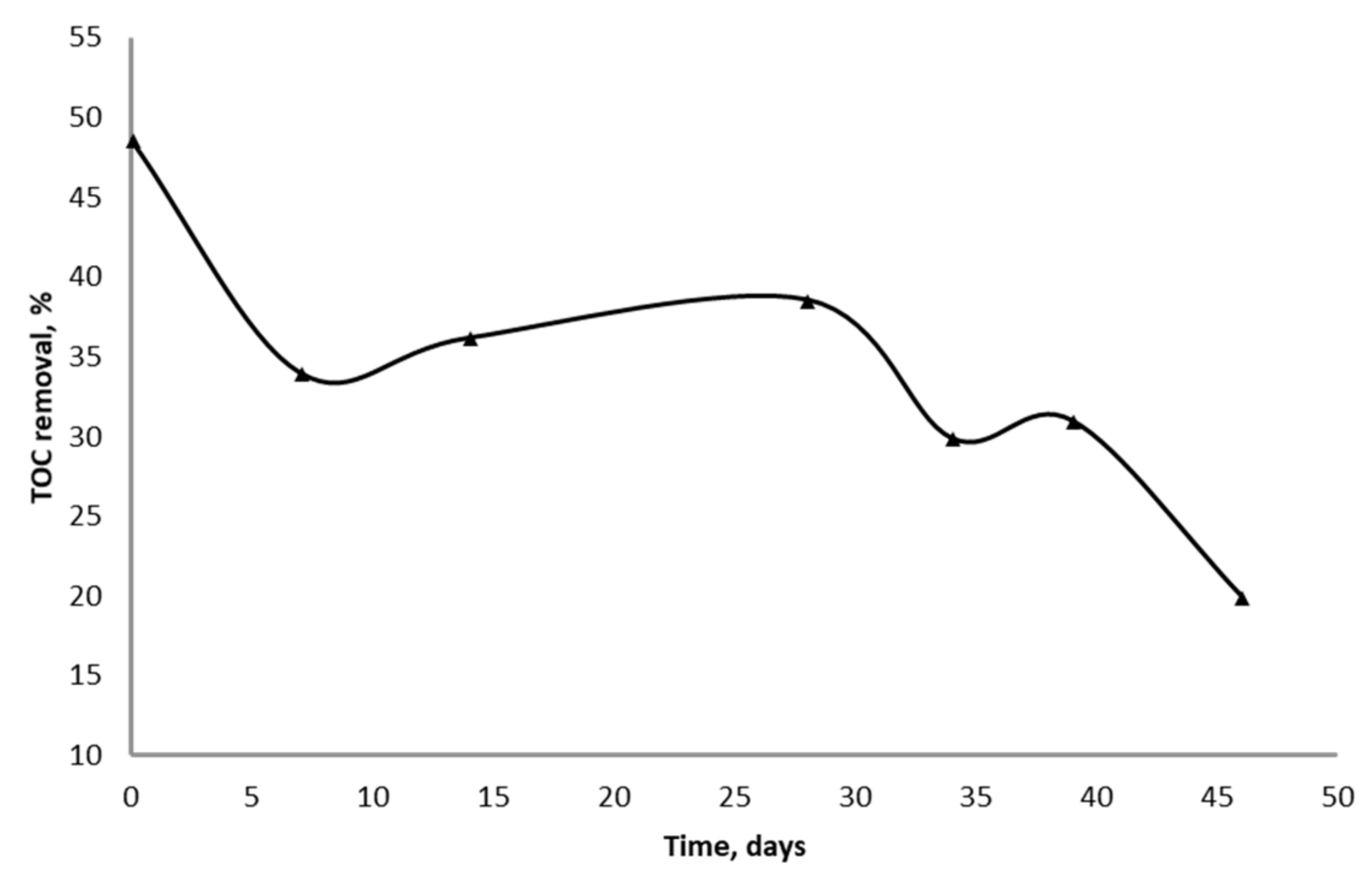
The efficiency of TOC removal during the continuous treatment of wastewater with used iron ore decreased from 48 to 20% for 45 days (Figure 3).
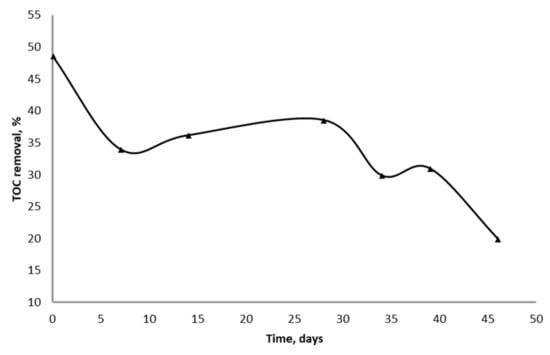
Figure 3.
The removal of TOC from wastewater during continuous process in the column reactors with used iron ore.
Therefore, the treatment of sucralose production wastewater using bioreduction of iron ore requires the approximately monthly regeneration of iron ore surface, notwithstanding permanent bioreduction and dissolution of this surface with production of Fe2+ ions. The concentration of total iron in effluent was 12 ± 0.5 mg/L, so almost all Fe2+ produced in anaerobic columns was hydrolyzed and precipitated in aerobic reactors.
4. Discussion
It is well known that sucralose itself did not degrade in aerobic or anaerobic biological reactors, either metabolically or co-metabolically (in the presence of sucrose), after 42–62 days of experiments [26]. The same could be expected for the by-products of sucralose production. In our experiments, biodegradability of the compounds in sucralose production wastewater was dismal, but under the conditions of bioreduction and biodissolution of iron ore, almost 70% of organic matter of this wastewater can be removed. The mechanism of recalcitrant organics removal from the wastewater of sucralose production is an adsorption of the polarized chlorinated organic substances on the positively charged particles of iron (hydr)oxides produced after the dissolution of the iron ore surface. An adsorption capacity of iron (hydr)oxide particles without bioreduction and biodissolution was about 20% of initial TOC, only because of the low specific surface of the particles. However, iron-reducing bacteria permanently renew the surface of iron ore particles and produce from iron ore small particles of ferrous and ferric hydroxides with a high specific surface, which adsorb chlorinated organics and ensure removal of TOC on the level of 70% of the initial value. Due to adsorption on renewal iron hydroxide surfaces, the biotreatment with iron ore can significantly diminish the quantity of granulated activated carbon that is used currently (or is planned to be used) for the removal of recalcitrant organics from the wastewater of sucralose production. According to the data of the sucralose manufacturer, adsorption capacity of GAC before the break point (after concentration of COD in effluent exceeding 100 mg/L) was 33 mg of COD per 1 g of GAC. Thus, treatment of 1 m3 of sucralose wastewater with COD concentration about 2000 mg/L requires 60 kg GAC, while wastewater biotreatment with iron ore, that is removing at least 50% COD, will require only 30 kg GAC/m3 thus saving at least $30/m3 of the wastewater, i.e., ensuring from $5 million to $25 million in annual savings for a sucralose production plant.
This process could be designed even more effectively because iron oxide can be regenerated by thermal treatment. In our experiments, the thermal treatment of used iron ore at 500 °C for 1 h increased adsorption capacity by 150% in comparison with fresh iron ore. COD removal capacity of iron ore in one cycle of the treatment was about 0.21 mg of COD per 1 g of iron ore, but due to 150 regenerations the total capacity could be 32 mg of COD per 1 g of iron ore, which is the same value as the adsorption capacity of GAC. However, the price for iron ore is about US$100/metric ton while the price for GAC (“activated charcoal”) is about US$2000/metric ton. The dosage of iron ore considering 150 regeneration cycles could be 46.7 g/L of wastewater. So, the savings with iron ore biocolumns will be determined by the difference of the costs of GAC plus disposal of GAC after treatment versus the cost of iron ore plus the cost of four columns and their thermal regeneration system. The three columns will work in continuous regime and one column will be out of flow for thermal regeneration and batch growth of iron-reducing bacteria biomass.
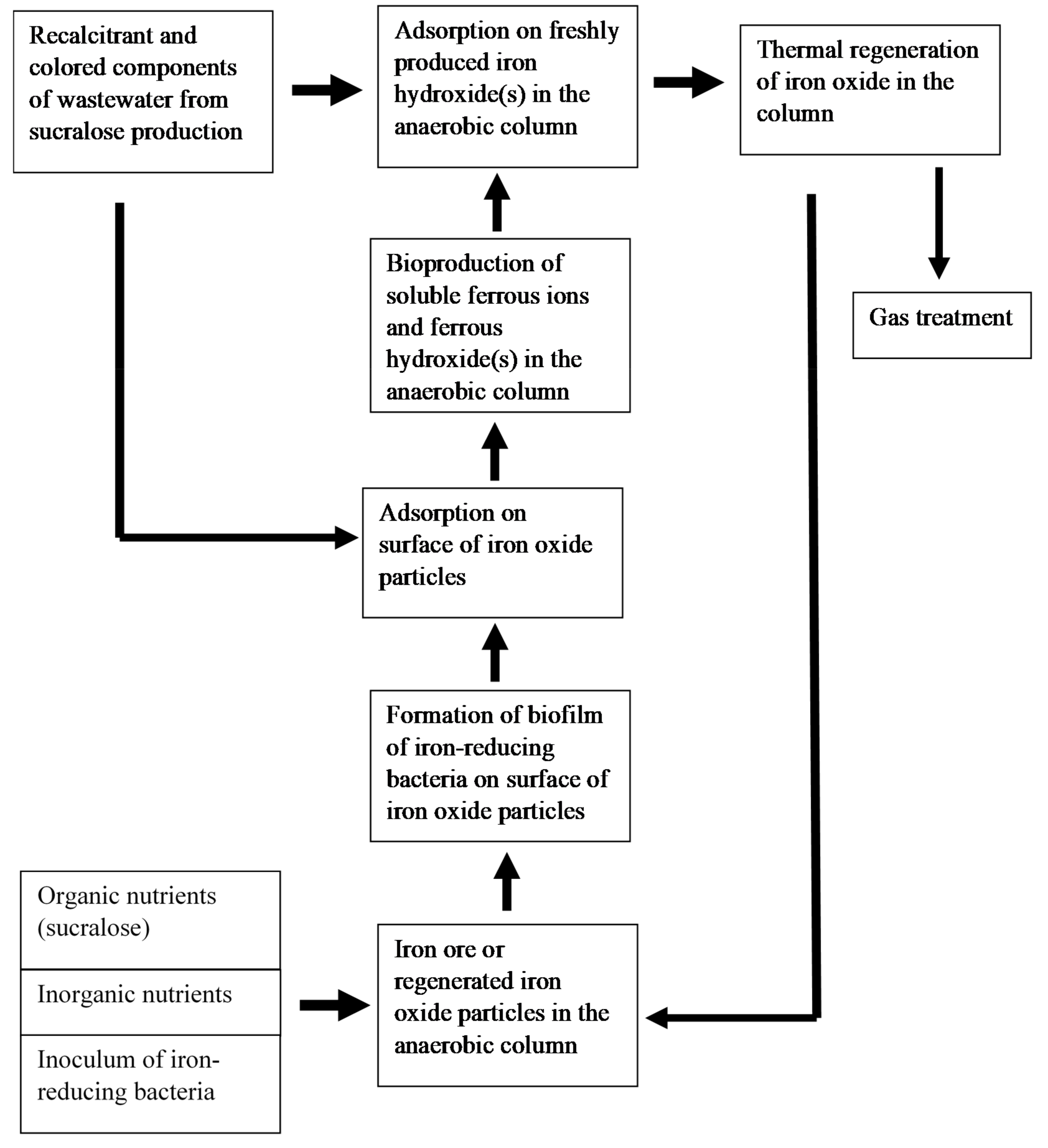
The conceptual scheme for the removal of xenobiotics from the wastewater of sucralose production plant using regenerated iron oxide adsorbent is shown in Figure 4.
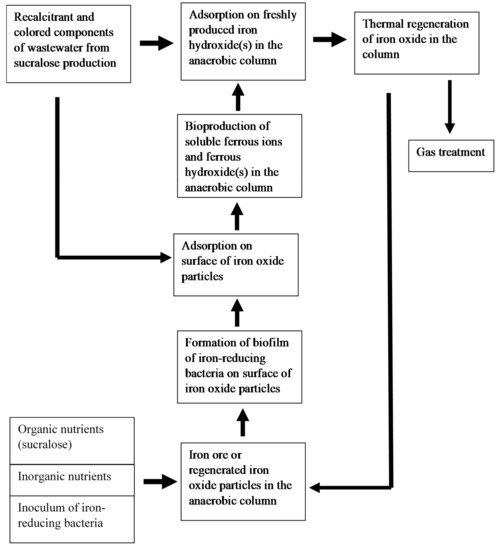
Figure 4.
The conceptual scheme for the removal of xenobiotics from the wastewater of sucralose production plant.
5. Conclusions
The removal of TOC using biotreatment with fresh iron ore was 60–70% after 14 days of batch cultivation. The removal of TOC using biotreatment with fresh iron ore was about 50% after 14 days of continuous process with HRT 3–5 days.
About 20% of recalcitrant organic compounds were removed quickly by non-treated iron ore particles and (b) adsorption of other (30–50% of recalcitrant organic compounds) was due to adsorption on ferrous/ferric hydroxides that are slowly produced by the microbial reduction of iron ore.
The pre-treatment of wastewater using bioreductin/oxidation of iron ore diminishes the quantity of granulated activated carbon (GAC) for post treatment by up to 70%.
Author Contributions
Conceptualization, J.H.T.; Methodology, V.S.; Formal Analysis, V.S.; Investigation, V.S.; Writing-Original Draft Preparation, V.I.; Project Administration, V.I.
Funding
Experiments were supported by Sembcorp Industries Ltd. and Nanyang Technological University, Singapore, data analysis and manuscript preparation was supported by National University of Food Technology, Ukraine.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Ivanov, V.; Stabnikov, V.; Guo, C.H.; Stabnikova, O.; Ahmed, Z.; Kim, I.S.; Shuy, E.B. Wastewater engineering applications of BioIronTech process based on the biogeochemical cycle of iron bioreduction and (bio)oxidation. AIMS Environ. Sci. 2014, 1, 53–66. [Google Scholar] [CrossRef]
- Ivanov, V. Environmental Microbiology for Engineers, 2nd ed.; CRC Press, Taylor & Francis Group: Abingdon, UK, 2015. [Google Scholar]
- Tay, J.H.; Tay, S.T.L.; Ivanov, V.; Stabnikova, O.; Wang, J.-Y. Compositions and Methods for the Treatment of Wastewater and Other Waste. U.S. Patent 7,393,452, 1 July 2008. [Google Scholar]
- Tay, J.H.; Tay, S.T.L.; Ivanov, V.; Hung, Y.C. Application of biotechnology for industrial waste treatment. In Handbook of Industrial Wastes Treatment, 2nd ed.; Revised and Expanded by Marcel Dekker; CRC Press, Taylor & Francis Group: New York, NY, USA, 2004; pp. 585–618. [Google Scholar]
- Roussel, J.; Carliell-Marquet, C. Significance of vivianite precipitation on the mobility of iron in anaerobically digested sludge. Front. Environ. Sci. 2016, 4, 60. [Google Scholar] [CrossRef]
- Guo, C.H.; Stabnikov, V.; Ivanov, V. The removal of phosphate from wastewater using anoxic reduction of iron ore in the rotating reactor. Biochem. Eng. J. 2009, 46, 223–226. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, J.; Chen, B.; Liu, L. Effectiveness of phosphate removal during anaerobic digestion of waste activated sludge by dosing iron (III). J. Environ. Manag. 2017, 193, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.; Lim, J.J.W.; Stabnikova, O.; Gin, K.Y.-H. Biodegradation of estrogens by facultative anaerobic iron-reducing bacteria. Process Biochem. 2010, 45, 284–287. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Z.; Zhang, Y. Sustainable strategy for enhancing anaerobic digestion of waste activated sludge: Driving dissimilatory iron reduction with fenton sludge. ACS Sustain. Chem. Eng. 2018, 6, 2220–2230. [Google Scholar] [CrossRef]
- Ivanov, V.; Stabnikova, E.V.; Stabnikov, V.P.; Kim, I.S.; Zubair, A. Effects of iron compounds on the treatment of fat-containing wastewaters. Appl. Biochem. Microbiol. 2002, 38, 255–258. [Google Scholar] [CrossRef]
- Ivanov, V.; Wang, J.-Y.; Stabnikov, V.; Xing, Z.; Tay, J.-H. Improvement of sludge quality by iron-reducing bacteria. J. Residuals Sci. Tech. 2004, 1, 165–168. [Google Scholar]
- Stabnikov, V.P.; Ivanov, V.N. The effect of various iron hydroxide concentrations on the anaerobic fermentation of sulfate-containing model wastewater. Appl. Biochem. Microbiol. 2006, 42, 284–288. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Chang, J.; Quan, X.; Li, Q. Biological sulfate reduction in the acidogenic phase of anaerobic digestion under dissimilatory Fe (III)-reducing conditions. Water Res. 2013, 47, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Yuzir, A.; Yaacob, S.S.; Tijani, H.I.; Abdullah, N.; Ahmed, Z. Addition of ferric chloride in anaerobic digesters to enhance sulphide removal and methanogenesis. Desalin. Water Treat. 2017, 79, 64–72. [Google Scholar] [CrossRef]
- Ivanov, V.; Stabnikov, V. Construction Biotechnology: Biogeochemistry, Microbiology and Biotechnology of Construction Materials and Processes; Springer Science & Business Media: Singapore, 2017; 317p. [Google Scholar]
- Stabnikov, V.; Ivanov, V. Biotechnological production of biogrout from iron ore and cellulose. J. Chem. Technol. Biotechnol. 2017, 92, 180–187. [Google Scholar] [CrossRef]
- Fitch, C.; Keim, K. Position of the Academy of Nutrition and Dietetics: Use of nutritive and nonnutritive sweeteners. J. Acad. Nutr. Diet. 2012, 112, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, J.; Eaton, A.; Badruzzaman, M.; Haghani, A.W.; Jacangelo, J.G. Occurrence and suitability of sucralose as an indicator compound of wastewater loading to surface waters in urbanized regions. Water Res. 2011, 45, 4019–4027. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Jiang, Y.; Tsoi, Y.-K.; Leung, K.S.-Y. Evaluating the environmental impact of artificial sweeteners: A study of their distributions, photodegradation and toxicities. Water Res. 2014, 52, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Singer, H.; Berg, M.; Müller, B.; Pernet-Coudrier, B.; Liu, H.; Qu, J. Elimination of polar micropollutants and anthropogenic markers by wastewater treatment in Beijing, China. Chemosphere 2015, 119, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Schenck, K.; Rosenblum, L.; Ramakrishan, B.; Carson, J., Jr.; Macke, D.; Nietcha, C. Correlation of trace contaminants to wastewater management practices in small watersheds. Environ. Sci. Process. Impacts 2015, 17, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.; Gawlik, B.; Boettcher, K.; Locoro, G.; Contini, S.; Bidoglio, G. Sucralose screening in European surface waters using a solid-phase extraction-liquid chromatography–triple quadrupole mass spectrometry method. J. Chromatogr. A 2009, 1216, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Mead, R.N.; Morgan, J.B.; Avery, G.B.; Kieber, R.J.; Kirk, A.M.; Skrabal, S.A.; Willey, J.D. Occurrence of the artificial sweetener sucralose in coastal and marine waters of the United States. Mar. Chem. 2009, 116, 13–17. [Google Scholar] [CrossRef]
- Tollefsen, K.E.; Nizzettj, L.; Huggett, D.B. Presence, fate and effects of the intense sweetener sucralose in the aquatic environment. Sci. Total Environ. 2012, 438, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-L.; Mahan, T.A.; Farley, E. Microbial Consortia for Biodegradation of Sucralose and Other Chlorinated Carbohydrates. U.S. Patent 8,557,976B2, 15 October 2013. [Google Scholar]
- Torres, C.I.; Ramakrishna, S.; Chiu, C.-A.; Nelson, K.G.; Westerhoff, P.; Krajmalnik-Brown, R. Fate of sucralose during wastewater treatment. Environ. Eng. Sci. 2011, 28, 325–331. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).