Evaluation of the Pharmaceutical Properties and Value of Astragali Radix
Abstract
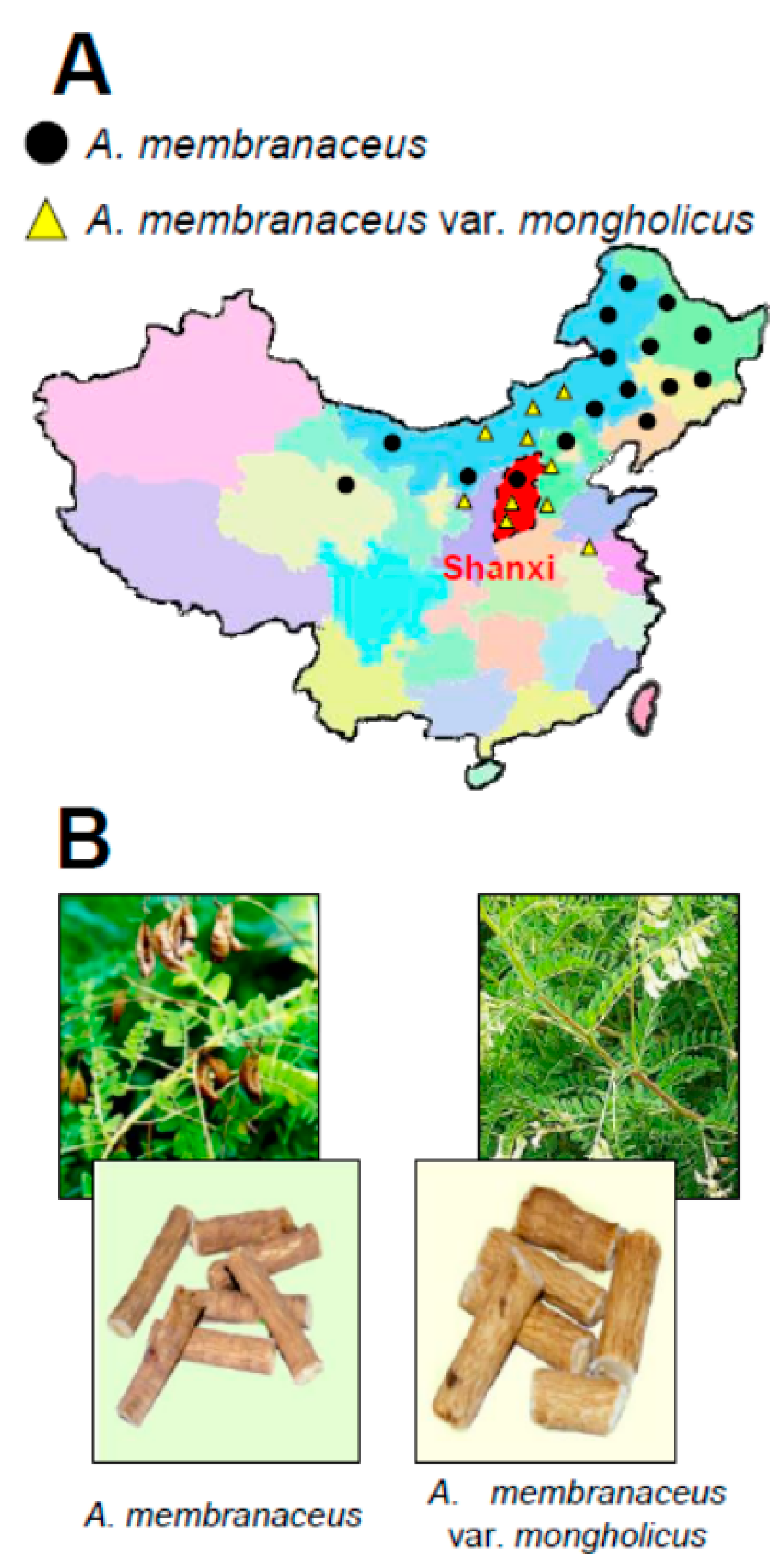
:1. Introduction
2. Chemical Determination of Different Plant Parts of Astragali Radix
3. The Optimization of Extraction of Astragali Radix
4. The Pharmaceutical Value of AR Extract and AR Major Ingredients
4.1. The Anti-Oxidative Actions of Astragali Radix and Its Major Constituents
4.2. The Immune Functions of Astragali Radix and Its Biological Ingredients
4.3. Protective Effects on Cardiovascular Diseases
4.4. Therapeutic Effects of Astragali Radix on Liver Fibrosis
4.5. The Erythropoietic Functions of Astragali Radix and Its Major Constituents
4.6. Other Pharmaceutical Properties of AR and Its Ingredients
5. Discussion
6. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid |
| ALT | Alanine aminotransferase |
| AR | Astragali Radix |
| ASR | Angelica Sinensis Radix |
| AST | Aspartate aminotransferase |
| BYHWD | Buyang Huanwu decoction |
| CEC | Circulating endothelial cells |
| CMM | Chinese Materia Medica |
| DBT | Danggui Buxue Tang |
| DDP | Cisplatin |
| DMN | Dimethylnitrosamine |
| DPPH | 2,2-Diphenyl-1-Picrylhydrazyl |
| EF | Epimedii Folium |
| EPO | Erythropoietin |
| ET-1 | Endothelin-1 |
| FRAP | Ferric reducing ability of plasma |
| GABA | γ-aminobutyric acid |
| HA | Hexadecenoic acid |
| HIF-1α | Hypoxia-induced factor |
| HNPCE | Homogenization-assisted negative pressure cavitation extraction |
| Hyp | Hydroxyproline |
| IDO | Indoleamine 2,3-dioxygenase |
| LN | Laminin |
| MAAH | Microwave-assisted acidic hydrolysis |
| MDA | Malondialdehyde |
| MMP | Matrix metalloproteinases |
| NSCLC | Non-small-cell lung cancer |
| PCIII | Procollagen type III |
| PLE | Pressurized liquid extraction |
| TCM | Traditional Chinese medicine |
| YPFS | Yu Ping Feng San |
References
- Fu, K.T. Flora of Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1998; Volume 42, pp. 78–347. [Google Scholar]
- Ma, X.Q.; Shi, Q.; Duan, J.A.; Dong, T.T.; Tsim, K.W. Chemical analysis of Radix Astragali (Huangqi) in China: A comparison with its adulterants and seasonal variations. J. Agric. Food Chem. 2002, 50, 4861–4866. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.L. Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 1990; pp. 127–274. [Google Scholar]
- Ma, X.Q.; Duan, J.A.; Zhu, D.Y.; Dong, T.T.; Tsim, K.W. Species identification of Radix Astragali (Huangqi) by DNA sequence of its 5S-rRNA spacer domain. Phytochemistry 2000, 54, 363–368. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.B.; Guo, B.L.; Zhao, Z.Z.; Liang, Z.T.; Yi, T. Study of the relationship between genetics and geography in determining the quality of Astragali Radix. Biol. Pharm. Bull. 2011, 34, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.H.; Lin, H.M.; Wu, R.Y. Identification of Astragalus medicines using scar markers. J. Food Drug Anal. 2008, 16, 57–62. [Google Scholar]
- Yu, K.Z.; Liu, J.; Guo, P.L.; Zhao, Z.Z.; Hong, H.; Chen, H.B.; Cai, S.Q. Microscopic research on a multi-source traditional Chinese medicine, Astragali Radix. J. Nat. Med. 2014, 68, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, P.; Wang, D.; Cheng, Y.Y. Micellar electrokinetic chromatography for the quantitative analysis of flavonoids in the Radix of Astragalus membranaceus var. mongholicus. Planta Med. 2008, 74, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Thwe, A.A.; Li, X.; Tuan, P.A.; Lee, S.; Lee, J.W.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.U. Accumulation of astragalosides and related gene expression in different organs of Astragalus membranaceus Bge. var mongholicus (Bge.). Molecules 2014, 19, 10922–10935. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Thwe, A.A.; Li, X.; Tuan, P.A.; Zhao, S.; Park, C.G.; Lee, J.W.; Park, S.U. Accumulation of flavonoids and related gene expressions in different organs of Astragalus membranaceus Bge. Appl. Biochem. Biotechnol. 2014, 173, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Thwe, A.A.; Li, X.H.; Kim, Y.J.; Kim, J.K.; Arasu, M.V.; AI-Dhabi, N.A.; Park, S.U. Triterpene and flavonoid biosynthesis and metabolic profiling of hairy roots, adventitious roots, and seedling roots of Astragalus membranaceus. J. Agric. Food Chem. 2015, 63, 8862–8869. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Hwang, J.; Lee, S.K.; Park, Y.D. Astragaloside content in the periderm, cortex, and xylem of Astragalus membranaceus root. J. Nat. Med. 2013, 67, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Choi, S.; Lee, D.; Noh, H.; Lee, S.; Choi, J.; Kim, S. Effects of ethanol extracts and compounds from Astragali Radix on chondrocytes and MIA-induced osteoarthritis model in rat. Planta Med. 2014, 80, 1493–1494. [Google Scholar] [CrossRef]
- Lv, G.P.; Hu, D.J.; Cheong, K.I.; Li, Z.Y.; Qing, X.M.; Zhao, J.; Li, S.P. Decoding glycome of Astragalus membranaceus based on pressurized liquid extraction, microwave-assisted hydrolysis and chromatographic analysis. J. Chromatogr. A 2015, 1409, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Wei, F.Y.; Gai, Q.Y.; Wang, W.; Luo, M.; Fu, Y.J.; Ma, W. A pilot-scale homogenization-assisted negative pressure cavitation extraction of Astragalus polysaccharides. Int. J. Biol. Macromol. 2014, 67, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.Y.; Jiang, Z.H.; Yu, H.; Xie, M.Y.; Hsiao, W.L.; Lu, A.P.; Han, Q.B. A new application of an aqueous diphase solvent system in one-step preparation of polysaccharide from the crude water extract of Radix Astragali by high-speed counter-current chromatography. J. Chromatogr. A 2012, 1262, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, Z.H.; Huang, L.F.; Zheng, S.H.; Wang, D.M.; Chen, S.L.; Zhang, H.T.; Yang, S.H. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.; Shabbir, A.; Wojcikowski, K.; Wohlmuth, H.; Gobe, G.C. The antioxidant effects of Radix Astragali (Astragalus membranaceus and related species) in protecting tissues from injury and disease. Curr. Drug Targets 2016, 17, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Toda, S.; Shirataki, Y. Inhibitory effects of Astragali Radix, a crude drug in oriental medicines, on lipid peroxidation and protein oxidative modification by copper. J. Ethnopharmacol. 1999, 68, 331–333. [Google Scholar] [CrossRef]
- Jia, X.; Sun, C.S.; Li, G.Y.; Li, G.B.; Chen, G.L. Effects of progressive drought stress on the physiology, antioxidative enzymes and secondary metabolites of Radix Astragali. Acta Physiol. Plant 2015, 37, 26. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Z.; Sun, Y.; Liu, X.; Tong, T. The two isomers of HDTIC compounds from Astragali Radix slow down telomere shortening rate via attenuating oxidative stress and increasing DNA repair ability in human fetal lung diploid fibroblast cells. DNA Cell Biol. 2010, 29, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Lee, J.S.; Choi, M.K.; Han, J.M.; Son, C.G. Ethanolic extract of Astragali Radix and Salviae Radix prohibits oxidative brain injury by psycho-emotional stress in whisker removal rat model. PLoS ONE 2014, 9, e98329. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.J.; Zhang, Y.; Qiu, L.; Dong, X.; Jiang, B. Schistosomicidal and antioxidant flavonoids from Astragalus englerianus. Planta Med. 2014, 80, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.J.; Wang, D.M.; Zhou, D. Structural characterization and evaluation of the antioxidant activity of phenolic compounds from Astragalus taipaishanensis and their structure-activity relationship. Sci. Rep. 2015, 5, 13914. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Zu, Y.G.; Fu, Y.J.; Luo, M.; Zhao, C.J.; Wang, W.; Zhao, B.S.; Li, J.; Efferth, T. Preparation and antioxidant activity of Radix Astragali residues extracts rich in calycosin and formononetin. Biochem. Eng. J. 2011, 56, 84–93. [Google Scholar] [CrossRef]
- He, Y.X.; Shi, H.L.; Huang, F.; Liu, H.S.; Wu, H.; Zhang, B.B.; Dou, W.; Wu, X.J.; Wang, Z.T. Astragalosides from Radix Astragali benefits experimental autoimmune encephalomyelitis in C57BL/6 mice at multiple levels. BMC Complement. Altern. Med. 2014, 14, 313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gong, A.G.; Riaz, K.; Deng, J.Y.; Ho, C.M.; Lin, H.Q.; Dong, T.T.; Lee, Y.K.; Tsim, K.W. A novel combination of four flavonoids derived from Astragali Radix relieves the symptoms of cyclophosphamide-induced anemic rats. FEBS Open Biol. 2017, 7, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, L.; Wang, S.; Zhang, Z. Astragaloside IV attenuates inflammatory reaction via activating immune function of regulatory T-cells inhibited by HMGB1 in mice. Pharm. Biol. 2016, 54, 3217–3225. [Google Scholar] [CrossRef] [PubMed]
- Murata, I.; Abe, Y.; Yaginuma, Y.; Yodo, K.; Kamakari, Y.; Miyazaki, Y.; Baba, D.; Shinoda, Y.; Iwasaki, T.; Takahashi, K.; et al. Astragaloside-IV prevents acute kidney injury and inflammation by normalizing muscular mitochondrial function associated with a nitric oxide protective mechanism in crush syndrome rats. Ann. Intensive Care 2017, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, H.X.; Zhang, Y.J.; Yang, Y.H.; Lu, M.L.; Zhang, J.; Li, S.T.; Zhang, S.P.; Li, G. Astragaloside IV attenuates inflammatory cytokines by inhibiting TLR4/NF-кB signaling pathway in isoproterenol-induced myocardial hypertrophy. J. Ethnopharmacol. 2013, 150, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhou, J.; Zhang, Y.; Chen, Y.; Yang, Z.; Huang, G.; Chen, Y.; Yuan, Z.; Peng, Y.; Cao, T. Astragaloside-IV alleviates heat-induced inflammation by inhibiting endoplasmic reticulum stress and autophagy. Cell. Physiol. Biochem. 2017, 42, 824–837. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, W.C.; Wang, W.P.; Tian, W.Y.; Zhang, X.G. Antioxidant activity of Astragalus polysaccharides and anti-tumor activity of the polysaccharides and siRNA. Carbohydr. Polym. 2010, 82, 240–244. [Google Scholar] [CrossRef]
- Ma, J.W.; Qiao, Z.Y.; Xiang, X. Aqueous extract of Astragalus mongholicus ameliorates high cholesterol diet induced oxidative injury in experimental rats models. J. Med. Plants Res. 2011, 5, 855–858. [Google Scholar]
- Xu, X.Y.; Li, F.; Zhang, X.; Li, P.C.; Zhang, X.; Wu, Z.X.; Li, D.P. In vitro synergistic antioxidant activity and identification of antioxidant components from Astragalus membranaceus and Paeonia lactiflora. PLoS ONE 2014, 9, e96780. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.H.; Hu, Q.L. Effect of Astragalus membranaceus polysaccharides on oxidative damage in skeletal muscle of exhaustive exercise rats. Afr. J. Agric. Res. 2011, 6, 4086–4090. [Google Scholar]
- Li, X.T.; Zhang, Y.K.; Kuang, H.X.; Jin, F.X.; Liu, D.W.; Gao, M.B.; Liu, Z.; Xin, X.J. Mitochondrial protection and anti-aging activity of Astragalus polysaccharides and their potential mechanism. Int. J. Mol. Sci. 2012, 13, 1747–1761. [Google Scholar] [CrossRef] [PubMed]
- Block, K.I.; Mead, M.N. Immune system effects of Echinacea, Ginseng, and Astragalus: A review integrative cancer therapies. Integr. Cancer Ther. 2003, 2, 247. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.; Lam, F.Y.; Cheung, A.P. Amelioration of experimental colitis by Astragalus membranaceus through anti-oxidation and inhibition of adhesion molecule synthesis. World J. Gastroenterol. 2005, 11, 5787–5794. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.; Chik, C.W. The protective action of radix Astragalus membranaceus against hapten-induced colitis through modulation of cytokines. Cytokine 2009, 47, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.J.; Jeney, G.; Racz, T.; Xu, P.; Jun, X.; Jeney, Z. Effect of two Chinese herbs (Astragalus Radix and Scutellaria Radix) on non-specific immune response of tilapia, Oreochromis niloticus. Aquaculture 2006, 253, 39–47. [Google Scholar] [CrossRef]
- Denzler, K.; Moore, J.; Harrington, H.; Morrill, K.; Huynh, T.; Jacobs, B.; Waters, R.; Langland, J. Characterization of the physiological response following in vivo administration of Astragalus membranaceus. Evid. Based Complement. Altern. Med. 2016, 2016, 6861078. [Google Scholar] [CrossRef] [PubMed]
- Li, L.K.; Kuang, W.J.; Huang, Y.F.; Xie, H.H.; Chen, G.; Zhou, Q.C.; Wang, B.R.; Wan, L.H. Anti-tumor effects of Astragalus on hepatocellular carcinoma in vivo. Indian J. Pharmacol. 2012, 44, 78–81. [Google Scholar] [PubMed]
- Lin, J.; Dong, H.F.; Oppenheim, J.J.; Howard, O.M. Effects of Astragali Radix on the growth of different cancer cell lines. World J. Gastroenterol. 2003, 9, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lee, W.B.; Lee, J.W.; Min, B.I.; Baek, S.K.; Lee, H.S.; Cho, S.H. Traditional herbal medicine as adjunctive therapy for breast cancer: A systematic review. Complement. Ther. Med. 2015, 23, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Qi, Y.G.; Liu, A.J.; Zhang, X.W.; Teng, A.G.; Tang, J.; Zhang, W.J.; Shi, L.G. Protective effect of herbal compound beverage of Radix Astragali on immunosuppressed mice induced by cyclophosphamide. Adv. Mater. Res. 2013, 721, 603–607. [Google Scholar] [CrossRef]
- Yin, J.Y.; Chan, B.C.; Yu, H.; Lau, I.Y.; Han, X.Q.; Cheng, S.W.; Wong, C.K.; Lau, C.B.; Xie, M.Y.; Fung, K.P.; et al. Separation, structure characterization, conformation and immunomodulating effect of a hyperbranched heteroglycan from Radix Astragali. Carbohydr. Polym. 2012, 87, 667–675. [Google Scholar] [CrossRef]
- Zhao, L.H.; Ma, Z.X.; Zhu, J.; Yu, X.H.; Weng, D.P. Characterization of polysaccharide from Astragalus Radix as the macrophage stimulator. Cell. Immunol. 2011, 271, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Bai, S.P.; Zhao, L.; Wang, X.H. Astragalus polysaccharide injection integrated with vinorelbine and cisplatin for patients with advanced non-small cell lung cancer: Effects on quality of life and survival. Med. Oncol. 2012, 29, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ito, H. Enhancing effect of antitumor polysaccharide from Astragalus or Radix Hedysarum on C3 cleavage production of macrophages in mice. Jpn. J. Pharmacol. 1988, 51, 432–434. [Google Scholar] [CrossRef]
- Kajimura, K.; Takagi, Y.; Miyano, K.; Sawabe, Y.; Mimura, M.; Sakagami, Y.; Yokoyama, H.; Yoneda, K. Polysaccharide of Astragali Radix enhances IgM antibody production in aged mice. Biol. Pharm. Bull. 1997, 20, 1178–1182. [Google Scholar] [CrossRef] [PubMed]
- Ercolini, A.M.; Miller, S.D. The role of infections in autoimmune disease. Clin. Exp. Immunol. 2009, 155, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Navegantes, K.; Gomes, R.; Pereira, P.; Czaikoski, P.; Heitmann, C.; Azevedo, M.; Monteiro, M. Immune modulation of some autoimmune diseases: The critical role of macrophages and neutrophils in the innate and adaptive immunity. J. Transl. Med. 2017, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Li, Y.L.; Yang, X.J.; Yao, J.H. Astragalus polysaccharide reduces inflammatory response by decreasing permeability of LPS-infected CaCO2 cells. Int. J. Biol. Macromol. 2013, 61, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.K.; Chan, J.Y.; Cheng, L.; Lau, C.P.; Han, S.Q.; Leung, P.C.; Fung, K.P.; Lau, C.B. Isolation of anti-inflammatory fractions and compounds from the root of Astragalus membranaceus. Phytother. Res. 2013, 27, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.G.; Zhang, L.M.; Lam, C.T.; Xu, M.L.; Wang, H.Y.; Lin, H.Q.; Dong, T.T.; Tsim, K.W. Polysaccharide of Danggui Buxue Tang, an ancient Chinese herbal decoction, induces expression of pro-inflammatory cytokines possibly via activation of NFκB signaling in cultured Raw 264.7 cells. Phytother. Res. 2017, 31, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.B.; Qiu, J.D.; Yang, L.H.; He, J.P.; Smith, G.W.; Li, H.Q. Therapeutic effects of Astragalus polysaccharides on inflammation and synovial apoptosis in rats with adjuvant-induced arthritis. Int. J. Rheum. Dis. 2010, 13, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Liu, H.K.; Hsiao, P.C.; Kuo, L.M.; Lee, I.J.; Wu, T.S.; Chiou, W.F.; Kuo, Y.H. New isoflavonoid glycosides and related constituents from Astragali Radix (Astragalus membranaceus) and their inhibitory activity on nitric oxide production. J. Agric. Food Chem. 2011, 59, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Y.N.; Yan, X.T.; Yang, S.Y.; Kim, S.; Lee, Y.M.; Koh, Y.S.; Kim, Y.H. Flavonoids from Astragalus membranaceus and their inhibitory effects on LPS-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Arch. Pharm. Res. 2014, 37, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; He, B.; Zheng, Z.G.; Wang, R.S.; Gu, F.; Duan, T.T.; Cheng, H.Q.; Zhu, Q. Inhibitory effects of two major isoflavonoids in Radix Astragali on high glucose-induced mesangial cells proliferation and AGEs-induced endothelial cells apoptosis. Planta Med. 2011, 77, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wen, J.; Yu, X. Influence of flavonoid of Astragalus membranaceus’s stem and leaves on the function of cell mediated immunity in mice. Chin. J. Integr. Tradit. West. Med. 1999, 19, 356–358. [Google Scholar]
- Gao, Q.T.; Cheung, J.K.; Li, J.; Jiang, Z.Y.; Chu, G.K.; Duan, R.; Cheung, A.W.; Zhao, K.J.; Choi, R.C.; Dong, T.T.; et al. A Chinese herbal decoction, Danggui Buxue Tang, activates extracellular signal-regulated kinase in cultured T-lymphocytes. FEBS Lett. 2007, 581, 5087–5093. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Q.; Zhuang, Y.; Tian, Y.P.; Thomas, G.N.; Ying, M.Z.; Tomlinson, B. Study of the effects of total flavonoids of Astragalus on atherosclerosis formation and potential mechanisms. Oxid. Med. Cell. Longev. 2012, 2012, 282383. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xu, H.Y.; Xu, L.; Wang, S.S.; Zhang, X.M. In vivo and in vitro immunomodulatory and anti-inflammatory effects of total flavonoids of Astragalus. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, G.; Liu, S.Y.; Li, J.B.; Wang, J.F.; Bo, L.L.; Qian, L.R.; Sun, X.J.; Deng, X.M. Hydrogen-rich saline protects against renal ischemia/reperfusion injury in rats. J. Surg. Res. 2011, 167, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.B.; Yan, J.C.; Su, H.L.; Yuan, W.; Xu, L.J. The effect of CD137-CD137 ligand interaction on the expression of nuclear factor of activated T cells c1 in apolipoprotein E-deficient mice atherosclerotic plaque model. Zhonghua Xin Xue Guan Bing Za Zhi 2012, 40, 775–779. [Google Scholar] [PubMed]
- Yang, Q.Y.; Chen, K.J.; Lu, S.; Sun, H.R. Research progress on mechanism of action of Radix Astragalus in the treatment of heart failure. Chin. J. Integr. Med. 2012, 18, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Jia, H.Y.; Guo, S.S.; Chen, T.H.; Wang, H.D. Protective effects of Radix Astragalus on vascular endothelial cells of patients with Binswanger’s disease. Chin. J. Integr. Med. 1999, 5, 46–49. [Google Scholar]
- Zhou, Y.; Tong, X.; Ren, S.; Wang, X.; Chen, J.; Mu, Y.; Sun, M.; Chen, G.; Zhang, H.; Liu, P. Synergistic anti-liver fibrosis actions of total Astragalus saponins and glycyrrhizic acid via TGF-β1/Smads signaling pathway modulation. J. Ethnopharmacol. 2016, 190, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.L.; Yuan, J.Y. Effect of Chinese herbal compound on liver fibrosis in rabbits with schistosomiasis by B-ultrasound. Asian Pac. J. Trop. Med. 2013, 6, 658–662. [Google Scholar] [CrossRef]
- Hu, G.; Mahady, B.; Hoi, M.P.; Wang, Y.H.; Lee, S.M. Polysaccharides from Astragali Radix restore chemical-induced blood vessel loss in zebrafish. Vasc. Cell 2012, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.Y.; Choi, R.C.; Cheung, A.W.; Guo, A.J.; Bi, C.W.; Zhu, K.Y.; Fu, Q.; Du, Y.; Zhang, W.L.; Zhan, J.Y.; et al. Flavonoids from Radix Astragali induce the expression of erythropoietin in cultured cells: A signaling mediated via the accumulation of hypoxia-inducible factor-1α. J. Agric. Food Chem. 2011, 59, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Juan, Y.C.; Kuo, Y.H.; Chang, C.C.; Zhang, L.J.; Lin, Y.Y.; Hsu, C.Y.; Liu, H.K. Administration of a decoction of sucrose- and polysaccharide-rich Radix Astragali (Huang qi) ameliorated insulin resistance and fatty liver but affected Beta-cell function in type 2 diabetic rats. Evid. Based Complement. Altern. Med. 2011, 2011, 349807. [Google Scholar] [CrossRef] [PubMed]
- Agyemang, K.; Han, L.; Liu, E.; Zhang, Y.; Wang, T.; Gao, X. Recent advances in Astragalus membranaceus anti-diabetic research: Pharmacological effects of its phytochemical constituents. Evid. Based Complement. Altern. Med. 2013, 2013, 654643. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wu, K.; Mao, X.; Wu, Y.; Ouyang, J. Astragalus polysaccharide improves insulin sensitivity in KKAy mice: Regulation of PKB/GLUT4 signaling in skeletal muscle. J. Ethnopharmacol. 2010, 127, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Tin, M.; Cho, C.H.; Ko, H. The antitumorigenic potential of total saponins from Radix Astragalus membranaceus as chemotherapeutic adjuvant in treating colon cancer. Acta Pharmacol. Sin. 2006, 27, 68. [Google Scholar]
- Auyeung, K.K.; Mok, N.I.; Wong, C.M.; Cho, C.H.; Ko, J.K. Astragalus saponins modulate mTOR and ERK signaling to promote apoptosis through the extrinsic pathway in HT-29 colon cancer cells. Int. J. Mol. Med. 2010, 26, 341–349. [Google Scholar] [PubMed]
- Law, P.C.; Auyeung, K.K.; Chan, L.Y.; Ko, J.K. Astragalus saponins downregulate vascular endothelial growth factor under cobalt chloride-stimulated hypoxia in colon cancer cells. BMC Complement. Altern. Med. 2012, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zheng, Y.; Que, Z.; Zhang, L.; Lin, S.; Le, V.; Liu, J.; Tian, J. Astragaloside IV inhibits progression of lung cancer by mediating immune function of Tregs and CTLs by interfering with IDO. J. Cancer Res. Clin. Oncol. 2014, 140, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.F.; Yao, Y.M.; Li, J.F.; Zhang, S.W.; Li, W.X.; Dong, N.; Yu, Y.; Sheng, Z.Y. The effect of Astragaloside IV on immune function of regulatory T cell mediated by high mobility group box 1 protein in vitro. Fitoterapia 2012, 83, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.H.; Niu, Y.B.; Song, X.M.; Zhao, D.D.; Wang, J.; Wu, X.L.; Zhang, R.; Mei, Q.B. Astragaloside II induces osteogenic activities of osteoblasts through the bone morphogenetic protein-2/MAPK and Smad1/5/8 pathways. Int. J. Mol. Med. 2012, 29, 1090–1098. [Google Scholar] [PubMed]
- Di Cesare Mannelli, L.; Pacini, A.; Micheli, L.; Femia, A.P.; Maresca, M.; Zanardelli, M.; Vannacci, A.; Gallo, E.; Bilia, A.R.; Caderni, G.; et al. Astragali radix: Could it be an adjuvant for oxaliplatin-induced neuropathy? Sci. Rep. 2017, 7, 42021. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Zanardelli, M.; Bartolucci, G.; Karioti, A.; Bilia, A.R.; Vannacci, A.; Mugelli, A.; Ghelardini, C. In vitro evidence for the use of astragali radix extracts as adjuvant against oxaliplatin-induced neurotoxicity. Planta Med. 2015, 81, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M.; Micheli, L.; Cinci, L.; Bili, A.R.; Ghelardini, C.; Di Cesare Mannelli, L. Pain relieving and protective effects of Astragalus hydroalcoholic extract in rat arthritis models. J. Pharm. Pharmacol. 2017, 69, 1858–1870. [Google Scholar] [CrossRef] [PubMed]
- Du, C.Y.; Choi, R.C.; Zheng, K.Y.; Dong, T.T.; Lau, D.T.; Tsim, K.W. Yu Ping Feng San, an ancient Chinese herbal decoction containing Astragali radix, Atractylodis macrocephalae rhizoma and Saposhnikoviae radix, regulates the release of cytokines in murine macrophages. PLoS ONE 2013, 8, e78622. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.S.; Yan, L.; Bi, C.W.; Chan, G.K.; Wu, Q.Y.; Liu, Y.L.; Huang, Y.; Yao, P.; Du, C.Y.; Dong, T.T.; et al. Yu Ping Feng San reverses cisplatin-induced multi-drug resistance in lung cancer cells via regulating drug transporters and p62/TRAF6 signaling. Sci. Rep. 2016, 6, 31926. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Namgung, U. Facilitating effects of Buyang Huanwu decoction on axonal regeneration after peripheral nerve transection. J. Ethnopharmacol. 2017, 213, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.D.; Wang, J.H.; Jin, G.R.; Zhao, Y.; Zhang, H.J. Neuroprotective effect of Buyang Huanwu Decoction against focal cerebral ischemia/reperfusion injury in rats-Time window and mechanism. J. Ethnopharmacol. 2012, 140, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.C.; Liu, P.; Hu, X.; Gao, H.; Zheng, X.; Huang, H. Neuroprotective effects of Buyang Huanwu decoction on cerebral ischemia-induced neuronal damage. Neural Regen. Res. 2014, 9, 1621–1627. [Google Scholar] [PubMed]
- Yang, J.H.; Gao, F.; Zhang, Y.K.; Liu, Y.; Zhang, D. Buyang Huanwu Decoction (BYHWD) enhances angiogenic effect of mesenchymal stem cell by upregulating VEGF expression after focal cerebral ischemia. J. Mol. Neurosci. 2015, 56, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Q.; Gong, A.G.; Wang, H.Y.; Duan, R.; Dong, T.T.; Zhao, K.J.; Tsim, K.W. Danggui Buxue Tang (Astragali radix and Angelicae sinensis radix) for menopausal symptoms: A review. J. Ethnopharmacol. 2017, 199, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huang, C.G.; Du, S.Y.; Yang, S.P.; Zhang, X.; Liu, J.Y.; Luo, X.Q.; Xu, J.H. Effect of Danggui Buxue Tang on immune-mediated aplastic anemia bone marrow proliferation mice. Phytomedicine 2014, 21, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Kim, M.H.; Hong, J.; Kim, K.; Yang, W.M. Effect of Dangguibohyul-Tang, a mixed extract of Astragalus membranaceus and Angelica sinensis, on allergic and inflammatory skin reaction compared with single extracts of Astragalus membranous Angelica Sinensis. Evid. Based Complement. Altern. Med. 2016, 2016, 5936354. [Google Scholar] [CrossRef] [PubMed]
- Chan, K. The evolutional development of traditional Chinese medicine (TCM) outside China: Challenges, training, practice, research, and future development. World J. Tradit. Chin. Med. 2016, 2, 6–28. [Google Scholar] [CrossRef]
| Working Parts | Biological Functions | Model | References |
|---|---|---|---|
| Astragaloside | Against oxidation of linoleic acid | In vitro | [19] |
| Enhancing anti-oxidant enzymes activities and accumulating osmotic agents | In vitro | [20] | |
| Improving DNA repair abilities | In vitro | [21] | |
| Upregulating lipideroxidation levels | In vivo | [22] | |
| Flavonoids | Enhancing free radical scavenging activities | In vitro | [23] |
| Stimulating lipid peroxidation inhibition levels | In vitro | [24,25,26] | |
| Decreasing SOD and GSH-Px contents | In vivo | [27] | |
| Saponins | Declining high-mobility group box 1 protein content | In vivo | [28] |
| Preventing renal and mitochondrial oxidative-induced dysfunctions, possibly through the TLR4/NF-κB pathway | In vivo; In vivo | [29,30] | |
| Polysaccharides | Decline of SOD and GSH-Px levels | In vivo | [33,35,36] |
| Decrease of SOD, GSH-Px, and catalase activities | In vitro | [34] |
| Working Parts | Biological Functions | Model | References |
|---|---|---|---|
| Astragalosides | Decrease colonic lesion area and histological damage | In vivo | [38,39] |
| Enhance non-specific immune response | In vivo | [40] | |
| Increase of monocytes, neutrophils, and lymphocytes counts | In vivo | [41] | |
| Increase Bax/Bcl-2 ratio | In vitro | [42] | |
| Suppress proliferation of various cancer cell types | In vitro | [43] | |
| Enhance breast cancer patients’ life span and increase their life quality | In vivo | [44] | |
| Polysaccharides | T cell and B cell proliferation | In vivo | [45] |
| Cytokine upregulation | In vitro | [46] | |
| Regulation of GM-CSF and NO productions and modulation of Akt pathway | In vitro | [47] | |
| Prolong cancer patient’s lifespan | In vivo | [48] | |
| Stimulate tumor cell apoptosis | In vitro | [49] | |
| Enhance IgM antibody production | In vivo | [50] | |
| Suppress chronic inflammation cytokine level | In vitro | [56,57,58,59,60] | |
| Increase synovial cell apoptosis rate | In vivo | [61] | |
| Flavonoids | Suppress NO and chronic inflammatory mediator release | In vitro | [62] |
| Inhibit LPS-stimulated cytokine production in bone marrow-derived dendritic cells | In vitro | [58] | |
| Accelerate cancer apoptosis rate | In vitro | [59] | |
| Prolong cancer patient’s lifespan | In vivo | [48] | |
| Stimulate lymphocyte proliferation | In vitro | [60,62,63] |
| Parts | Biological Functions | Model | References |
|---|---|---|---|
| Polysaccharides | Anti-obesity | In vitro; In vivo | [72,73,74] |
| Saponins | Reduction of tumor size | In vivo | [75] |
| Downregulation of mTOR expression and interference with DNA binding activity | In vitro | [76] | |
| Suppression of VEGF and bFGF levels and downregulation of p-Akt, p-mTaOR, VEGF, VEGFR1, and VEGFR2 | In vitro; In vivo | [77] | |
| Enhancement of immune response | In vitro; In vivo | [78,79] | |
| Induction of BMP-2 and Smad1/5/8 expressions | In vitro | [80] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, A.G.W.; Duan, R.; Wang, H.Y.; Kong, X.P.; Dong, T.T.X.; Tsim, K.W.K.; Chan, K. Evaluation of the Pharmaceutical Properties and Value of Astragali Radix. Medicines 2018, 5, 46. https://doi.org/10.3390/medicines5020046
Gong AGW, Duan R, Wang HY, Kong XP, Dong TTX, Tsim KWK, Chan K. Evaluation of the Pharmaceutical Properties and Value of Astragali Radix. Medicines. 2018; 5(2):46. https://doi.org/10.3390/medicines5020046
Chicago/Turabian StyleGong, Amy G. W., Ran Duan, Huai Y. Wang, Xiang P. Kong, Tina T. X. Dong, Karl W. K. Tsim, and Kelvin Chan. 2018. "Evaluation of the Pharmaceutical Properties and Value of Astragali Radix" Medicines 5, no. 2: 46. https://doi.org/10.3390/medicines5020046
APA StyleGong, A. G. W., Duan, R., Wang, H. Y., Kong, X. P., Dong, T. T. X., Tsim, K. W. K., & Chan, K. (2018). Evaluation of the Pharmaceutical Properties and Value of Astragali Radix. Medicines, 5(2), 46. https://doi.org/10.3390/medicines5020046








