Oral Intake of Royal Jelly Has Protective Effects Against Tyrosine Kinase Inhibitor-Induced Toxicity in Patients with Renal Cell Carcinoma: A Randomized, Double-Blinded, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Study Design
2.3. Protocol
2.4. Statistical Analyses
3. Results
3.1. Patient Background
3.2. Adverse Events
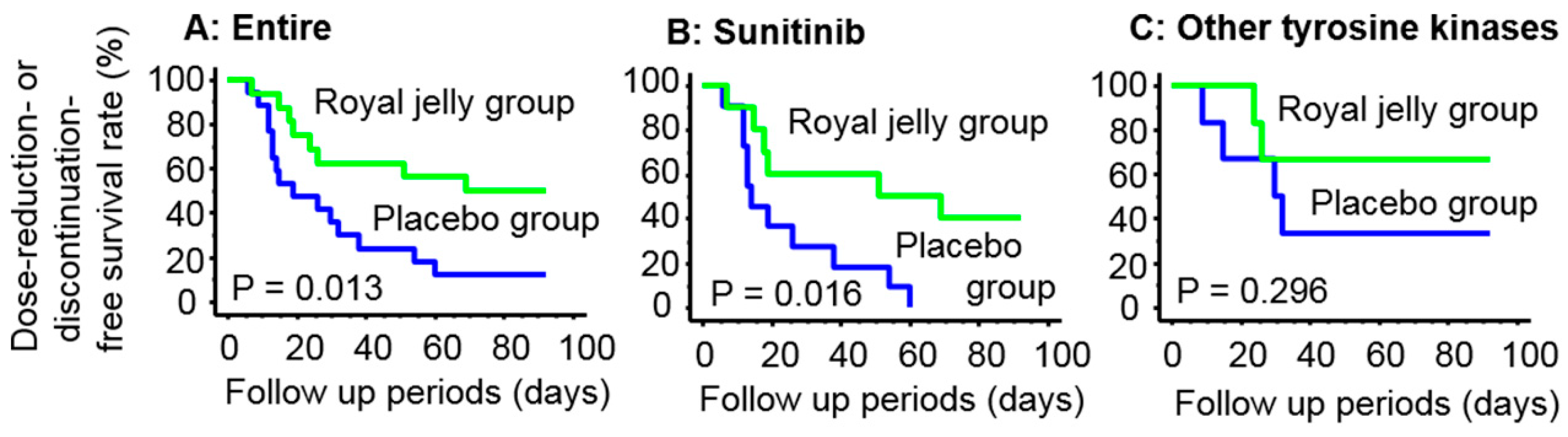
3.3. Dose Reduction or Discontinuation of Tyrosine Kinase Inhibitors
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bedke, J.; Gauler, T.; Grünwald, V.; Hegele, A.; Herrmann, E.; Hinz, S.; Janssen, J.; Schmitz, S.; Schostak, M.; Tesch, H.; et al. Systemic therapy in metastatic renal cell carcinoma. World J. Urol. 2017, 35, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Zhao, Z.; Zeng, T.; Liang, X.; Chen, D.; Duan, X.; Zeng, G.; Wu, W. Crosstalk between VEGFR and other receptor tyrosine kinases for TKI therapy of metastatic renal cell carcinoma. Cancer Cell Int. 2018, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Zarrabi, K.; Wu, S. Current and emerging therapeutic targets for metastatic renal cell carcinoma. Curr. Oncol. Rep. 2018, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Pooleri, G.K.; Nair, T.B.; Sanjeevan, K.V.; Thomas, A. Neo adjuvant treatment with targeted molecules for renal cell cancer in current clinical practise. Indian J. Surg. Oncol. 2012, 3, 114–119. [Google Scholar] [CrossRef]
- Rizzo, M.; Porta, C. Sunitinib in the treatment of renal cell carcinoma: An update on recent evidence. Ther. Adv. Urol. 2017, 9, 195–207. [Google Scholar] [CrossRef]
- Wentink, M.Q.; Verheul, H.M.W.; Pal, S.K.; George, S.; Voortman, J.; Danchaivijitr, P.; Adelaiye, R.; Poslinski, D.; Groman, A.; Hutson, A.; et al. Phase I Study of dalteparin in combination with sunitinib in patients with metastatic clear cell renal carcinoma. Clin. Genitourin Cancer 2017. [Google Scholar] [CrossRef]
- Atkins, M.B.; Plimack, E.R.; Puzanov, I.; Fishman, M.N.; McDermott, D.F.; Cho, D.C.; Vaishampayan, U.; George, S.; Olencki, T.E.; Tarazi, J.C.; et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: A non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol. 2018, 19, 405–415. [Google Scholar] [CrossRef]
- Clarke, J.M.; George, D.J.; Lisi, S.; Salama, A. Immune checkpoint blockade: The new frontier in cancer treatment. Target Oncol. 2018, 13, 1–20. [Google Scholar] [CrossRef]
- Guo, J.; Jin, J.; Oya, M.; Uemura, H.; Takahashi, S.; Tatsugami, K.; Rha, S.Y.; Lee, J.L.; Chung, J.; Lim, H.Y.; et al. Safety of pazopanib and sunitinib in treatment-naive patients with metastatic renal cell carcinoma: Asian versus non-Asian subgroup analysis of the COMPARZ trial. J. Hematol. Oncol. 2018, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pello, S.; Hofmann, F.; Tahbaz, R.; Marconi, L.; Lam, T.B.; Albiges, L.; Bensalah, K.; Canfield, S.E.; Dabestani, S.; Giles, R.H.; et al. A systematic review and meta-analysis comparing the effectiveness and adverse effects of different systemic treatments for non-clear cell renal cell carcinoma. Eur. Urol. 2017, 71, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.; Stein, D.; Teltsch, D.Y.; Tao, S.; Cisar, L.; Ramaswamy, K. Real-world chart review study of adverse events management in patients taking tyrosine kinase inhibitors to treat metastatic renal cell carcinoma. J. Oncol. Pharm. Pract. 2018, 24, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Bi, F.; Ren, X.; Cheng, Y.; Wang, J.; Rosbrook, B.; Jiang, M.; Guo, J. First-line axitinib versus sorafenib in Asian patients with metastatic renal cell carcinoma: Exploratory subgroup analyses of Phase III data. Future Oncol. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Vidal, M.J.; Martínez Ortega, E.; Montesa Pino, A.; Pérez Valderrama, B.; Viciana, R. Management of adverse events of targeted therapies in normal and special patients with metastatic renal cell carcinoma. Cancer Metastasis Rev. 2012, 31, S19–27. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Porta, C.; Bono, P.; Powles, T.; Eisen, T.; Sternberg, C.N.; Gschwend, J.E.; De Giorgi, U.; Parikh, O.; Hawkins, R.; et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES Study. J. Clin. Oncol. 2014, 32, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.; Conti, A.; De Giorgi, U.; Iacovelli, R.; Pantano, F.; Burattini, L.; Muzzonigro, G.; Berardi, R.; Santini, D.; Cascinu, S. Risk of gastrointestinal events with sorafenib, sunitinib and pazopanib in patients with solid tumors: A systematic review and meta-analysis of clinical trials. Int. J. Cancer 2014, 135, 763–773. [Google Scholar] [CrossRef]
- Chrisoulidou, A.; Mandanas, S.; Margaritidou, E.; Mathiopoulou, L.; Boudina, M.; Georgopoulos, K.; Pazaitou-Panayiotou, K. Treatment compliance and severe adverse events limit the use of tyrosine kinase inhibitors in refractory thyroid cancer. Onco. Targets Ther. 2015, 8, 2435–2442. [Google Scholar]
- Que, Y.; Liang, Y.; Zhao, J.; Ding, Y.; Peng, R.; Guan, Y.; Zhang, X. Treatment-related adverse effects with pazopanib, sorafenib and sunitinib in patients with advanced soft tissue sarcoma: A pooled analysis. Cancer Manag. Res. 2018, 10, 2141–2150. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.; Rizzo, M.; Burattini, L.; Farfariello, V.; Berardi, R.; Santoni, G.; Carteni, G.; Cascinu, S. Present and future of tyrosine kinase inhibitors in renal cell carcinoma: Analysis of hematologic toxicity. Recent Pat. Antiinfect. Drug Discov. 2012, 7, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.; Miller, K.B.; Jaffe, I.Z. Molecular mechanisms for vascular complications of targeted cancer therapies. Clin. Sci. (Lond.) 2016, 130, 1763–1779. [Google Scholar] [CrossRef] [PubMed]
- Teppo, H.R.; Soini, Y.; Karihtala, P. Reactive oxygen species-mediated mechanisms of action of targeted cancer therapy. Oxid. Med. Cell. Longev. 2017, 2017, 1485283. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.R.; Yang, Y.C.; Shi, L.S.; Peng, C.C. Antioxidant properties of royal jelly associated with larval age and time of harvest. J. Agric. Food Chem. 2008, 56, 11447–11452. [Google Scholar] [CrossRef] [PubMed]
- Kolayli, S.; Sahin, H.; Can, Z.; Yildiz, O.; Malkoc, M.; Asadov, A. A member of complementary medicinal food: Anatolian royal jellies, their chemical compositions, and antioxidant properties. J. Evid. Based Complement. Altern. Med. 2016, 21, NP43–NP48. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Chou, W.M.; Widowati, D.A.; Lin, I.P.; Peng, C.C. 10-hydroxy-2-decenoic acid of royal jelly exhibits bactericide and anti-inflammatory activity in human colon cancer cells. BMC Complement. Altern. Med. 2018, 18, 202. [Google Scholar] [CrossRef] [PubMed]
- Hajra, S.; Patra, A.R.; Basu, A.; Bhattacharya, S. Prevention of doxorubicin (DOX)-induced genotoxicity and cardiotoxicity: Effect of plant derived small molecule indole-3-carbinol (I3C) on oxidative stress and inflammation. Biomed. Pharmacother. 2018, 101, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, A.; Edison, T.N.J.I.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamy, I. Toxicity of Doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Kaynar, L.; Cetin, A.; Hacioglu, S.K.; Eser, B.; Koçyigit, İ.; Canöz, Ö.; Tasdemir, A.; Karadag, C.; Kurnaz, F.; Saraymen, R.; et al. Efficacy of royal jelly on methotrexate-induced systemic oxidative stress and damage to small intestine in rats. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Suemaru, K.; Cui, R.; Li, B.; Watanabe, S.; Okihara, K.; Hashimoto, K.; Yamada, H.; Araki, H. Topical application of royal jelly has a healing effect for 5-fluorouracil-induced experimental oral mucositis in hamsters. Methods Find. Exp. Clin. Pharmacol. 2008, 30, 103–106. [Google Scholar] [CrossRef]
- Karadeniz, A.; Simsek, N.; Karakus, E.; Yildirim, S.; Kara, A.; Can, I.; Kisa, F.; Emre, H.; Turkeli, M. Royal jelly modulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxid. Med. Cell. Longev. 2011, 2011, 981793. [Google Scholar] [CrossRef]
- Ibrahim, A.; Eldaim, M.A.; Abdel-Daim, M.M. Nephroprotective effect of bee honey and royal jelly against subchronic cisplatin toxicity in rats. Cytotechnology 2016, 68, 1039–1048. [Google Scholar] [CrossRef]
- Erdem, O.; Güngörmüş, Z. The effect of royal jelly on oral mucositis in patients undergoing radiotherapy and chemotherapy. Holist. Nurs. Pract. 2014, 28, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Kogashiwa, Y.; Moro, Y.; Kohno, N. The effect of topical application of royal jelly on chemoradiotherapy-induced mucositis in head and neck cancer: A preliminary study. Int. J. Otolaryngol. 2014, 2014, 974967. [Google Scholar] [CrossRef]
- Mofid, B.; Rezaeizadeh, H.; Termos, A.; Rakhsha, A.; Mafi, A.R.; Taheripanah, T.; Ardakani, M.M.; Taghavi, S.M.; Moravveji, S.A.; Kashi, A.S. Effect of processed honey and royal jelly on cancer-related fatigue: A double-blind randomized clinical trial. Electron Phys. 2016, 8, 2475–2482. [Google Scholar] [CrossRef] [PubMed]
- Osama, H.; Abdullah, A.; Gamal, B.; Emad, D.; Sayed, D.; Hussein, E.; Mahfouz, E.; Tharwat, J.; Sayed, S.; Medhat, S.; et al. Effect of honey and royal jelly against cisplatin-induced nephrotoxicity in patients with cancer. J. Am. Coll. Nutr. 2017, 36, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Matsuo, T.; Araki, K.; Nakamura, Y.; Sagara, Y.; Ohba, K.; Sakai, H. Anticancer effects of green tea and the underlying molecular mechanisms in bladder cancer. Medicines (Basel) 2018, 5, 87. [Google Scholar] [CrossRef]
- Ohba, K.; Miyata, Y.; Watanabe, S.; Hayashi, T.; Kanetake, H.; Kanda, S.; Sakai, H. Clinical significance and predictive value of prostaglandin E2 receptors (EPR) 1–4 in patients with renal cell carcinoma. Anticancer Res. 2011, 31, 597–605. [Google Scholar]
- Silici, S.; Ekmekcioglu, O.; Kanbur, M.; Deniz, K. The protective effect of royal jelly against cisplatin-induced renal oxidative stress in rats. World J. Urol. 2011, 29, 127–132. [Google Scholar] [CrossRef]
- Yamaura, K.; Tomono, A.; Suwa, E.; Ueno, K. Topical royal jelly alleviates symptoms of pruritus in a murine model of allergic contact dermatitis. Pharmacogn. Mag. 2013, 9, 9–13. [Google Scholar] [CrossRef]
- Khazaei, M.; Ansarian, A.; Ghanbari, E. New findings on biological actions and clinical applications of royal jelly: A review. J. Diet. Suppl. 2018, 15, 757–775. [Google Scholar] [CrossRef]
- Kamakura, M.; Mitani, N.; Fukuda, T.; Fukushima, M. Antifatigue effect of fresh royal jelly in mice. J. Nutr. Sci. Vitaminol. (Tokyo) 2001, 47, 394–401. [Google Scholar] [CrossRef]
- Del Fabbro, E. Current and future care of patients with the cancer anorexia-cachexia syndrome. Am. Soc. Clin. Oncol. Educ. Book 2015, e229–e237. [Google Scholar] [CrossRef] [PubMed]
- Macciò, A.; Madeddu, C.; Mantovani, G. Current pharmacotherapy options for cancer anorexia and cachexia. Expert Opin. Pharmacother. 2012, 13, 2453–2472. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Imai, J.; Fujiwara, M.; Yaeshima, T.; Kawashima, T.; Kobayashi, K. A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J. Biol. Chem. 1990, 265, 11333–11337. [Google Scholar] [PubMed]
- Watanabe, K.; Shinmoto, H.; Kobori, M.; Tsushida, T.; Shinohara, K.; Kanaeda, J.; Yonekura, M. Stimulation of cell growth in the U-937 human myeloid cell line by honey royal jelly protein. Cytotechnology 1998, 26, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Araki, Y.; Hata, T.; Ichihara, K.; Ito, M.; Tanaka, M.; Honda, S. 10-Hydroxy-2-decenoic acid, the major Lipid component of royal jelly, extends the lifespan of caenorhabditis elegans through dietary restriction and target of rapamycin signaling. J. Aging Res. 2015, 2015, 425261. [Google Scholar] [CrossRef] [PubMed]
| Variables | Entire (n = 33) | Placebo (n = 17) | Royal Jelly (n = 16) | p Value |
|---|---|---|---|---|
| Age | 0.101 | |||
| Mean ± SD, years | 67.6 ± 6.6 | 65.8 ± 8.8 | 69.6 ± 5.9 | |
| Gender; n (%) | 0.909 | |||
| Male/Female | 23/10 (30.3) | 12/5 (29.4) | 11/5 (31.3) | |
| Performance Status | 0.598 | |||
| 0/1 | 16/17 (51.5) | 9/8 (47.1) | 7/9 (56.3) | |
| Pathological Type | 0.446 | |||
| Conventional | 29 (87.9) | 16 (94.1) | 13 (81.3) | |
| Fuhrman Grade | 0.425 | |||
| 1 or 2/3 or 4 | 6/27 (81.8) | 2/15 (88.2) | 4/12 (75.0) | |
| pT stage | 0.201 | |||
| 1 or 2/3 or 4 | 9/24 (72.7) | 3/14 (82.4) | 6/10 (62.5) | |
| Lymph Node Metastasis | 0.881 | |||
| Presence | 19 (57.6) | 10 (58.8) | 9 (56.3) | |
| Distant Metastasis | 0.325 | |||
| Presence | 27 (81.8) | 15 (88.2) | 12 (75.0) | |
| Neo-Adjuvant Setting | 0.965 | |||
| Yes | 2 (6.1) | 1 (5.9) | 1 (6.3) | |
| Past Therapy Used TKI | 0.948 | |||
| Presence | 4 (12.1) | 2 (11.8) | 2 (12.5) | |
| TKIs | 0.539 | |||
| Sunitinib | 21 (63.6) | 11 (64.7) | 10 (62.5) | |
| Pazopanib | 7 (21.2) | 3 (17.6) | 4 (25.0) | |
| Axitinib | 4 (12.1) | 3 (17.6) | 1 (6.3) | |
| Sorafenib | 1 (3.0) | 0 (0.0) | 1 (6.3) |
| Variables | Placebo | Royal Jelly | p Value |
|---|---|---|---|
| Sunitinib | n = 11 | n =10 | |
| Age (mean ± SD), years | 66.5 ± 8.3 | 67.8 ± 5.8 | 0.673 |
| Gender; Male/Female, n (%) | 6/5 (45.5) | 8/2 (20.0) | 0.217 |
| Performance Status; 0/1 | 7/4 (36.4) | 6/4 (40.0) | 0.864 |
| Pathological Type; Conventional | 11 (100.0) | 8 (80.0) | 0.297 |
| Fuhrman grade; 2/3+4 | 1/10 (90.9) | 2/8 (80.0) | 0.476 |
| pT stage; 1+2/3+4 | 2/9 (81.8) | 3/7 (70.0) | 0.525 |
| Lymph Node Metastasis; Presence | 7 (63.6) | 6 (60.0) | 0.864 |
| Distant Metastasis; Presence | 9 (81.8) | 8 (80.0) | 0.916 |
| Neo-Adjuvant Setting; Yes | 1 (9.1) | 0 (0.0) | 0.329 |
| Past Therapy Used TKI; Presence | 1 (9.1) | 0 (0.0) | 0.329 |
| Others | n = 6 | n = 6 | |
| Age (mean ± SD); years | 64.5 ± 3.8 | 72.5 ± 5.3 | 0.013 |
| Gender; Male / Female | 6/0 (0.0) | 3/3 (50.0) | 0.182 |
| Performance Status; 0/1 | 2/4 (66.7) | 1/5 (83.3) | 0.505 |
| Pathological Type; Conventional | 5 (83.3) | 5 (83.3) | 0.999 |
| Fuhrman grade; 2/3+4 | 1/5 (83.3) | 3/3 (50.0) | 0.221 |
| pT Stage; 1+2/3+4 | 1/5 (83.3) | 2/4 (66.7) | 0.501 |
| LN Metastasis; Presence | 3 (50.0) | 3 (50.0) | 0.999 |
| Distant Metastasis; Presence | 6 (100.0) | 4 (66.7) | 0.121 |
| Neo-Adjuvant Setting; Yes | 0 (0.0) | 1 (16.7) | 0.296 |
| Past Therapy Used TKI; Presence | 1 (16.7) | 2 (33.3) | 0.505 |
| Adverse Events | Entire | Sunitinib | Others | |||
|---|---|---|---|---|---|---|
| Placebo (n = 17) | RJ (n = 16) | Placebo (n = 11) | RJ (n = 10) | Placebo (n = 6) | RJ (n = 6) | |
| Hypertension | ||||||
| Nothing | 6 (35.3) | 4 (25.0) | 5 (45.5) | 3 (30.0) | 1 (16.7) | 1 (16.7) |
| Mild | 10 (58.8) | 12 (75.0) | 6 (54.5) | 7 (70.0) | 4 (66.7) | 5 (83.3) |
| Severe | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| p value | 0.460 | 0.466 | 0.574 | |||
| Fatigue | ||||||
| Nothing | 2 (11.8) | 11 (68.8) | 1 (9.1) | 6 (60.0) | 1 (16.7) | 5 (83.3) |
| Mild | 13 (76.5) | 5 (31.3) | 9 (81.8) | 4 (40.0) | 4 (66.7) | 1 (16.7) |
| Severe | 2 (11.8) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| p value | 0.003 | 0.040 | 0.065 | |||
| Anorexia | ||||||
| Nothing | 4 (23.5) | 11 (68.8) | 3 (27.3) | 8 (80.0) | 1 (16.7) | 3 (50.0) |
| Mild | 9 (52.9) | 5 (31.3) | 5 (45.5) | 2 (20.0) | 4 (66.7) | 3 (50.0) |
| Severe | 4 (23.5) | 0 (0.0) | 3 (27.3) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| p value | 0.015 | 0.038 | 0.343 | |||
| Digestive Symptoms | ||||||
| Nothing | 6 (35.3) | 11 (68.8) | 3 (27.3) | 7 (70.0) | 3 (50.0) | 4 (66.7) |
| Mild | 8 (47.1) | 5 (31.3) | 6 (54.5) | 3 (30.0) | 2 (33.3) | 2 (33.3) |
| Severe | 3 (17.6) | 0 (0.0) | 2 (18.2) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| p value | 0.077 | 0.102 | 0.565 | |||
| Dysgeusia | ||||||
| Nothing | 7 (41.2) | 9 (56.3) | 5 (45.5) | 5 (50.0) | 2 (33.3) | 4 (66.7) |
| Mild | 9 (52.9) | 7 (43.8) | 5 (45.5) | 5 (50.0) | 4 (66.7) | 2 (33.3) |
| Severe | 1 (5.9) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| p value | 0.479 | 0.621 | 0.248 | |||
| Hand–Foot Syndrome | ||||||
| Nothing | 7 (41.2) | 8 (50.0) | 3 (27.3) | 4 (40.0) | 4 (66.7) | 4 (66.7) |
| Mild | 9 (52.9) | 8 (50.0) | 8 (72.7)) | 6 (60.0) | 1 (16.7) | 2 (33.3) |
| Severe | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| p value | 0.578 | 0.537 | 0.513 | |||
| Oral Mucositis | ||||||
| Nothing | 9 (52.9) | 11 (68.8) | 7 (63.6) | 7 (70.0) | 2 (33.3) | 4 (66.7) |
| Mild | 8 (47.1) | 5 (31.3) | 4 (36.4) | 3 (30.0) | 4 (66.7) | 2 (33.3) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| p value | 0.353 | 0.757 | 0.248 | |||
| Adverse Events | Entire | Sunitinib | Others | |||
|---|---|---|---|---|---|---|
| Placebo (n = 17) | RJ (n = 16) | Placebo (n = 11) | RJ (n = 10) | Placebo (n = 6) | RJ (n = 6) | |
| Leukopenia | ||||||
| Nothing | 11 (35.3) | 11 (25.0) | 5 (45.5) | 6 (60.0) | 6 (100.0) | 5 (83.3) |
| Low | 5 (58.8) | 5 (75.0) | 5 (45.5) | 4 (40.0) | 0 (0.0) | 1 (16.7) |
| High | 1 (5.9) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| p value | 0.616 | 0.561 | 0.296 | |||
| Anemia | ||||||
| Nothing | 10 (58.8) | 14 (87.5) | 4 (36.4) | 8 (80.0) | 6 (100.0) | 6 (100.0) |
| Mild | 6 (35.3) | 2 (12.5) | 4 (36.4) | 2 (20.0) | 0 (0.0) | 0 (0.0) |
| Severe | 1 (5.9) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| p value | 0.162 | 0.117 | > 0.999 | |||
| Platelets | ||||||
| Nothing | 5 (29.4) | 9 (56.3) | 2 (18.2) | 5 (50.0) | 3 (50.0) | 4 (66.7) |
| Mild | 8 (47.1) | 4 (25.0) | 6 (54.5) | 3 (30.0) | 2 (33.3) | 1 (16.7) |
| Severe | 4 (23.5) | 3 (18.8) | 3 (27.3) | 2 (20.0) | 1 (16.7) | 1 (16.7) |
| p value | 0.274 | 0.295 | 0.788 | |||
| Renal Dysfunction | ||||||
| Nothing | 8 (47.1) | 10 (62.5) | 5 (45.5) | 6 (60.0) | 3 (50.0) | 4 (66.7) |
| Mild | 9 (52.9) | 6 (37.5) | 6 (54.5) | 4 (40.0) | 3 (50.0) | 2 (33.3) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| p value | 0.373 | 0.505 | 0.558 | |||
| Liver Dysfunction | ||||||
| Nothing | 12 (70.6) | 14 (87.5) | 7 (63.6) | 9 (90.0) | 5 (83.3) | 5 (83.3) |
| Mild | 4 (23.5) | 1 (6.3) | 4 (36.4) | 1 (10.0) | 0 (0.0) | 0 (0.0) |
| Severe | 1 (5.9) | 1 (6.3) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (16.7) |
| p value | 0.382 | 0.621 | 0.999 | |||
| Thyroid Abnormality | ||||||
| Nothing | 9 (52.9) | 10 (62.5) | 7 (63.6) | 7 (70.0) | 2 (33.3) | 3 (50.0) |
| Mild | 7 (41.2) | 6 (37.5) | 4 (36.4) | 3 (30.0) | 3 (50.0) | 3 (50.0) |
| Severe | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| P value | 0.577 | 0.757 | 0.549 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araki, K.; Miyata, Y.; Ohba, K.; Nakamura, Y.; Matsuo, T.; Mochizuki, Y.; Sakai, H. Oral Intake of Royal Jelly Has Protective Effects Against Tyrosine Kinase Inhibitor-Induced Toxicity in Patients with Renal Cell Carcinoma: A Randomized, Double-Blinded, Placebo-Controlled Trial. Medicines 2019, 6, 2. https://doi.org/10.3390/medicines6010002
Araki K, Miyata Y, Ohba K, Nakamura Y, Matsuo T, Mochizuki Y, Sakai H. Oral Intake of Royal Jelly Has Protective Effects Against Tyrosine Kinase Inhibitor-Induced Toxicity in Patients with Renal Cell Carcinoma: A Randomized, Double-Blinded, Placebo-Controlled Trial. Medicines. 2019; 6(1):2. https://doi.org/10.3390/medicines6010002
Chicago/Turabian StyleAraki, Kyohei, Yasuyoshi Miyata, Kojiro Ohba, Yuichiro Nakamura, Tomohiro Matsuo, Yasushi Mochizuki, and Hideki Sakai. 2019. "Oral Intake of Royal Jelly Has Protective Effects Against Tyrosine Kinase Inhibitor-Induced Toxicity in Patients with Renal Cell Carcinoma: A Randomized, Double-Blinded, Placebo-Controlled Trial" Medicines 6, no. 1: 2. https://doi.org/10.3390/medicines6010002
APA StyleAraki, K., Miyata, Y., Ohba, K., Nakamura, Y., Matsuo, T., Mochizuki, Y., & Sakai, H. (2019). Oral Intake of Royal Jelly Has Protective Effects Against Tyrosine Kinase Inhibitor-Induced Toxicity in Patients with Renal Cell Carcinoma: A Randomized, Double-Blinded, Placebo-Controlled Trial. Medicines, 6(1), 2. https://doi.org/10.3390/medicines6010002






