Pomegranate Juice Extract Decreases Cisplatin Toxicity on Peripheral Blood Mononuclear Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Pomegranate Fruit Extract
2.2. Chemical Tests
2.2.1. Phytochemical Screening Test
2.2.2. Antioxidant Test (DPPH Radical Scavenging Method)
2.2.3. Carbohydrates Test
2.2.4. Phenol Content Test
2.2.5. Dry Matter Test
2.3. Cell Culture and Treatment
2.3.1. A549 Cells
2.3.2. Control Normal Cells
2.3.3. Treatment of Cells
2.4. Viability Tests
Neutral Red Uptake Assay
2.5. Statistical Analysis
3. Results
3.1. Phytochemical Screening Test
3.2. Antioxidant Test (DPPH Radical Scavenging Method)
3.3. Carbohydrate Test
3.4. Phenol Content Test
3.5. Dry Matter Test
3.6. Cell Viability Results
3.6.1. Neutral Red Assay
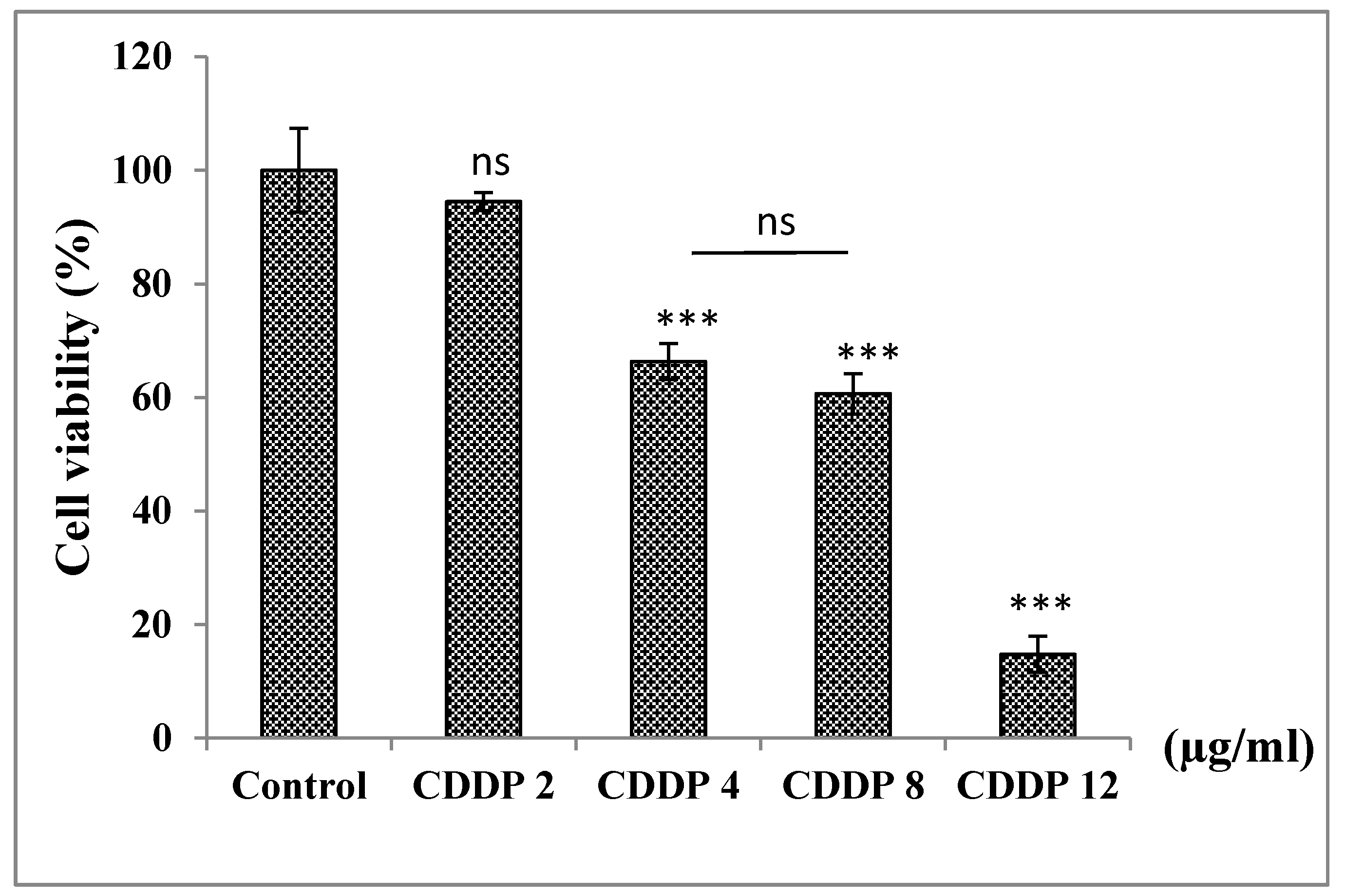
3.6.2. Treatment of A549 Cells with CDDP in a Dose−Dependent Manner
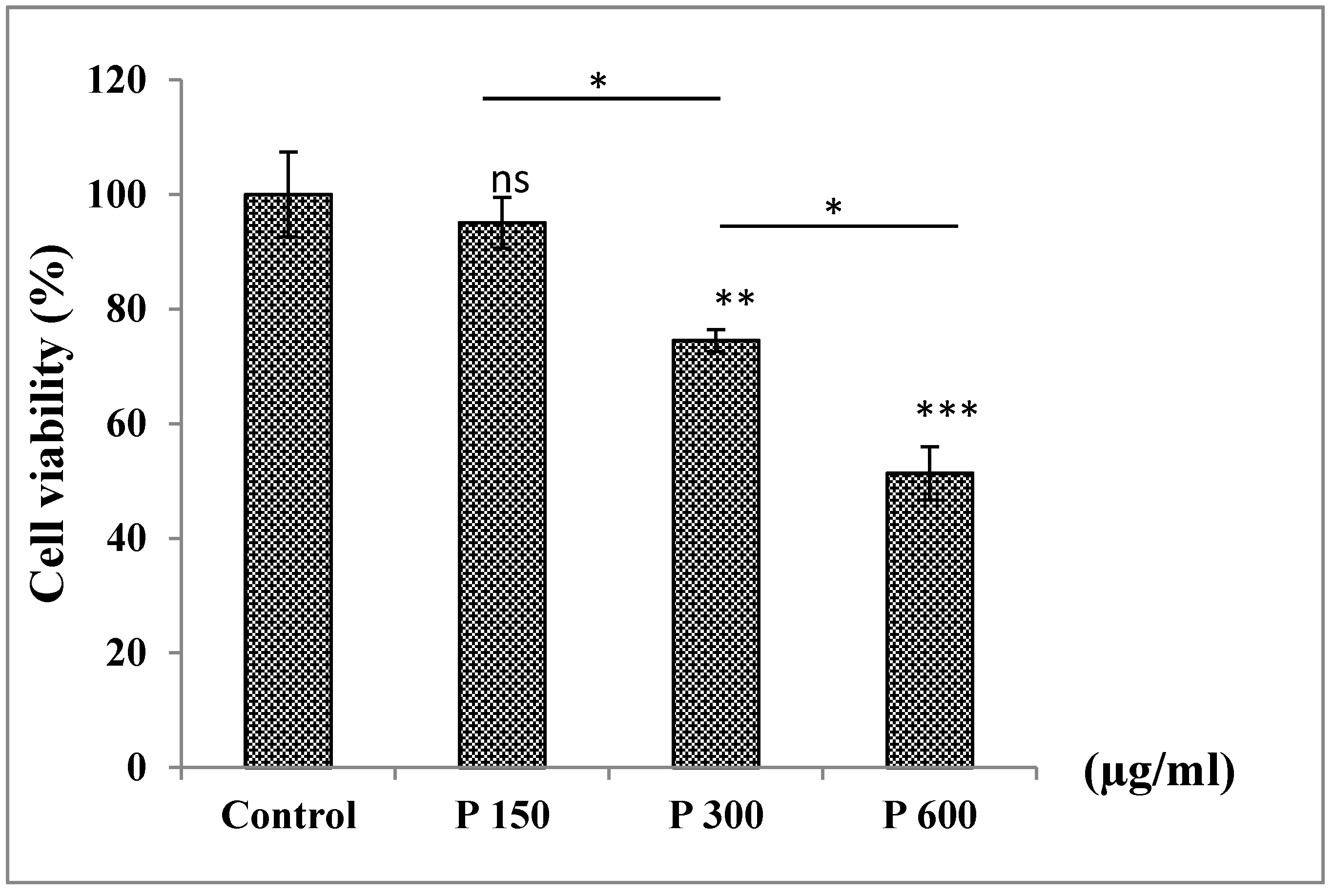
3.6.3. Treatment of A549 Cells with Pomegranate in a Dose−Dependent Manner
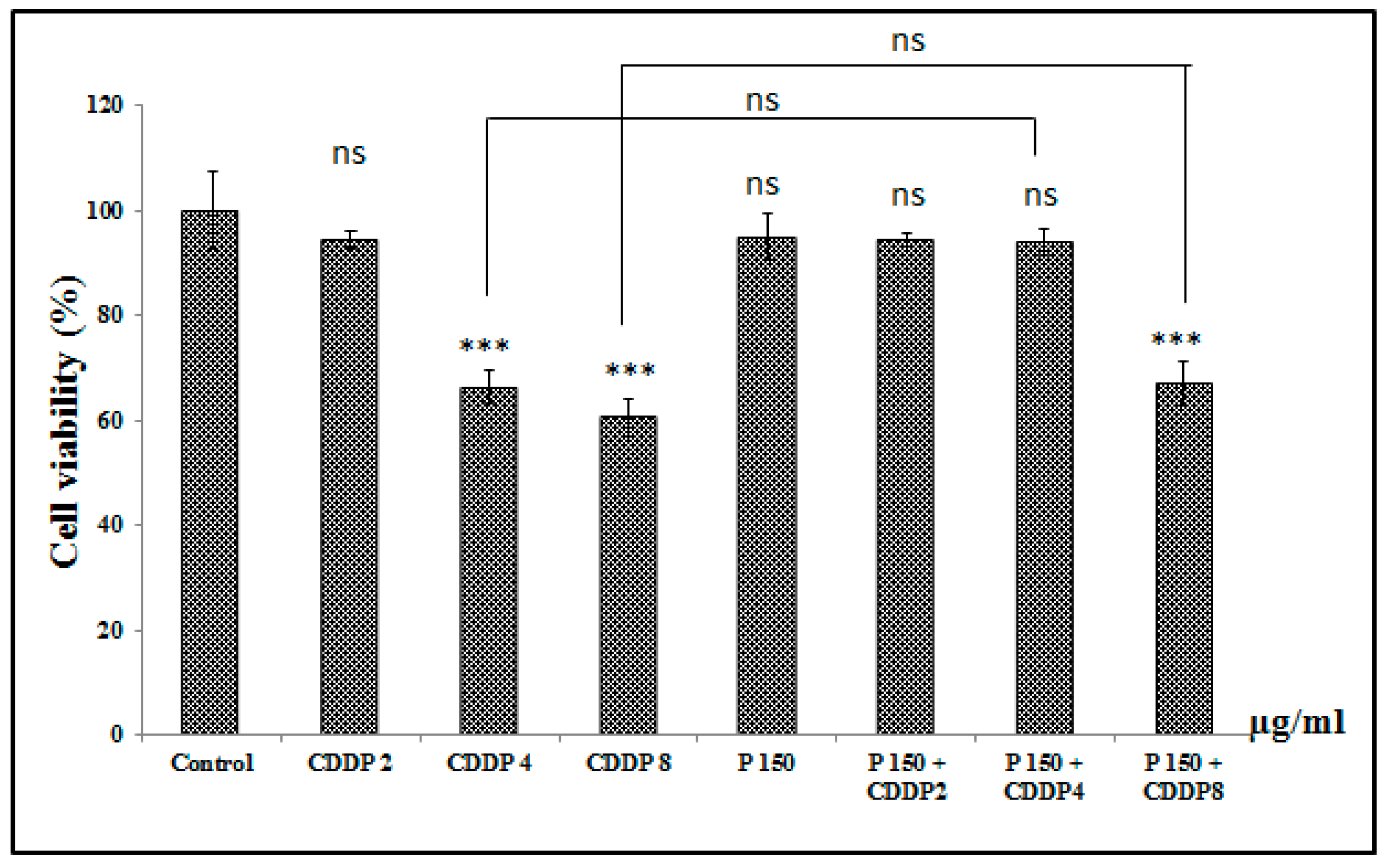
3.6.4. Effect of Combination of Pomegranate with CDDP on A549 Cell Viability
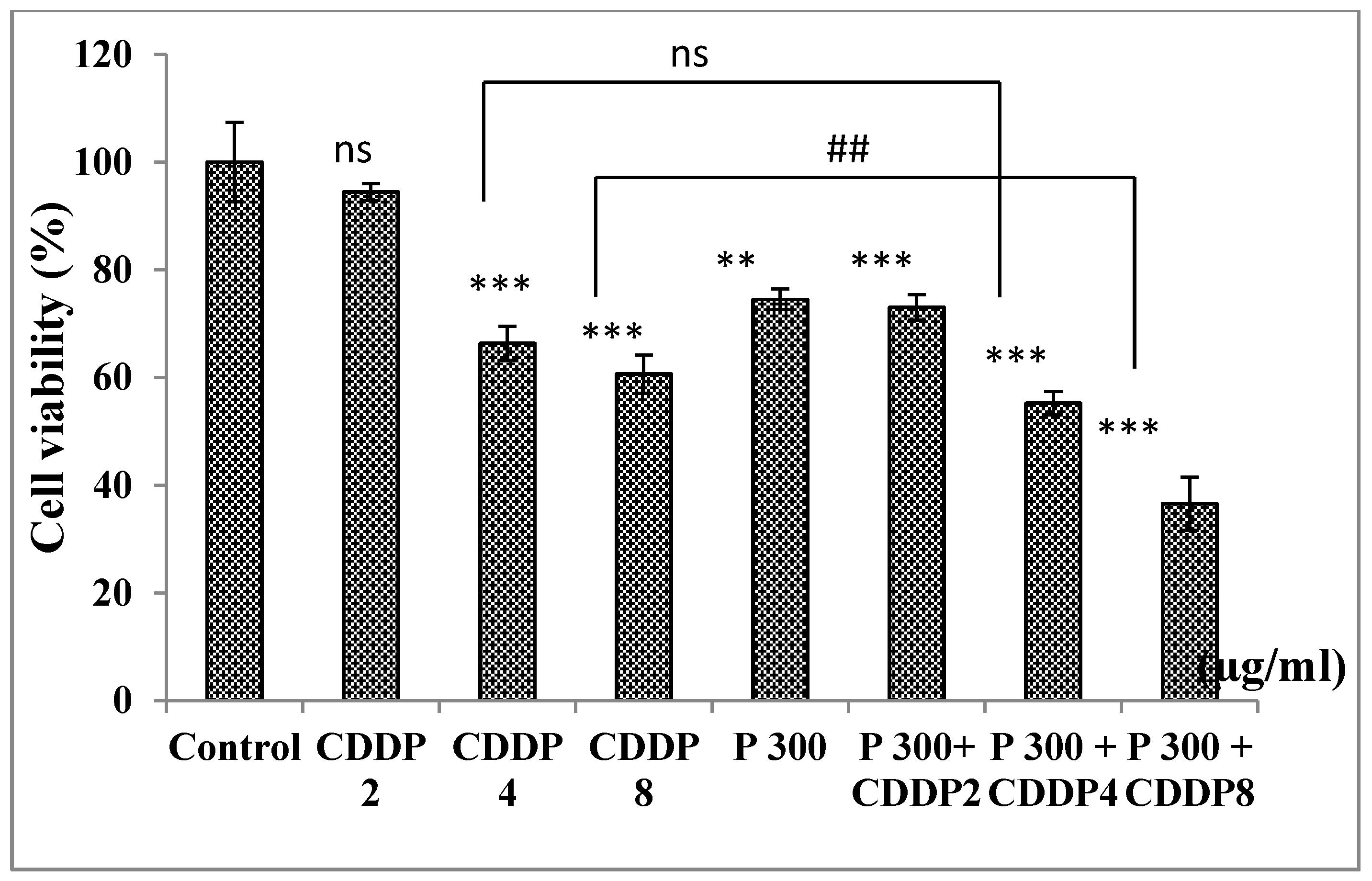
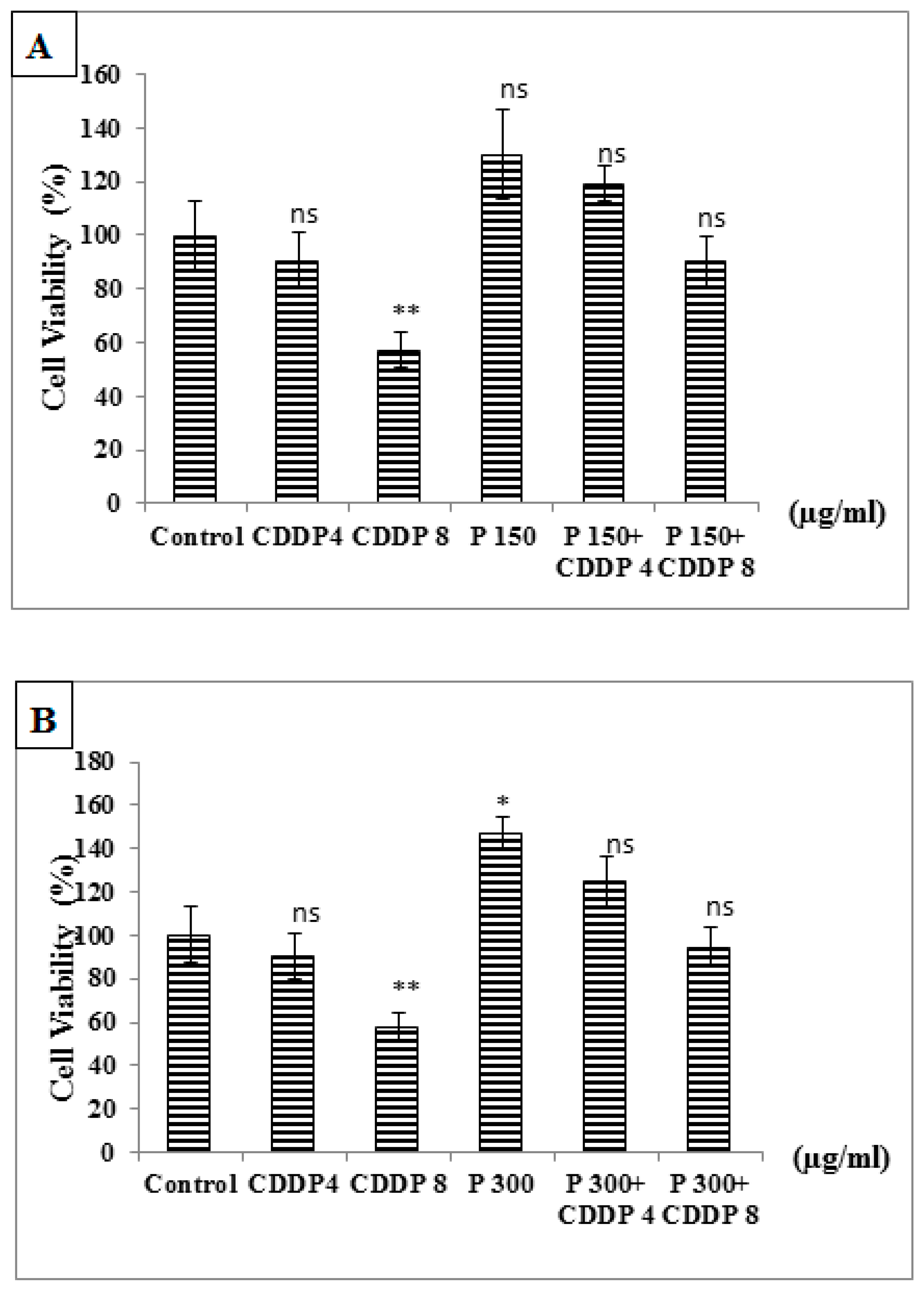
3.6.5. Effect of Combination of Pomegranate and CDDP on Normal PBMC
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2008; Volume 83, pp. 584–594. [Google Scholar] [CrossRef]
- Evans, M. Lung cancer: Needs assessment, treatment and therapies. Br. J. Nurs. 2013, 22, S15–S16. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Florea, A.M.; Busselberg, D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, L.; Ghiselli, S.; Guaran, V.; Chicca, M.; Simoni, E.; Olivetto, E.; Lelli, G.; Martini, A. Correlation of adverse effects of cisplatin administration in patients affected by solid tumours: A retrospective evaluation. Oncol. Rep. 2013, 29, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Nasser, M.; Cheikh-Ali, H.; Hijazi, A.; Merah, O.; Al-Rekaby, A.; Awada, R. Phytochemical Profile, Antioxidant and Antitumor Activities of Green Grape Juice. Processes 2020, 8, 507. [Google Scholar] [CrossRef]
- Makki, R.; Rammal, H.; Farhan, H.; Nasser, M.; el Dirani, Z.; Hijazi, A.; Badran, B. The antioxidant and anti-tumor activities of the Lebanese Centranthus Longiflorus LThe antioxidant and anti-tumor activities of the Lebanese Centranthus Longiflorus L. World J. Pharm. Sci. 2015, 3, 347–354. [Google Scholar]
- Nasser, G.; Sabbah, A.; Chokeir, N.; Hijazi, A.; Rammal, H.; Issa, M. Chemical composition and antioxidant capacity of Lebanese molasses pomegranate. Am. J. Pharm. Tech. Res. 2017, 7, 191–204. [Google Scholar]
- Turrini, E.; Ferruzzi, L.; Fimognari, C. Potential Effects of Pomegranate Polyphenols in Cancer Prevention and Therapy. Oxid. Med. Cell Longev. 2015, 2015, 938475. [Google Scholar] [CrossRef]
- Al-Dujaili, E.; Abu Hajleh, M. Article Anti-Cancer Activity of Pomegranate and its Biophenols. EC Nutr. 2016, 6, 28–52. [Google Scholar]
- Bassiri-Jahromi, S. Punica granatum (Pomegranate) activity in health promotion and cancer prevention. Oncol. Rev. 2018, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Hijazi, A.; Bouchra, S.; Zeinab, J.; Sajida, I.; Rammal, H.; Abd-El-Ameer, A.; Mohammed, N. Efficiency of combining pomegranate juice with low-doses of cisplatin and taxotere on A549 human lung adenocarcinoma cells. Asian Pac. J. Trop. Biomed. 2018, 8, 19. [Google Scholar] [CrossRef]
- Mohamad, N.; As-sadi, F.; Fatima, J.; Hussein, K.; Hijazi, A.; Ali, C.; Hassan, R. Antibacterial, antioxidant and antiproliferative activities of the hydroalcoholic extract of the lebanese plant: Ephedra campylopoda. Int Res. J. Pharm. 2017, 7, 23–29. [Google Scholar] [CrossRef]
- Brand, W.; Cuvelier, W.; Berset, M.E. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Farhan, H.; Malli, F.; Rammal, H.; Hijazi, A.; Bassal, A.; Ajouz, N.; Badran, B. Phytochemical screening and antioxidant activity of Lebanese Eryngium creticum L. Asian Pac. J. Trop. Biomed. 2012, 2, S1217–S1220. [Google Scholar] [CrossRef]
- Mannerström, M.; Toimela, T.; Sarkanen, J.R.; Heinonen, T. Human BJ Fibroblasts is an Alternative to Mouse BALB/c 3T3 Cells in in vitro Neutral Red Uptake Assay. Basic Clin. Pharmacol. Toxicol. 2017, 121, 109–115. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A Comparative Review on the Extraction, Antioxidant Content and Antioxidant Potential of Different Parts of Walnut (Juglans regia L.) Fruit and Tree. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Arruebo, M.; Vilaboa, N.; Saez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; Gonzalez-Fernandez, A. Assessment of the evolution of cancer treatment therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef]
- Panth, N.; Manandhar, B.; Paudel, K.R. Anticancer Activity of Punica granatum (Pomegranate): A Review. Phytother. Res. 2017, 31, 568–578. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; Khedr, E.G.; El-Bahrawy, H.A.; Abd El-Fattah, E.E. Effect of Pomegranate Hull Extract on Liver Neoplastic Changes in Rats: More than an Antioxidant. Nutr. Cancer 2016, 68, 1044–1051. [Google Scholar] [CrossRef]
- Jaganathan, S.; Vellayappan, M.V.; Narasimhan, G.; Supriyanto, E. Role of pomegranate and citrus fruit juices in colon cancer prevention. WJG 2014, 20, 4618–4625. [Google Scholar] [CrossRef]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tian, L. Diverse Phytochemicals and Bioactivities in the Ancient Fruit and Modern Functional Food Pomegranate (Punica granatum). Molecules 2017, 22. [Google Scholar] [CrossRef]
- Ricci, D.; Giamperi, L.; Bucchini, A.; Fraternale, D. Antioxidant activity of Punica granatum fruits. Fitoterapia 2006, 77, 310–312. [Google Scholar] [CrossRef]
- Sayed, B.; Talou, T.; Saad, Z.; Hijazi, A.; Cerny, M.; Kanaan, H.; Chokr, A.; Merah, O. Fennel oil and by-products seed characterization and their potential applications. Ind. Crops Prod. 2018, 111, 92–98. [Google Scholar] [CrossRef]
- Caravaca, A.M.G.; Verardo, V.; Toselli, M.; Segura Carretero, A.; Fernández-Gutiérrez, A.; Caboni, M. Determination of the major phenolic compounds in pomegranate juices by HPLC–DAD–ESI-MS. J. Agric. Food Chem. 2013, 61. [Google Scholar] [CrossRef]
- Zhao, F.; Pang, W.; Zhang, Z.; Zhao, J.; Wang, X.; Liu, Y.; Wang, X.; Feng, Z.; Zhang, Y.; Sun, W.; et al. Pomegranate extract and exercise provide additive benefits on improvement of immune function by inhibiting inflammation and oxidative stress in high-fat-diet-induced obesity in rats. J. Nutr. Biochem. 2016, 32, 20–28. [Google Scholar] [CrossRef]
- Magrone, T.; Jirillo, E.; Spagnoletta, A.; Magrone, M.; Russo, M.A.; Fontana, S.; Laforgia, F.; Donvito, I.; Campanella, A.; Silvestris, F.; et al. Immune Profile of Obese People and in vitro Effects of Red Grape Polyphenols on Peripheral Blood Mononuclear Cells. Oxid. Med. Cell. Longev. 2017, 2017, 9210862. [Google Scholar] [CrossRef] [PubMed]

| Plant Constituents | Reagents Added | Color |
|---|---|---|
| Phenolic acids | FeCl3 (1%) + K3 (Fe(CN)6) (1%) | Greenish Blue color |
| Terpenoids | Chloroform + concentrated sulfuric acid | Reddish brown color in the surface |
| Flavonoids | KOH (potassium hydroxide 50%) | Yellow |
| Quinones | HCl concentrated | Precipitate or yellow color |
| Alkaloids | Dragendroff reagent | Reddish Orange precipitate/turbidity |
| Tannins | (FeCl2 (1%)) | Blue color |
| Resins | Acetone + water + agitation | Turbidity |
| Saponins | Vigorous shaking | Layer of foam |
| Reducing sugar | Water + fehlings (A+B) + boil | Brick-red precipitate |
| Anthraquinones | HCl (10%) + boil | Precipitate |
| Proteins and amino acids | Ninhydrin (0.25%) + boil | Blue color |
| Phlabotannins | HCl (1%) + boil 5 min + cooling | Red precipitate |
| Flavanones | H2SO4 concentrated | Purple red color |
| Diterpenes | Copper sulfate | Green color |
| Sterols and steroids | Chloroform + H2SO4 concentrated | Red color of upper layer + greenish yellow fluorescence in acidic layer |
| Anthocyanins | NaOH (10%) | Blue color |
| Lignines | Safranin | Pink color |
| Cardiac glycosides | Acetic acid glacial + FeCl3 (5%) + H2SO4 concentrated | Purple ring + brown ring + green ring |
| Fixed oils and fatty acids | Spot test | Oil spot |
| Bioactive Molecule | Result |
|---|---|
| Reducing sugar | + |
| Anthraquinones | − |
| Proteins and amino acids | − |
| Phlabotannins | − |
| Alkaloids | + |
| Tanins | + |
| Resins | − |
| Terpenoids | + |
| Flavonoids | + |
| Quinones | − |
| Sterols et steroids | + |
| Diterpenes | − |
| Anthocyanins | + |
| Flavanones | + |
| Lignins | + |
| Cardiac glycosides | − |
| Saponins | − |
| Phenols | + |
| Fixed oils and fatty acids | − |
| Test | Content |
|---|---|
| Amount of carbohydrates | 69.29% |
| Total phenol content | 6.93 mg GAE/g |
| The percentage of dry matter | 81.1463% |
| Classes | Phytochemicals |
|---|---|
| Ellagitannins, gallotannins, and derivatives | Brevifolin, Brevifolin carboxylic acid, Brevifolin carboxylic acid 10−monopotassium sulphate, Castalagin, Casuariin, Casuarinin, Corilagin, Isocorilagin, Hippomanin A, Gemin D |
| Flavonoids | Rutin (Quercetin−3−O−rutinoside), Quercetin−3,40−dimethyl ether 7−O−_−L−arabinofuranosyl(1−6)−_−D−glucoside, Cyanidin, Chrysanthemin (Cyanidin−3−O−glucoside), Cyanin (Cyanidin−3,5−di−O−glucoside), (Procyanidin A2, Procyanidin B1, Procyanidin B2, Procyanidin B3 |
| Lignans | Conidendrin, Isohydroxymatairesinol, Isolariciresinol, Matairesinol |
| Triterpenoids and phytosterols | Punicanolic acid, Ursolic acid, Campesterol, Cholesterol |
| Fatty acids and lipids | Caproic acid (Hexanoic acid), Caprylic acid (Octanoic acid), Capric acid |
| Akaloids and indolamines | N−(2′,5′−Dihydroxyphenyl)pyridinium chloride, Hygrine, Norhygrine, Pelletierine, N−Methylpelletierine, Norpseudopelletierine, Pseudopelletierine, 2−(2′−Hydroxypropyl)−∆1piperideine, 2−(2′−Propenyl)−∆1piperideine, Punigratane (2,5−Diheptyl−N−methylpyrrolidine), Sedridine, Melatonin, Serotonin, Tryptamine |
| Organic acids and phenolic acids | Ascorbic acid, Citric acid, Fumaric acid, L−Malic acid, Oxalic acid, Quinic acid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasser, M.; Damaj, Z.; Hijazi, A.; Merah, O.; Al-Khatib, B.; Hijazi, N.; Trabolsi, C.; Damaj, R.; Nasser, M. Pomegranate Juice Extract Decreases Cisplatin Toxicity on Peripheral Blood Mononuclear Cells. Medicines 2020, 7, 66. https://doi.org/10.3390/medicines7100066
Nasser M, Damaj Z, Hijazi A, Merah O, Al-Khatib B, Hijazi N, Trabolsi C, Damaj R, Nasser M. Pomegranate Juice Extract Decreases Cisplatin Toxicity on Peripheral Blood Mononuclear Cells. Medicines. 2020; 7(10):66. https://doi.org/10.3390/medicines7100066
Chicago/Turabian StyleNasser, Mohamad, Ziad Damaj, Akram Hijazi, Othmane Merah, Batoul Al-Khatib, Nadine Hijazi, Christine Trabolsi, Raghida Damaj, and Mouhamad Nasser. 2020. "Pomegranate Juice Extract Decreases Cisplatin Toxicity on Peripheral Blood Mononuclear Cells" Medicines 7, no. 10: 66. https://doi.org/10.3390/medicines7100066
APA StyleNasser, M., Damaj, Z., Hijazi, A., Merah, O., Al-Khatib, B., Hijazi, N., Trabolsi, C., Damaj, R., & Nasser, M. (2020). Pomegranate Juice Extract Decreases Cisplatin Toxicity on Peripheral Blood Mononuclear Cells. Medicines, 7(10), 66. https://doi.org/10.3390/medicines7100066







