The Multitarget Activity of Natural Extracts on Cancer: Synergy and Xenohormesis
Abstract
1. Introduction
2. Natural Compounds, Hormesis and Xenohormesis
3. Combined Therapies, Multitargeting and Synergy
- Drug selection: The right selection of the drugs for synergy studies is the first step to succeed. There are multiple available drugs for a single disease, but not all are suitable for a synergy study. Drugs must be selected considering different molecular targets. If not, antagonism or other undesired pharmacological interactions can be obtained. These different molecular targets can be located in different molecules, in distinguished epitopes of the same molecule or even in molecules of different pathways.
- Biological assay: According to the selected method for synergy studies, a robust and reliable biological assay must be selected that allows testing a high number of samples with a large variability in composition. Survival or viability tests are diverse and allow high throughput screening approaches [28]. They are commonly used for anticancer compound research, but also for most of the other areas of drug discovery in which synergy is topical, such as antimicrobial drug discovery.
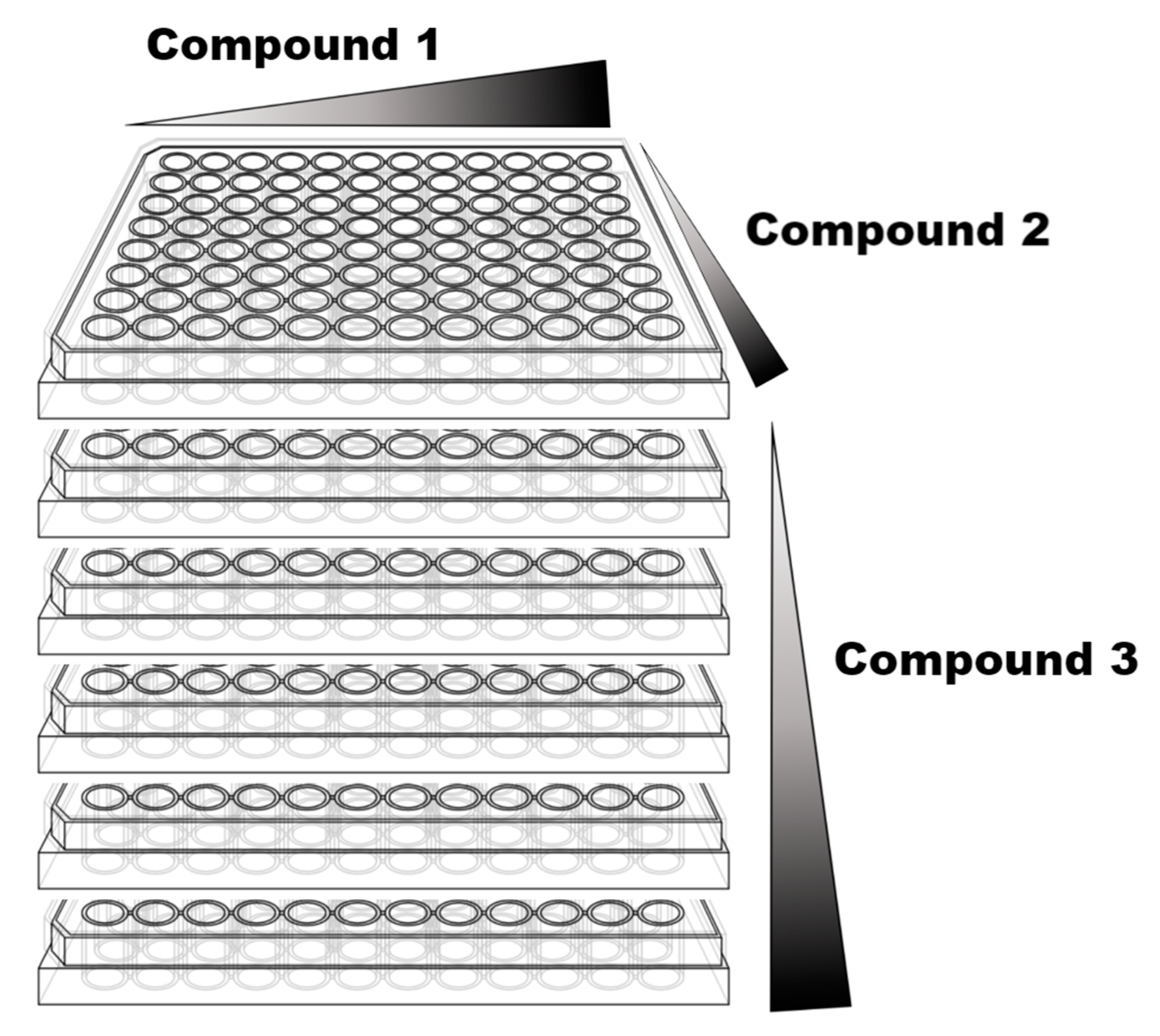
- Sample testing: Once the test is selected, an adequate design of the plates is also crucial. Checkboard plate design is probably the best approach for pairwise combinations using multi-wells plates. This strategy can be used not only for pairwise combinations but also for 3-drug combinations as shown in Figure 1.
4. Examples of Synergy Studies
5. Drawbacks of Using Natural Compounds
6. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Diamandis, M.; White, N.M.A.; Yousef, G.M. Personalized medicine: Marking a new epoch in cancer patient management. Mol. Cancer Res. 2010, 8, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Personalized medicine. Curr. Opin. Mol. Ther. 2002, 4, 548–558. [Google Scholar] [PubMed]
- Olopade, O.I.; Grushko, T.A.; Nanda, R.; Huo, D. Advances in breast cancer: Pathways to personalized medicine. Clin. Cancer Res. 2008, 14, 7988–7999. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Zaman, N.; McGee, S.; Milanese, J.S.; Masoudi-Nejad, A.; O’Connor-McCourt, M. Predictive genomics: A cancer hallmark network framework for predicting tumor clinical phenotypes using genome sequencing data. Semin. Cancer Biol. 2015, 30, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.M.; Surana, R.; Wang, S. Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 2010, 10, 317–327. [Google Scholar] [CrossRef]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef]
- Arora, A.; Scholar, E.M. Role of tyrosine kinase inhibitors in cancer therapy. J. Pharmacol. Exp. Ther. 2005, 315, 971–979. [Google Scholar] [CrossRef]
- Edelman, L.B.; Eddy, J.A.; Price, N.D. In silico models of cancer. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 438–459. [Google Scholar] [CrossRef]
- Ruiz-Torres, V.; Losada-Echeberría, M.; Herranz-López, M.; Barrajón-Catalán, E.; Galiano, V.; Micol, V.; Encinar, A.J. New Mammalian Target of Rapamycin (mTOR) Modulators Derived from Natural Product Databases and Marine Extracts by Using Molecular Docking Techniques. Mar. Drugs 2018, 16, 385. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, W.T. Prediction of Response to Drug Therapy of Cancer: A Review of In Vitro Assays. Drugs 1992, 44, 690–708. [Google Scholar] [CrossRef] [PubMed]
- Nyga, A.; Cheema, U.; Loizidou, M. 3D tumour models: Novel in vitro approaches to cancer studies. J. Cell Commun. Signal. 2011, 5, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Barrajon-Catalan, E.; Herranz-Lopez, M.; Joven, J.; Segura-Carretero, A.; Alonso-Villaverde, C.; Menendez, J.A.; Micol, V. Molecular promiscuity of plant polyphenols in the management of age-related diseases: Far beyond their antioxidant properties. Adv. Exp. Med. Biol. 2014, 824, 141–159. [Google Scholar] [PubMed]
- Joven, J.; Rull, A.; Rodriguez-Gallego, E.; Camps, J.; Riera-Borrull, M.; Hernández-Aguilera, A.; Martin-Paredero, V.; Segura-Carretero, A.; Micol, V.; Alonso-Villaverde, C.; et al. Multifunctional targets of dietary polyphenols in disease: A case for the chemokine network and energy metabolism. Food Chem. Toxicol. 2013, 51, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Rejhová, A.; Opattová, A.; Čumová, A.; Slíva, D.; Vodička, P. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Sinclair, D.A. Xenohormesis: Sensing the chemical cues of other species. Cell 2008, 133, 387–391. [Google Scholar] [CrossRef]
- Menendez, J.A.; Joven, J.; Aragones, G.; Barrajon-Catalan, E.; Beltran-Debon, R.; Borras-Linares, I.; Camps, J.; Corominas-Faja, B.; Cufi, S.; Fernandez-Arroyo, S.; et al. Xenohormetic and anti-aging activity of secoiridoid polyphenols present in extra virgin olive oil: A new family of gerosuppressant agents. Cell Cycle 2013, 12, 555–578. [Google Scholar] [CrossRef]
- Ruiz-Torres, V.; Encinar, J.; Herranz-López, M.; Pérez-Sánchez, A.; Galiano, V.; Barrajón-Catalán, E.; Micol, V. An Updated Review on Marine Anticancer Compounds: The Use of Virtual Screening for the Discovery of Small-Molecule Cancer Drugs. Molecules 2017, 22, 1037. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Skalicka-Wozniak, K.; Orhan, I.E.; Xiao, J.; Locatelli, M.; Piwowarski, J.P.; Granica, S.; Tomczyk, M. A comprehensive review of agrimoniin. Ann. N. Y. Acad. Sci. 2017, 1401, 166–180. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Locatelli, M.; Granica, S.; Cacciagrano, F.; Tomczyk, M. A Review on the Dietary Flavonoid Tiliroside. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1395–1421. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Menor, L.; Barrajon-Catalan, E.; Segura-Carretero, A.; Marti, N.; Saura, D.; Menendez, J.A.; Joven, J.; Micol, V. The promiscuous and synergic molecular interaction of polyphenols in bactericidal activity: An opportunity to improve the performance of antibiotics? Phytother. Res. 2015, 29, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Roell, K.R.; Reif, D.M.; Motsinger-Reif, A.A. An Introduction to Terminology and Methodology of Chemical Synergy-Perspectives from Across Disciplines. Front. Pharmacol. 2017, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the chou-talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Greco, W.R.; Bravo, G.; Parsons, J.C. The search for synergy: A critical review from a response surface perspective. Pharmacol. Rev. 1995, 47, 331–385. [Google Scholar] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Stoddart, M.J. Cell viability assays: Introduction. Methods Mol. Biol. (Clifton, N.J.) 2011, 740, 1–6. [Google Scholar]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef]
- Lansky, E.P.; Jiang, W.; Mo, H.; Bravo, L.; Froom, P.; Yu, W.; Harris, N.M.; Neeman, I.; Campbell, M.J. Possible synergistic prostate cancer suppression by anatomically discrete pomegranate fractions. Invest. New Drugs 2005, 23, 11–20. [Google Scholar] [CrossRef]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007, 224, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Reddivari, L.; Sclafani, R.; Das, U.N.; Vanamala, J. Resveratrol potentiates grape seed extract induced human colon cancer cell apoptosis. Front. Biosci. Elite 2011, 3E, 1509–1523. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Agulló-Chazarra, L.; Herranz-López, M.; Valdés, A.; Cifuentes, A.; Micol, V. Rosemary (Rosmarinus officinalis) extract causes ROS-induced necrotic cell death and inhibits tumor growth in vivo. Sci. Rep. 2018. [Google Scholar] [CrossRef]
- Brahmbhatt, M.; Gundala, S.R.; Asif, G.; Shamsi, S.A.; Aneja, R. Ginger phytochemicals exhibit synergy to inhibit prostate cancer cell proliferation. Nutr. Cancer 2013, 65, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gundala, S.R.; Mukkavilli, R.; Vangala, S.; Reid, M.D.; Aneja, R. Synergistic interactions among flavonoids and acetogenins in Graviola (Annona muricata) leaves confer protection against prostate cancer. Carcinogenesis 2015, 36, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Einbond, L.S.; Wu, H.A.; Kashiwazaki, R.; He, K.; Roller, M.; Su, T.; Wang, X.; Goldsberry, S. Carnosic acid inhibits the growth of ER-negative human breast cancer cells and synergizes with curcumin. Fitoterapia 2012, 83, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Morré, D.J.; Morré, D.M. Synergistic Capsicum-tea mixtures with anticancer activity. J. Pharm. Pharmacol. 2003, 55, 987–994. [Google Scholar] [CrossRef]
- Zhou, J.R.; Li, L.; Pan, W. Dietary soy and tea combinations for prevention of breast and prostate cancers by targeting metabolic syndrome elements in mice. Am. J. Clin. Nutr. 2007, 86, 882S–888S. [Google Scholar] [CrossRef]
- Zhou, J.R.; Yu, L.; Zhong, Y.; Blackburn, G.L. Soy phytochemicals and tea bioactive components synergistically inhibit androgen-sensitive human prostate tumors in mice. J. Nutr. 2003, 133, 516–521. [Google Scholar] [CrossRef]
- Malongane, F.; McGaw, L.J.; Mudau, F.N. The synergistic potential of various teas, herbs and therapeutic drugs in health improvement: A review. J. Sci. Food Agric. 2017, 97, 4679–4689. [Google Scholar] [CrossRef]
- Mertens-Talcott, S.U.; Percival, S.S. Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett. 2005, 218, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Mertens-Talcott, S.U.; Bomser, J.A.; Romero, C.; Talcott, S.T.; Percival, S.S. Ellagic acid potentiates the effect of quercetin on p21 waf1/cip1, p53, and MAP-kinases without affecting intracellular generation of reactive oxygen species in vitro. J. Nutr. 2005, 135, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Linnewiel-Hermoni, K.; Khanin, M.; Danilenko, M.; Zango, G.; Amosi, Y.; Levy, J.; Sharoni, Y. The anti-cancer effects of carotenoids and other phytonutrients resides in their combined activity. Arch. Biochem. Biophys. 2015, 572, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, U.; Gorlach, S.; Owczarek, K.; Hrabec, E.; Szewczyk, K. Synergistic interactions between anticancer chemotherapeutics and phenolic compounds and anticancer synergy between polyphenols. Postepy Hig. Med. Dosw. 2014, 68, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Penugonda, K. Pomegranate juice: A heart-healthy fruit juice. Nutr. Rev. 2009, 67, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.; Hawthorne, S. Ellagic acid, pomegranate and prostate cancer—A mini review. J. Pharm. Pharmacol. 2008, 60, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Larrosa, M.; García-Conesa, M.T.; Espín, J.C.; Tomás-Barberán, F.A. Ellagitannins, ellagic acid and vascular health. Mol. Aspects Med. 2010, 31, 513–539. [Google Scholar] [CrossRef]
- Bishayee, A.; Bishayee, A. Pomegranate-derived constituents as inducers of cell death: Implications in cancer prevention and therapy. In Natural Compounds as Inducers of Cell Death; Springer: Dordrecht, The Netherlands, 2012; Volume 1, pp. 33–47. [Google Scholar]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Herzon, S.B.; Vanderwal, C.D. Introduction: Natural Product Synthesis. Chem. Rev. 2017, 117, 11649–11650. [Google Scholar] [CrossRef]
- Hale, K.J. (Ed.) The Chemical Synthesis of Natural Products; Willey: Hobolen, NJ, USA, 2000. [Google Scholar]
- Barrajon-Catalan, E.; Fernandez-Arroyo, S.; Roldan, C.; Guillen, E.; Saura, D.; Segura-Carretero, A.; Micol, V. A systematic study of the polyphenolic composition of aqueous extracts deriving from several Cistus genus species: Evolutionary relationship. Phytochem. Anal. 2011, 22, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Borrás-Linares, I.; Herranz-López, M.; Barrajón-Catalán, E.; Arráez-Román, D.; González-Álvarez, I.; Bermejo, M.; Gutiérrez, A.F.; Micol, V.; Segura-Carretero, A. Permeability study of polyphenols derived from a phenolic-enriched hibiscus sabdariffa extract by UHPLC-ESI-UHR-Qq-TOF-MS. Int. J. Mol. Sci. 2015, 16, 18396–18411. [Google Scholar] [CrossRef] [PubMed]
- Borrás-Linares, I.; Pérez-Sánchez, A.; Lozano-Sánchez, J.; Barrajón-Catalán, E.; Arráez-Román, D.; Cifuentes, A.; Micol, V.; Carretero, A.S. A bioguided identification of the active compounds that contribute to the antiproliferative/cytotoxic effects of rosemary extract on colon cancer cells. Food Chem. Toxicol. 2015, 80, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Vicente, M.; Barrajón-Catalán, E.; Herranz-López, M.; Segura-Carretero, A.; Joven, J.; Encinar, J.A.; Micol, V. Plant-derived polyphenols in human health: Biological activity, metabolites and putative molecular targets. Curr. Drug Metab. 2018, 19, 351–369. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds: A review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed]
- Khushnud, T.; Mousa, S.A. Potential role of naturally derived polyphenols and their nanotechnology delivery in cancer. Mol. Biotechnol. 2013, 55, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Lu, W.; Kelly, A.L.; Miao, S. Emulsion-based encapsulation and delivery systems for polyphenols. Trends Food Sci. Technol. 2016, 47, 1–9. [Google Scholar] [CrossRef]
- Mignet, N.; Seguin, J.; Chabot, G.G. Bioavailability of polyphenol liposomes: A challenge ahead. Pharmaceutics 2013, 5, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Aditya, N.P.; Espinosa, Y.G.; Norton, I.T. Encapsulation systems for the delivery of hydrophilic nutraceuticals: Food application. Biotechnol. Adv. 2017, 35, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Barrajón-Catalán, E.; Funes, L.; Herranz-López, M.; González-Álvarez, I.; Bermejo, M.V.M. Differential absorption of curcuminoids between free and liposomed curcumin formulations. In Curcumin: Clinical Uses, Health Effects and Potential Complications; Martin, V., Ed.; Nova Publishers: Hauppauge, NY, USA, 2016; pp. 99–110. [Google Scholar]
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Mimeault, M.; Hauke, R.; Batra, S.K. Recent advances on the molecular mechanisms involved in the drug resistance of cancer cells and novel targeting therapies. Clin. Pharmacol. Ther. 2008, 83, 673–691. [Google Scholar] [CrossRef] [PubMed]
| Extract/Compound | Synergy | Experimental Model (Cell Line) | Effect | References |
|---|---|---|---|---|
| Pomegranate extract | Among their compounds | Oral cancer (KB, CAL27), colon cancer (HT-29, HCT116, SW480, SW620) and prostate cancer (RWPE-1, 22Rv1) | Antiproliferative, apoptotic and antioxidant | [29] |
| Pomegranate extract | Among their compounds | Prostate cancer (DU 145) | Antiproliferative, antimetastatic and phospholipase A2 (PLA2) inhibition | [30] |
| Grape extract | Among their compounds and with Ara-C and tazofurin | Leukemia (HL-60) | Antiproliferative and apoptotic | [31] |
| Grape extract | Among their compounds | Colon cancer (HCT116) | Antiproliferative and apoptotic | [32] |
| Rosemary extract | Among their compounds | Colon cancer (HT-29) | Antiproliferative | [33] |
| Ginger extract | Among their compounds | Prostate cancer (PC-3) | Antiproliferative | [34] |
| Graviola flavonoids | Among their compounds | Prostate cancer (PC-3) | Antiproliferative | [35] |
| Turmeric extract | With rosemary compounds | Breast cancer (MDA-MB-453, MDA-MB-468, and MCF7) | Antiproliferative, G1 cell cycle arrest | [36] |
| Tea extract | With capsicum compounds | Cervical cancer (HeLa) and breast cancer (4T1) | Antiproliferative | [37] |
| Tea extract | With soy compounds | Mice in vivo model | Metabolic effect | [38] |
| Tea extract | With soy compounds | Prostate cancer (LnCAP) xenotrasplants | Antiproliferative | [39] |
| Tea extract | With others tea extracts | Review | Antioxidant, antimicrobial and antitumoral | [40] |
| Resveratrol | With quercetin and ellagic acid | Leukemia (MOLT-4) | Antiproliferative, apoptosis and cell cycle arrest | [41,42] |
| Carothenoids | With other phytochemicals | Prostate cancer LNCaP , PC-3 and DU-145) and breast cancer (MCF-7) | Antiproliferative | [43] |
| Genistein | With cisplatin, 5-fluorouracil, arsenic trioxide, doxorubicin, gemcitabine camptothecine and hidroxi-camptothecine | Pancreatic cancer (BxPC-3 xenograft, COL-357 and L3.6pl) colon cancer (HT29), hepatic cancer (HepG2, Hep3B, SK-Hep-1, HEpG2 xenograft), cervical cancer (HeLa) ovarian cancer (OAW-42), bladder cancer (TCC-SUP) and lung cancer (ME-180pt, UMSCC-5) | Antiproliferative | [44] |
| Curcumin | With 5-fluorouracil, oxaliplatin, cisplatin, etoposide, camptothecine and doxorubicine | Colon cancer (HT-29), ovarian cancer (2008 and C13) and human and rat glioblastoma cell lines | Antiproliferative | [44] |
| (-)-epìgallocatechin-3-gallate | With doxorubicin, gemcitabine and cisplatin | Carcinoma doxorubicin resistant (KB-A-1 xenograft), cholangiocarcinoma (Mz-ChA-1 cell line and xenograft) and ovarian cancer (SKOC3, CAOV3 and C200) | Antiproliferative | [44] |
| Quercetin | With doxorubicin, cisplatin, arsenic trioxide and temozolomide | Neuroblastoma and Edwing’s sarcoma cell lines, laryngeal cancer (Hep2), leukemia (U937 and HL-60) and astrocytoma | Antiproliferative | [44] |
| Resveratrol | With Cisplatin and doxorubicin | Acute leukemia (ML-2/DX30, AML-2/DX100 and AML-2/DX300) | Antiproliferative | [44] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herranz-López, M.; Losada-Echeberría, M.; Barrajón-Catalán, E. The Multitarget Activity of Natural Extracts on Cancer: Synergy and Xenohormesis. Medicines 2019, 6, 6. https://doi.org/10.3390/medicines6010006
Herranz-López M, Losada-Echeberría M, Barrajón-Catalán E. The Multitarget Activity of Natural Extracts on Cancer: Synergy and Xenohormesis. Medicines. 2019; 6(1):6. https://doi.org/10.3390/medicines6010006
Chicago/Turabian StyleHerranz-López, María, María Losada-Echeberría, and Enrique Barrajón-Catalán. 2019. "The Multitarget Activity of Natural Extracts on Cancer: Synergy and Xenohormesis" Medicines 6, no. 1: 6. https://doi.org/10.3390/medicines6010006
APA StyleHerranz-López, M., Losada-Echeberría, M., & Barrajón-Catalán, E. (2019). The Multitarget Activity of Natural Extracts on Cancer: Synergy and Xenohormesis. Medicines, 6(1), 6. https://doi.org/10.3390/medicines6010006







