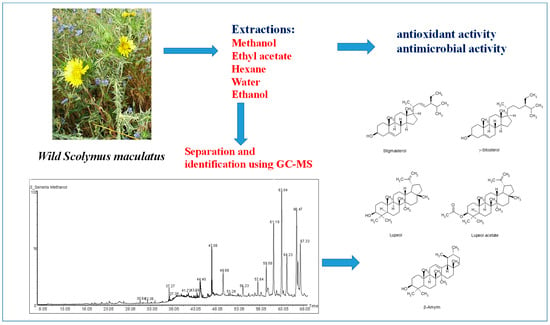
Phytochemical Composition and Biological Activities of Wild Scolymus maculatus L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection and Extract Preparation
2.2. Instrumentation
2.3. GC-MS Chromatographic Condition
2.4. Free Radical Scavenging Capabilities of Extracts
2.5. Antibacterial and Antifungal Activity
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DPPH | diphenylpicrylhydrazyl |
| AA | antioxidant activity |
| MIC | minimum inhibitory concentration |
| GC-MS | gas chromatography-mass spectrometry |
| EI | electron impact |
| BHI | brain heart infusion |
| EC50 | half-maximal effective concentration |
| TIC | total ion chromatogram |
References
- Aswad, M.; Rayan, M.; Abu-Lafi, S.; Falah, M.; Raiyn, J.; Abdallah, Z. Nature is the best source of anti-inflammatory drugs: Indexing natural products for their anti-inflammatory bioactivity. Inflamm. Res. 2018, 67, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.; Abu-Lafi, S.; Adawi, A.; Schwed, J.S.; Stark, H.; Rayan, A. From medicinal plant extracts to defined chemical compounds targeting the histamine H4 receptor: Curcuma longa in the treatment of inflammation. Inflamm. Res. 2017, 66, 923–929. [Google Scholar] [CrossRef]
- Zaid, H.; Raiyn, J.; Osman, M.; Falah, M.; Srouji, S.; Rayan, A. In silico modeling techniques for predicting the tertiary structure of human H4 receptor. Front. Biosci. 2016, 21, 597–619. [Google Scholar]
- Zeidan, M.; Rayan, M.; Zeidan, N.; Falah, M.; Rayan, A. Indexing natural products for their potential anti-diabetic activity: Filtering and mapping discriminative physicochemical properties. Molecules 2017, 22, 1563. [Google Scholar] [CrossRef]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Rayan, A.; Raiyn, J.; Falah, M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS ONE 2017, 12, e0187925. [Google Scholar] [CrossRef]
- Kacergius, T.; Abu-Lafi, S.; Kirkliauskiene, A.; Kirkliauskiene, A.; Gabe, V.; Adawi, A.; Rayan, M.; Qutob, M.; Stukas, R.; Utkus, A.; et al. Inhibitory capacity of Rhus coriaria L. extract and its major component methyl gallate on Streptococcus mutans biofilm formation by optical profilometry: Potential applications for oral health. Mol. Med. Rep. 2017, 16, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Gabe, V.; Kacergius, T.; Abu-Lafi, S.; Kalesinskas, P.; Masalha, M.; Falah, M.; Abu-Farich, B.; Melninkaitis, A.; Zeidan, M.; Rayan, A. Inhibitory Effects of Ethyl Gallate on Streptococcus mutans Biofilm Formation by Optical Profilometry and Gene Expression Analysis. Molecules 2019, 24, 529. [Google Scholar] [CrossRef] [PubMed]
- Masalha, M.; Abu-Lafi, S.; Abu-Farich, B.; Rayan, M.; Issa, N.; Zeidan, M.; Rayan, A. A New Approach for Indexing Honey for Its Heath/Medicinal Benefits: Visualization of the Concept by Indexing Based on Antioxidant and Antibacterial Activities. Medicines 2018, 5, 135. [Google Scholar] [CrossRef] [PubMed]
- Masalha, M.; Rayan, M.; Adawi, A.; Abdallah, Z.; Rayan, A. Capturing antibacterial natural products with in silico techniques. Mol. Med. Rep. 2018, 18, 763–770. [Google Scholar] [CrossRef]
- Rayan, M.; Abdallah, Z.; Abu-Lafi, S.; Masalha, M.; Rayan, A. Indexing natural products for their antifungal activity by filters-based approach: Disclosure of discriminative properties. Curr. Comput. Aided Drug Des. 2018, 15, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Abu-Lafi, S.; Makhamra, S.; Rayan, I.; Barriah, W.; Nasser, A.; Abu Farkh, B.; Rayan, A. Sesamin from Cuscuta palaestina natural plant extracts: Directions for new prospective applications. PLoS ONE 2018, 13, e0195707. [Google Scholar] [CrossRef] [PubMed]
- Said, O.; Khalil, K.; Fulder, S.; Azaizeh, H. Ethnopharmacological survey of medicinal herbs in Israel, the Golan Heights and the West Bank region. J. Ethnopharmacol. 2002, 83, 251–265. [Google Scholar] [CrossRef]
- Sawalha, A.F.; Sweileh, W.M.; Zyoud, S.H.; Jabi, S.W. Self-therapy practices among university students in Palestine: Focus on herbal remedies. Complement. Ther. Med. 2008, 16, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Azaizeh, H.; Saad, B.; Khalil, K.; Said, O. The state of the art of traditional arab herbal medicine in the eastern region of the mediterranean: A review. Evid. Based Complement. Alternat. Med. 2006, 3, 229–235. [Google Scholar] [CrossRef]
- Adnan, S.N.; Ibrahim, N.; Yaacob, W.A. Transcriptome analysis of methicillin-resistant Staphylococcus aureus in response to stigmasterol and lupeol. J. Glob. Antimicrob. Resist. 2017, 8, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Samani, M.; Rafieian-Kopaei, M.; Azimi, N. Gundelia: A systematic review of medicinal and molecular perspective. Pak. J. Biol. Sci. 2013, 16, 1238–1247. [Google Scholar]
- Raicht, R.F.; Cohen, B.I.; Fazzini, E.P.; Sarwal, A.N.; Takahashi, M. Protective effect of plant sterols against chemically induced colon tumors in rats. Cancer Res. 1980, 40, 403–405. [Google Scholar]
- Yasukawa, K.; Takido, M.; Matsumoto, T.; Takeuchi, M.; Nakagawa, S. Sterol and triterpene derivatives from plants inhibit the effects of a tumor promoter, and sitosterol and betulinic acid inhibit tumor formation in mouse skin two-stage carcinogenesis. Oncology 1991, 48, 72–76. [Google Scholar] [CrossRef]
- Ododo, M.M.; Choudhury, M.K.; Dekebo, A.H. Structure elucidation of beta-sitosterol with antibacterial activity from the root bark of Malva parviflora. SpringerPlus 2016, 5, 1210. [Google Scholar] [CrossRef]
- Ali, H.; Dixit, S.; Ali, D.; Alqahtani, S.M.; Alkahtani, S.; Alarifi, S. Isolation and evaluation of anticancer efficacy of stigmasterol in a mouse model of DMBA-induced skin carcinoma. Drug Des. Dev. Ther. 2015, 9, 2793–2800. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.A.; Saenz, M.T.; Garcia, M.D.; Fernandez, M.A. Study of the topical anti-inflammatory activity of Achillea ageratum on chronic and acute inflammation models. Z. Naturforsch. C 1999, 54, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Murtaza, I.; Tarapore, R.S.; Suh, Y.; Adhami, V.M.; Johnson, J.J.; Siddiqui, I.A.; Khan, N.; Asim, M.; Hafeez, B.B.; et al. Lupeol inhibits proliferation of human prostate cancer cells by targeting beta-catenin signaling. Carcinogenesis 2009, 30, 808–817. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M.; Palma De La Pena, N.; Pastor, N.; Martín-Cordero, C.; Navarro, E.; Cortés, F.; Ayuso, M.J.; Toro, M.V. Anti-tumour activity of Digitalis purpurea L. subsp. heywoodii. Planta. Med. 2003, 69, 701–704. [Google Scholar] [PubMed]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Al-Homaidan, A.A.; Ibraheem, I.B.M.; Al-Othman, M.R.; Hatamleh, A.A. Antibacterial β-amyrin isolated from Laurencia microcladia. Arab. J. Chem. 2015, 8, 32–37. [Google Scholar] [CrossRef]
- De Castilho, A.L.; da Silva, J.P.C.; Saraceni, C.H.C.; Collantes Díaz, I.E.; Paciencia, M.L.B.; Varella, A.D.; Suffredini, I.B. In vitro activity of Amazon plant extracts against Enterococcus faecalis. Braz. J. Microbiol. 2014, 45, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Ruiz, G.; Hernandez-Vazquez, L.; Luna, H.; Wacher-Rodarte, M.D.C.; Navarro-Ocana, A. Growth inhibition of Streptococcus from the oral cavity by alpha-amyrin esters. Molecules 2012, 17, 12603–12611. [Google Scholar] [CrossRef]
- Bisignano, G.; Lagana, M.G.; Trombetta, D.; Arena, S.; Nostro, A.; Uccella, N.; Mazzanti, G.; Saija, A. In vitro antibacterial activity of some aliphatic aldehydes from Olea europaea L. FEMS Microbiol. Lett. 2001, 198, 9–13. [Google Scholar] [CrossRef]
- Trombetta, D.; Saija, A.; Bisignano, G.; Arena, S.; Caruso, S.; Uccella, G.; Uccella, N.; Castelli, F. Study on the mechanisms of the antibacterial action of some plant alpha, beta-unsaturated aldehydes. Lett. Appl. Microbiol. 2002, 35, 285–290. [Google Scholar] [CrossRef]
- Wal, A.; Srivastava, R.S.; Wal, P.; Rai, A.; Sharma, S. Lupeol as a magical drug. Pharm. Biol. Eval. 2015, 2, 142–151. [Google Scholar]
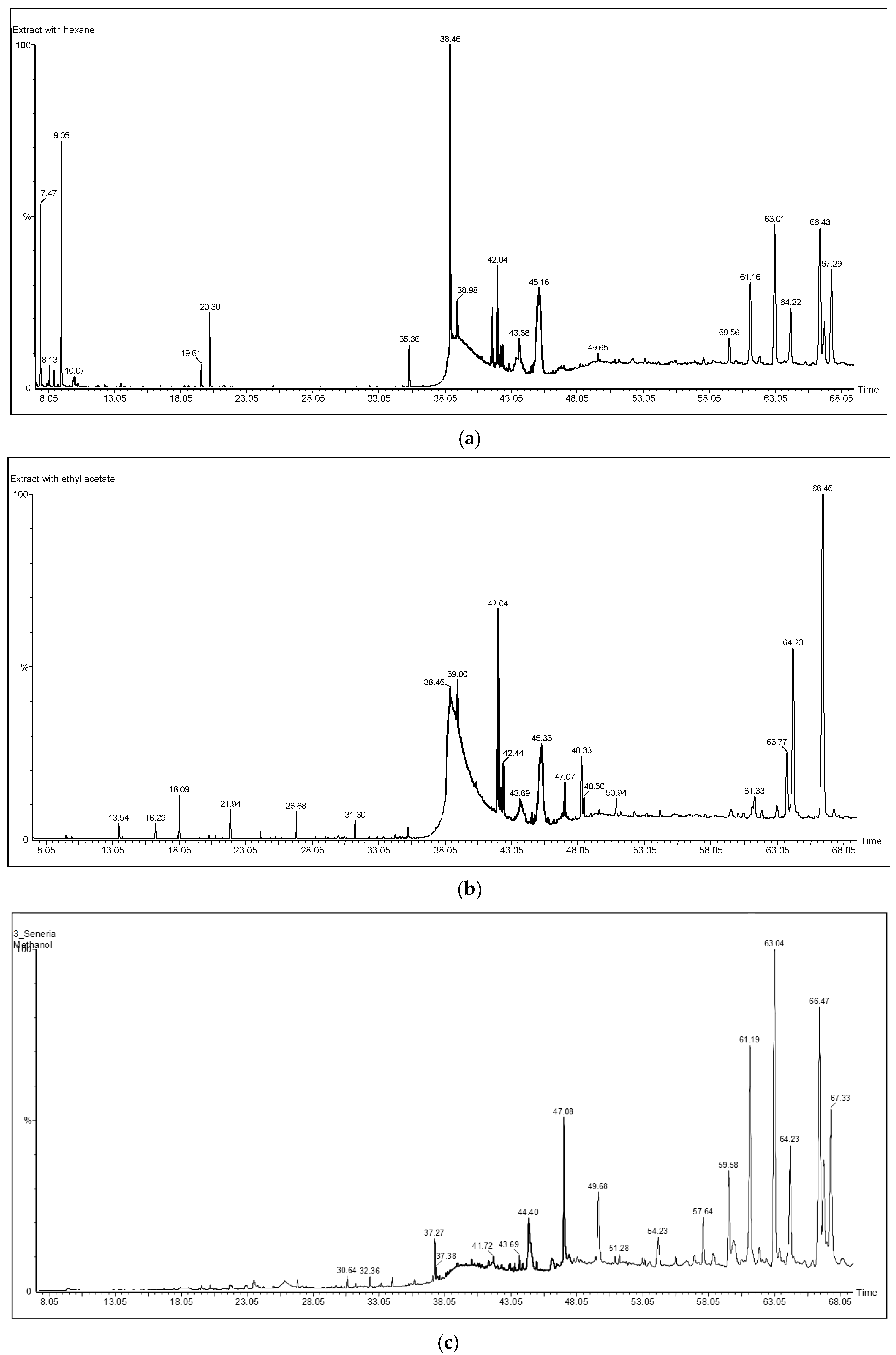
| Phytochemical Names and Retention Times | Area % | ||||
|---|---|---|---|---|---|
| No. | Compound Name | RT (min) | Methanol | Ethyl Acetate | n-Hexane |
| 1 | 1-Ethylbutyl hydroperoxide | 9.054 | 6.42 | ||
| 2 | 1-Ethyl-2-heptylcyclopropane | 16.29 | 0.32 | ||
| 3 | Acetoglyceride | 18.09 | 1.19 | ||
| 4 | 2,4-Decadienal | 20.303 | 1.78 | ||
| 5 | Nonylcyclopropane | 21.89 | 0.63 | ||
| 6 | (E)-3-Octadecene | 26.88 | 0.59 | ||
| 7 | Coniferyl alcohol | 30.64 | 0.37 | ||
| 8 | 6,10,14-Trimethyl-2-pentadecanone | 32.36 | 0.24 | 0.40 | |
| 9 | Ethyl palmitate | 35.36 | 0.99 | ||
| 10 | Methyl linoleate | 37.27 | 0.97 | ||
| 11 | 14-Octadecenoic acid, methyl ester | 37.38 | 0.30 | 0.24 | |
| 12 | Ethyl linolelaidate | 38.455 | 12.48 | ||
| 13 | Ethyl (9E)-9-octadecenoate | 38.554 | 2.35 | ||
| 14 | Palmitamide | 39.00 | 42.67 | 11.97 | |
| 15 | Doconexent | 40.08 | 0.69 | 2.61 | |
| 16 | Oleamide | 42.04 | 5.08 | 2.90 | |
| 17 | Octyl cyclohexanecarboxylate | 42.44 | 1.27 | 0.74 | |
| 18 | Tetracosane | 44.4 | 2.15 | 0.28 | |
| 19 | Diploptene | 45.16 | 15.68 | ||
| 20 | 2-Linoleoylglycerol | 47.08 | 5.87 | 1.46 | 0.39 |
| 21 | Spinacene | 48.49 | 0.47 | 0.32 | |
| 22 | Tetratriacontane | 49.68 | 2.34 | 1.01 | |
| 23 | Unknown | 54.23 | 2.04 | ||
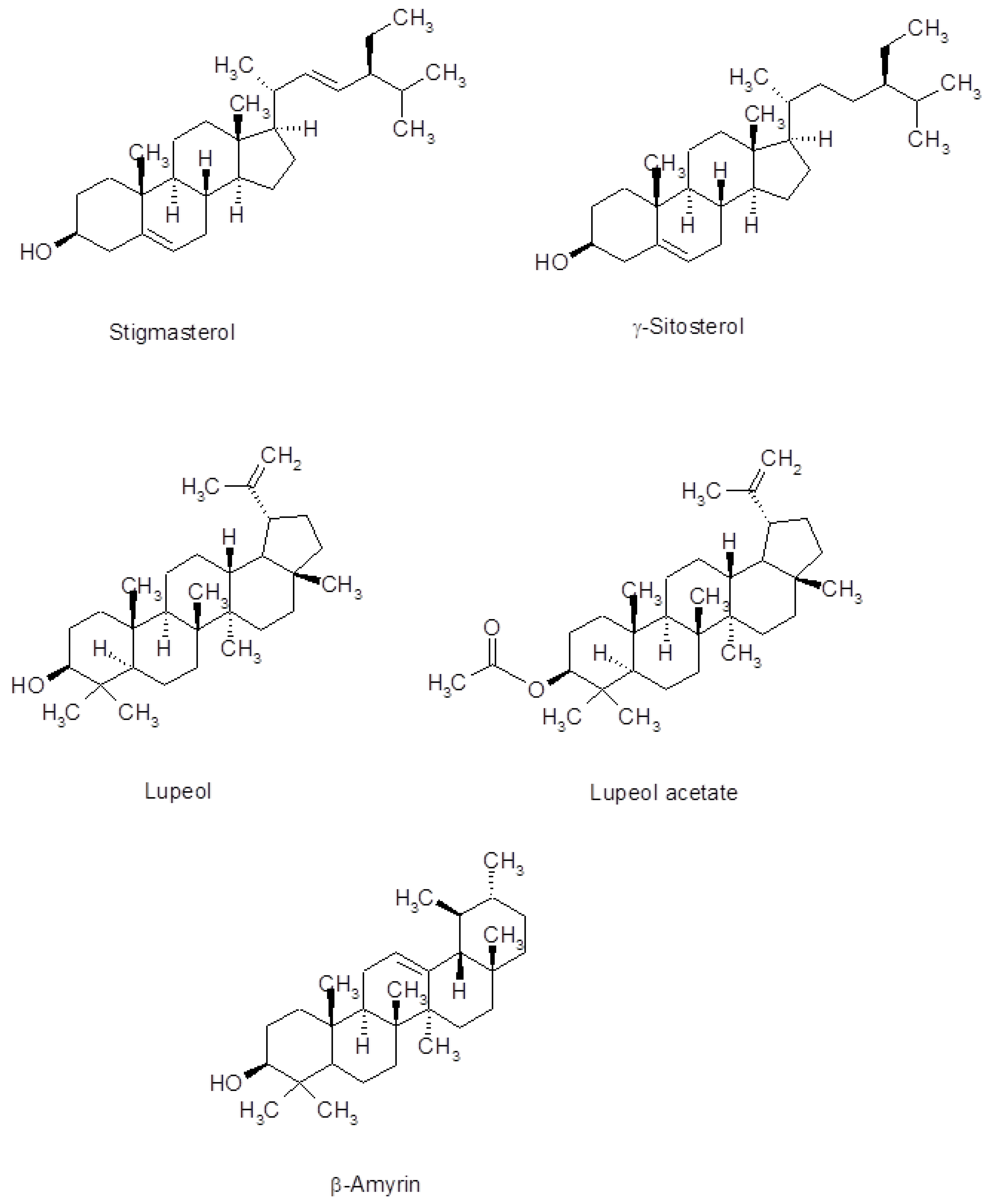
| 24 | Stigmasterol | 57.64 | 2.55 | 0.27 | |
| 25 | γ-Sitosterol | 59.58 | 5.73 | 0.22 | 1.59 |
| 26 | β-Amyrin | 61.19 | 15.98 | 0.61 | 5.44 |
| 27 | Stigmasterol acetate | 61.33 | 1.27 | ||
| 28 | Lupeol | 63.04 | 22.25 | 10.05 | |
| 29 | Stigmastan-3,5-diene | 63.76 | 4.21 | ||
| 30 | (3α)-12-Oleanen-3-yl acetate | 64.23 | 9.14 | 12.11 | 4.22 |
| 31 | Lupenyl acetate | 66.47 | 18.11 | 26.82 | 11.03 |
| 32 | Unknown | 67.33 | 11.26 | 0.16 | 7.78 |
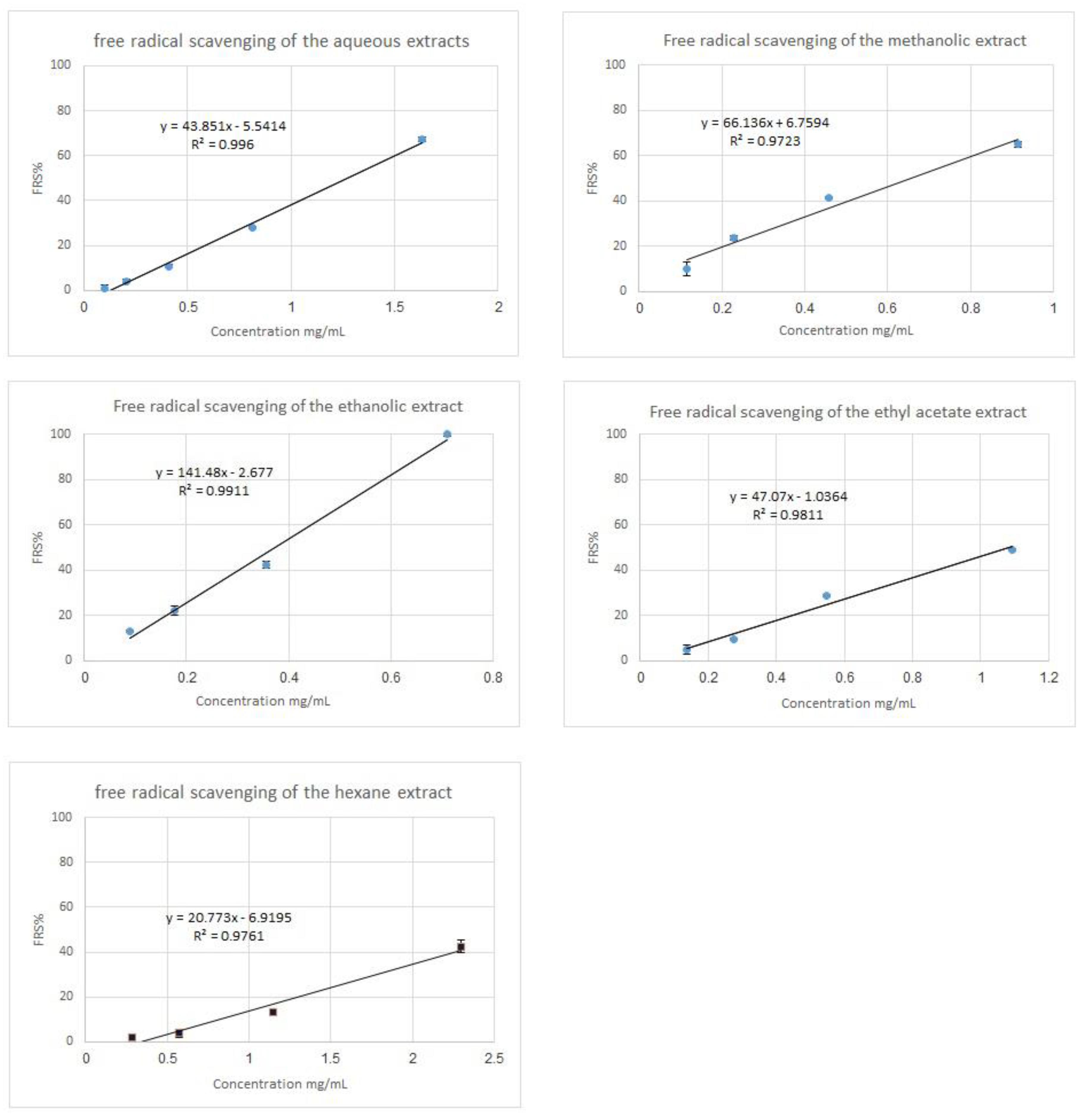
| S. maculatus Extract Type | EC50 (mg/mL) |
|---|---|
| Aqueous extract | 1.27 |
| Methanolic extract | 0.65 |
| Ethanolic extract | 0.37 |
| Ethyl acetate extract | 1.08 |
| n-hexane extract | >2.0 |
| Type of Extract/Microbial Strain | Staphylococcus aureus | Salmonella typhimurium | Candida albicans |
|---|---|---|---|
| Aqueous extract | 0.5 mg/mL | >4.0 mg/mL | 1.0 mg/mL |
| Methanolic extract | 0.5 mg/mL | >4.0 mg/mL | 0.5 mg/mL |
| Ethanolic extract | 0.5 mg/mL | 0.5 mg/mL | 0.5 mg/mL |
| Ethyl acetate extract | 0.5 mg/mL | 0.5 mg/mL | 0.5 mg/mL |
| n-hexane extract | 1.0 mg/mL | >4.0 mg/mL | 0.5 mg/mL |
| Tetracycline | - | 0.01 mg/mL | - |
| Erythromycin | 0.0078 mg/mL | - | - |
| Nystatin | - | - | 0.00312 mg/mL |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Lafi, S.; Rayan, M.; Masalha, M.; Abu-Farich, B.; Al-Jaas, H.; Abu-Lafi, M.; Rayan, A. Phytochemical Composition and Biological Activities of Wild Scolymus maculatus L. Medicines 2019, 6, 53. https://doi.org/10.3390/medicines6020053
Abu-Lafi S, Rayan M, Masalha M, Abu-Farich B, Al-Jaas H, Abu-Lafi M, Rayan A. Phytochemical Composition and Biological Activities of Wild Scolymus maculatus L. Medicines. 2019; 6(2):53. https://doi.org/10.3390/medicines6020053
Chicago/Turabian StyleAbu-Lafi, Saleh, Mahmoud Rayan, Mahmud Masalha, Basheer Abu-Farich, Hashem Al-Jaas, Malek Abu-Lafi, and Anwar Rayan. 2019. "Phytochemical Composition and Biological Activities of Wild Scolymus maculatus L." Medicines 6, no. 2: 53. https://doi.org/10.3390/medicines6020053
APA StyleAbu-Lafi, S., Rayan, M., Masalha, M., Abu-Farich, B., Al-Jaas, H., Abu-Lafi, M., & Rayan, A. (2019). Phytochemical Composition and Biological Activities of Wild Scolymus maculatus L. Medicines, 6(2), 53. https://doi.org/10.3390/medicines6020053