Biopsychosocial Assessment of Pain with Thermal Imaging of Emotional Facial Expression in Breast Cancer Survivors
Abstract
1. Introduction
2. Method
Participants
3. Measurement
3.1. Psychometric Measures
3.2. Psychobiological Measures
3.3. Procedure
3.4. Statistical Analysis
4. Results
4.1. Inflammatory Response IL-6
4.2. Psychophysiological Assessment through Thermal Infrared Imaging
4.2.1. Baseline
4.2.2. The Effect of the Emotional Facial Expressions on the Thermal Changes Associated with Facial Temperature in the ROIs
4.2.3. Thermography: Intra-Group Differences
4.3. Relationship between IL-6 and Temperature
4.4. Psychosocial Assessment of Pain
4.5. Relationship between IL-6 and Psychosocial Characteristics
4.6. The Relationship of Psychosocial Characteristics
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schreiber, K.L.; Kehlet, H.; Belfer, I.; Edwards, R.R. Predicting, preventing and managing persistent pain after breast cancer surgery: The importance of psychosocial factors. Pain Manag. 2014, 4, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Leysen, L.; Adriaenssens, N.; Aguilar Ferrandiz, M.E.; Devoogdt, N.; Tassenoy, A.; Ickmans, K.; Goubert, D.; van Wilgen, C.P.; Wijma, A.J.; et al. Pain following cancer treatment: Guidelines for the clinical classification of predominant neuropathic, nociceptive and central sensitization pain. Acta Oncol. 2016, 55, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Honerlaw, K.R.; Rumble, M.E.; Rose, S.L.; Coe, C.L.; Costanzo, E.S. Biopsychosocial predictors of pain among women recovering from surgery for endometrial cancer. Gynecol. Oncol. 2016, 140, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Bredal, I.S.; Smeby, N.A.; Ottesen, S.; Warncke, T.; Schlichting, E. Chronic Pain in Breast Cancer Survivors: Comparison of Psychosocial, Surgical, and Medical Characteristics between Survivors with and without Pain. J. Pain Symptom Manag. 2014, 48, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Novy, D.M.; Aigner, C.J. The biopsychosocial model in cancer pain. Curr. Opin. Support. Palliat. Care 2014, 8, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Belfer, I.; Schreiber, K.L.; Shaffer, J.R.; Shnol, H.; Blane, K.; Morando, A.; Englert, D.; Greco, C.; Brufsky, A.; Ahrendt, G.; et al. Persistent Postmastectomy Pain in Breast Cancer Survivors: Analysis of Clinical, Demographic, and Psychosocial Factors. J. Pain 2013, 14, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Yanez, B.; Thompson, E.H.; Stanton, A.L. Quality of life among Latina breast cancer patients: A systematic review of the literature. J. Cancer Surviv. 2011, 5, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Porro, M.; Andrés, M.; Rodríguez, S. Regulación Emocional y Cáncer: Utilización Diferencial de la Expresión y Supresión Emocional en Pacientes Oncológicos. Avances En Psicología Latinoamericana. 2012. Available online: http://revistas.urosaROI.edu.co/index.php/apl/article/view/1969/1967 (accessed on 28 November 2016).
- Wittig, R.M.; Crockford, C.; Weltring, A.; Langergraber, K.; Deschner, T.; Zuberbühler, K. Social support reduces stress hormone levels in wild chimpanzees across stressful events and everyday affiliations. Nat. Commun. 2016, 7, 13361. [Google Scholar] [CrossRef] [PubMed]
- DeVon, H.A.; Piano, M.R.; Rosenfeld, A.G.; Hoppensteadt, D.A. The Association of Pain with Protein Inflammatory Biomarkers. Nurs. Res. 2014, 63, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Bäckryd, E. Pain in the Blood? Envisioning Mechanism-Based Diagnoses and Biomarkers in Clinical Pain Medicine. Diagnostics 2015, 5, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Lasselin, J.; Elsenbruch, S.; Lekander, M.; Axelsson, J.; Karshikoff, B.; Grigoleit, J.; Engler, H.; Schedlowski, M.; Benson, S. Mood disturbance during experimental endotoxemia: Predictors of state anxiety as a psychological component of sickness behavior. Brain Behav. Immun. 2016, 57, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Shattuck, E.C.; Muehlenbein, M.P. Towards an integrative picture of human sickness behavior. Brain Behav. Immun. 2016, 57, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Jaremka, L.M.; Alfano, C.M.; Glaser, R.; Povoski, S.P.; Lipari, A.M.; Agnese, D.M.; Farrar, W.B.; Yee, L.D.; Carson, W.E.; et al. Social support predicts inflammation, pain, and depressive symptoms: Longitudinal relationships among breast cancer survivors. Psychoneuroendocrinology 2014, 42, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cuevas, I.; Marins, J.C.B.; Lastras, J.A.; Carmona, P.M.G.; Cano, S.P.; García-Concepción, M.Á.; Sillero-Quintana, M. Classification of factors influencing the use of infrared thermography in humans: A review. Infrared Phys. Technol. 2015, 71, 28–55. [Google Scholar] [CrossRef]
- McIntosh, N.D.; Zajonc, R.B.; Vig, P.S.; Emerick, S.W. Facial Movement, Breathing, Temperature, and Affect: Implications of the Vascular Theory of Emotional Efference. Cognit. Emot. 1997, 11, 171–196. [Google Scholar] [CrossRef]
- Rustemeyer, J.; Radtke, J.; Bremerich, A. Thermography and thermoregulation of the face. Head Face Med. 2007, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, S.; Gallese, V.; Merla, A. Thermal infrared imaging in psychophysiology: Potentialities and limits. Psychophysiology 2014, 51, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Ingleby, M.; Ward, R.D. Automated Facial Expression Classification and affect interpretation using infrared measurement of facial skin temperature variations. ACM Trans. Auton. Adapt. Syst. 2006, 1, 91–113. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Lv, S.; Lv, Y.; Wu, G.; Peng, P.; Chen, F.; Wang, X. A Natural Visible and Infrared Facial Expression Database for Expression Recognition and Emotion Inference. IEEE Trans. Multimed. 2010, 12, 682–691. [Google Scholar] [CrossRef]
- Jarlier, S.; Grandjean, D.; Delplanque, S.; N’Diaye, K.; Cayeux, I.; Velazco, M.; Sander, D.; Vuilleuimer, P.; Sherer, K. Thermal Analysis of Facial Muscles Contractions. IEEE Trans. Affect. Comput. 2011, 2, 2–9. [Google Scholar] [CrossRef]
- Levenson, R.W.; Ekman, P.; Friesen, W.V. Voluntary Facial Action Generates Emotion-Specific Autonomic Nervous System Activity. Psychophysiology 1990, 27, 363–384. [Google Scholar] [CrossRef] [PubMed]
- Kraft, T.L.; Pressman, S.D. Grin and bear it: The influence of manipulated facial expression on the stress response. Psychol. Sci. 2012, 23, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Price, T.F.; Harmon-Jones, E. Embodied emotion: The influence of manipulated facial and bodily states on emotive responses. Wiley Interdiscip. Rev. Cognit. Sci. 2015, 6, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Baudic, S.; Jayr, C.; Albi-Feldzer, A.; Fermanian, J.; Masselin-Dubois, A.; Bouhassira, D.; Attal, N. Effect of Alexithymia and Emotional Repression on Postsurgical Pain in Women with Breast Cancer: A Prospective Longitudinal 12-Month Study. J. Pain 2016, 17, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R. The short-form McGill pain questionnaire. Pain 1987, 30, 191–197. [Google Scholar] [CrossRef]
- Escalante, A.; Lichtenstein, M.; Ríos, N.; Hazuda, H. Measuring chronic rheumatic Pain in Mexican Americans: Cross-cultural adaptation of the McGill Pain Questionnaire. J. Clin. Epidemiol. 1996, 49, 1389–1399. [Google Scholar] [CrossRef]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF). Arthritis Care Res. 2011. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, D.; Raubertas, R.F.; Rosenthal, S.N. The short-form McGill Pain Questionnaire in chronic cancer pain. J. Pain Symptom Manag. 1993, 8, 191–195. [Google Scholar] [CrossRef]
- Cleeland, C. The Brief Pain Inventory User Guide. 2009. Available online: http://sosmanuals.com/manuals/cae48184b7bae3b1d85105ea85375b60.pdf (accessed on 4 April 2017).
- Galindo, O.; Benjet, C.; Juárez, F.; Rojas, E.; Riveros, A.; Aguilar, J.; Á lvarez, M.; Alvarado, S. Psychometric properties of the Hospital Anxiety and Depression Scale (HADS) in a Mexican population of cancer patients. Salud Ment. 2015, 38, 253–258. [Google Scholar] [CrossRef]
- Prins, A.; Ouimette, P.; Kimerling, R.; Cameron, R.; Hugelshofer, D.; Shaw-Hegwer, J.; Thrailkill, A.; Gusman, F.; Sheikh, J. The Primary Care PTSD Screen (PC-PTSD): Development and operating characteristics (PDF). Prim. Care Psychiatry 2003, 9, 9–14. [Google Scholar] [CrossRef]
- Tauben, D. Chronic Pain Management: Measurement-Based Step Care Solutions—IASP. 2012. Available online: http://www.iasp-pain.org/PublicationsNews/NewsletterIssue.aspx?ItemNumber=2064 (accessed on 4 April 2017).
- Oliva, F.; Hernández, M.; Calleja, N. Validation of the Mexican Version of the State-Trait Ander Expression Inventory (STAXI-2). Acta Colombiana de Psicología. 2010. Available online: http://www.redalyc.org/pdf/798/79819279010.pdf (accessed on 4 October 2015).
- Taylor, G.J.; Ryan, D.; Bagby, R.M. Toward the Development of a New Self-Report Alexithymia Scale. Psychother. Psychosom. 1985, 44, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Durán, W. Validación de la Escala de Alexitimia de Toronto (TAS-20). 2007. Available online: http://catarina.udlap.mx/u_dl_a/tales/documentos/lps/weisel_d_m/indice.html (accessed on 4 October 2015).
- Shibata, M.; Ninomiya, T.; Jensen, M.; Anno, K.; Yonmoto, K.; Makino, S.; Iwaki, R.; Yashimiro, K.; Yoshida, T.; Imada, Y.; et al. Alexithymia Is Associated with Greater Risk of Chronic Pain and Negative Affect and with Lower Life Satisfaction in a General Population: The Hisayama Study. PLoS ONE 2014, 9, e90984. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cabello, R.; Salguero, J.; Fernández-Berrocal, P.; Gross, J. A Spanish Adaptation of the Emotion Regulation Questionnaire. Eur. J. Psychol. Assess. 2013. [Google Scholar] [CrossRef]
- Russell, D. UCLA Loneliness Scale (Version 3): Reliability, Validity, and Factor Structure. J. Personal. Assess. 1996, 66, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Sillero, M.; Fernández, I.; Arnaiz, J.; Bouzas, J. Protocol for thermographic assessment in humans. In PreCongress XIII EAT Congress Course on “Medical Applications of Human Thermography”; TERMOINEF Group: Madrid, Spain, 2015. [Google Scholar] [CrossRef]
- Military Torture Interrogation Prank. In YouTube. 2011. Available online: https://www.youtube.com/watch?v=SF2g6oxIfQ (accessed on 4 October 2015).
- Cute Kid Gets Caught Buying Drinks for the Ladies. YouTube. 2011. Available online: https://www.youtube.com/watch?v=J_9UV3fiW0E (accessed on 4 October 2015).
- Domínguez Sánchez, F. La Alegria, la Tristeza y la Ira. In Psicología de la Emoció N; Fernández, E., García, B., Jiménez, M., Martín, M., Domínguez, F., Eds.; Centro de Estudios Ramón Areces: Madrid, Spain, 2010; pp. 280–289. [Google Scholar]
- Chambers, C.; Mogil, J. Ontogeny and phylogeny of facial expression of pain. Pain 2015, 156, 798–799. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.; Ambadar, Z.; Ekman, P. Observer-Based Measurement of Facial Expression with the Facial Action Coding System. In Handbook of Emotion Elicitation and Assessment; Cohan, J., Allen, J., Eds.; Oxford University Press: New York, NY, USA, 2007; pp. 203–221. Available online: https://pdfs.semanticscholar.org/eafd/a8a94e410f1ad53b3e193ec124e80d57d095.pdf (accessed on 4 October 2015).
- Boiten, F. Autonomic response patterns during voluntary facial action. Psychophysiology 1996, 33, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Tyler, W.J.; Boasso, A.M.; Mortimore, H.M.; Silva, R.S.; Charlesworth, J.D.; Marlin, M.A.; Aebersold, K.; Aven, L.; Wetmore, D.Z.; Pal, S.K. Transdermal neuromodulation of noradrenergic activity suppresses psychophysiological and biochemical stress responses in humans. Sci. Rep. 2015, 5, 13865. [Google Scholar] [CrossRef] [PubMed]
- Knüpfer, H.; Preiß, R. Significance of interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res. Treat. 2007, 102, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, P.; Perron, M.; Beaupré, M. The voluntary control of facial action units in adults. Emotion 2010, 10, 266. [Google Scholar] [CrossRef] [PubMed]
- Kreibig, S.D. Autonomic nervous system activity in emotion: A review. Biol. Psychol. 2010, 84, 394–421. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Courtois, I.; Van den Bergh, O.; Viaeyen, J.; Van Diest, I. Pain and respiration: A systematic review. Pain 2017, 158, 995–1006. [Google Scholar] [CrossRef] [PubMed]
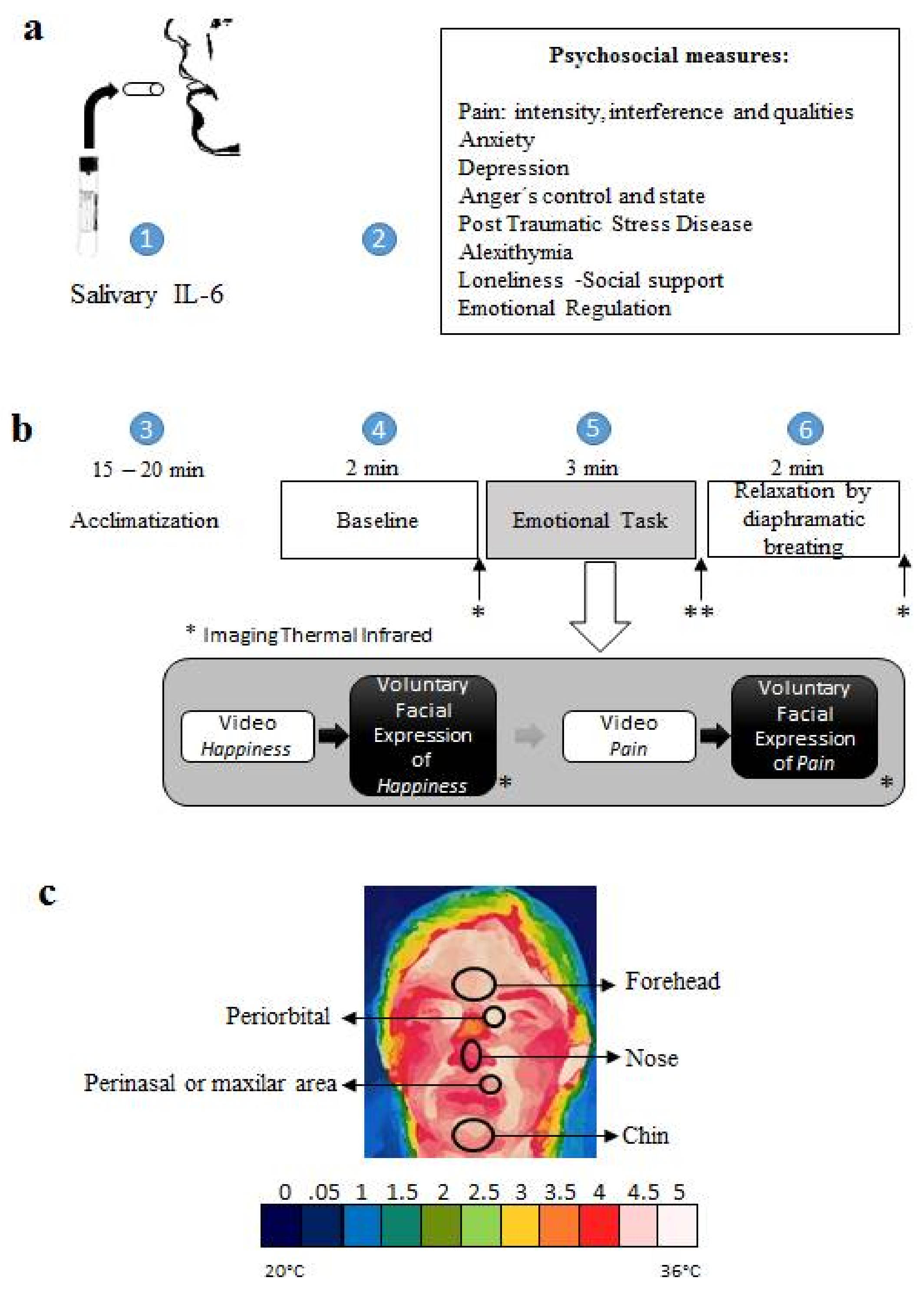

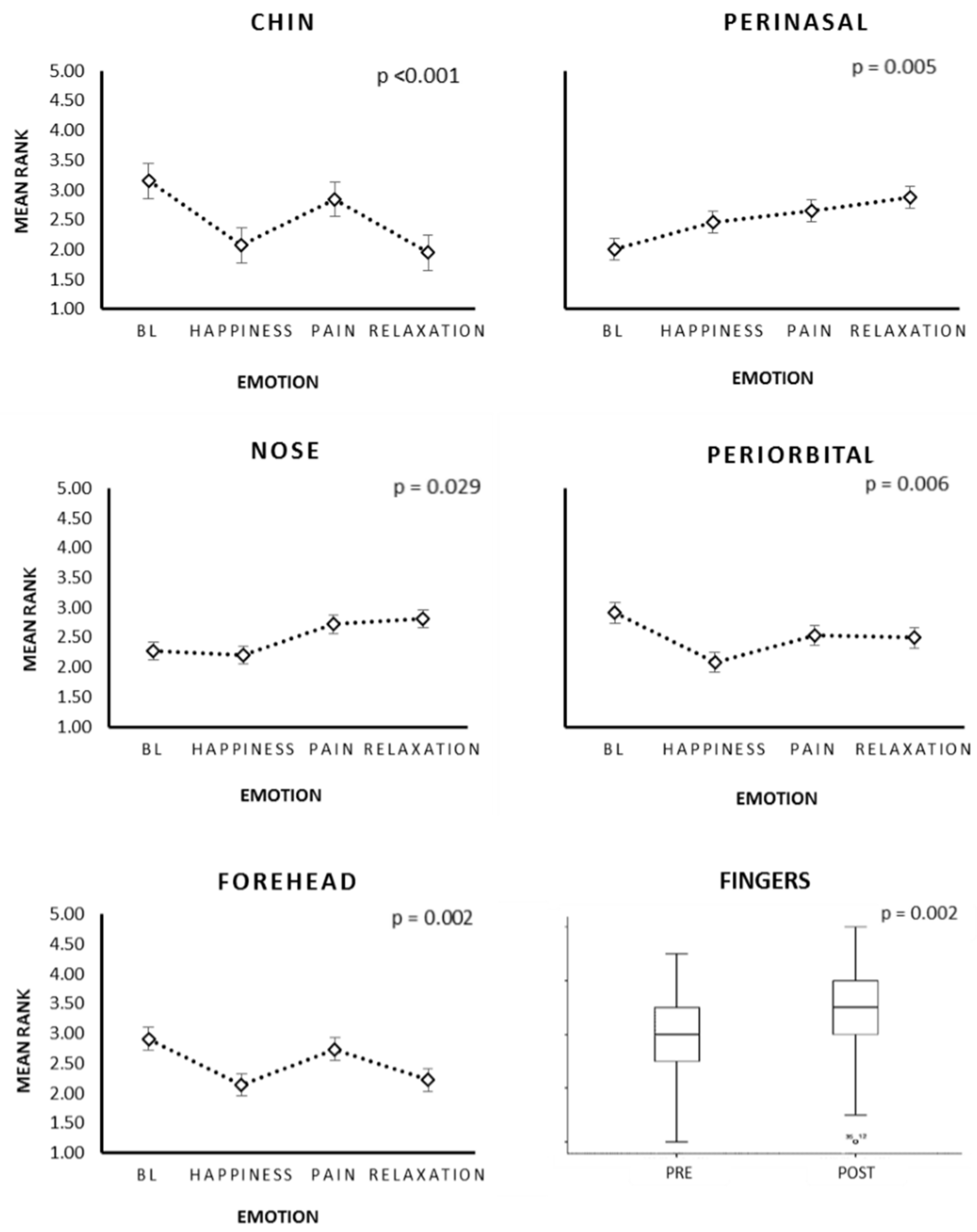

| ROI | CA—Mama Group | Healthy Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | Ha | Pa | Rlx | X2 | p | BL | Ha | Pa | Rlx | X2 | p | |
| Chin | 3.03 | 2 | 3.1 | 1.87 | 15.87 | 0.002 ** | 3.22 | 2.06 | 2.58 | 2.14 | 13.87 | 0.003 ** |
| Perinasal | 1.9 | 2.3 | 2.93 | 2.97 | 8.40 | 0.038 * | 2.14 | 2.5 | 2.44 | 2.92 | 5.33 | 0.149 |
| Nose | 1.93 | 2.2 | 3.1 | 2.77 | 10.15 | 0.017 * | 2.56 | 2.19 | 2.39 | 2.86 | 3.63 | 0.304 |
| Periorbital | 2.9 | 2.17 | 2.57 | 2.37 | 4.88 | 0.18 | 2.78 | 2.08 | 2.58 | 2.56 | 4.26 | 0.235 |
| Forehead | 2.8 | 1.93 | 2.9 | 2.37 | 9.93 | 0.019 * | 2.92 | 2.19 | 2.64 | 2.25 | 5.13 | 0.162 |
| Psychometric Outcomes | CA-Mama Group | Health Group | p-Value |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Pain (BPI) | |||
| Maximum | 4.73 (3.05) | 3.57 (3.10) | 0.418 |
| Minimum | 2.73 (2.12) | 2.14 (2.79) | 0.588 |
| Mean | 4.13 (2.77) | 3.42 (2.57) | 0.577 |
| Current | 2.53 (2.29) | 2.28 (3.14) | 0.836 |
| Relief (percentage) | 28 (37.64) | 24.28 (38.23) | 0.832 |
| Interference (BPI) | |||
| General Activity | 3.20 (3.07) | 2.14 (3.67) | 0.488 |
| Affective State | 3.33 (3.79) | 1.85 (3.07) | 0.380 |
| Walking | 3.91 (1.98) | 0.71 (1.88) | 0.017 * |
| Working | 2.26 (3.55) | 1.42 (3.77) | 0.281 |
| Relationship | 2.86 (3.66) | 1.71 (3.72) | 0.502 |
| Sleep | 2.73 (3.17) | 3.28 (4.27) | 0.737 |
| Enjoy | 2.53 (4.01) | 1.85 (3.18) | 0.767 |
| Pain’s Qualities (SF-MPQ) | |||
| Intensity (NAS) | 1.33 (1.17) | 0.727 (0.786) | 0.152 |
| Sensory | 5.00 (3.9) | 2.54 (3.07) | 0.102 |
| Affective | 0.600 (1.35) | 0.000 (0.000) | 0.108 |
| Anxiety (HADS-A) | 5.67 (3.13) | 6.22 (3.93) | 0.661 |
| Depression (HADS-D) | 3.00 (3.16) | 3.94 (3.65) | 0.464 |
| Anger (STAXI-2) | |||
| State | 2.67 (2.41) | 4.50 (4.09) | 0.137 |
| Temperament | 3.13 (2.10) | 3.82 (4.24) | 0.544 |
| External Control | 10.47 (4.92) | 11.47 (4.07) | 0.449 |
| Internal Control | 13.07 (5.63) | 10.67 (5.33) | 0.219 |
| Posttraumatic Stress (PC-PTSD) | 0.67 (0.90) | 1.55 (1.036) | 0.036 * |
| Loneliness (L-UCLA) | 30.40 (10.88) | 36.78 (9.22) | 0.044 * |
| Alexithymia (TAS) | |||
| Total | 26.20 (12.68) | 13.23 (8.66) | 0.008 ** |
| Identification | 14.50 (7.44) | 6.23 (4.74) | 0.004 ** |
| Expression | 11.70 (6.16) | 7.00 (5.38) | 0.065 |
| Emotional Regulation (EQR) | |||
| Cognitive Reappraisal | 27.10 (6.33) | 29.92 (6.13) | 0.293 |
| Suppression | 14.10 (6.38) | 11.00 (4.47) | 0.376 |
| RIO—Psychosocial Measure | Coefficient of Correlation | |
|---|---|---|
| Temp. Fingers—Temp. Nose | 0.450 | ** |
| —Interference General (BPI) | −0.390 | * |
| —Interference Affective (BPI) | −0.517 | * |
| —Interference Relationship (BPI) | −0.437 | * |
| —Interference Sleep (BPI) | −0.600 | *** |
| —Interference Enjoy (BPI) | −0.665 | *** |
| —Pain (NAS of SF-MPQ) | −0.584 | ** |
| —Loneliness (L-UCLA) | −0.487 | ** |
| Temp. Chin—Temp. Maxillofacial | 0.483 | ** |
| —Temp. Nose | 0.459 | ** |
| —Internal Control of Anger (STAXI-2) | 0.383 | * |
| —Alexithymia (TAS) | −0.446 | * |
| —Supression (EQR) | −0.481 | * |
| Temp. Maxillary—Temp. Nose | 0.821 | *** |
| —Interference Sleep (BPI) | −0.520 | ** |
| —Interference Enjoy (BPI) | −0.461 | ** |
| —Pain (NAS of SF-MPQ) | −0.504 | ** |
| Temp. Nose—Interference Sleep (BPI) | −0.443 | * |
| —Pain (NAS of SF-MPQ) | −0.413 | * |
| Temp. Periorbital—Temp. Forehead | 0.469 | ** |
| —Reappraisal (EQR) | −0.485 | * |
| Measure | Correlation of Coefficient | Measure | Correlation of Coefficient | ||
|---|---|---|---|---|---|
| Pain Maximum | Anxiety | ||||
| Minimum | 0.813 | *** | Depression | 0.687 | *** |
| Mean | 0.724 | *** | Interference Enjoy | 0.496 | ** |
| Current | 0.742 | *** | PTSD | 0.737 | *** |
| Interference General Activity | 0.651 | *** | Loneliness | 0.462 | ** |
| Interference Affective State | 0.614 | *** | State of anger | 0.478 | ** |
| Interference Walking | 0.708 | *** | Temperament anger | 0.446 | ** |
| Interference Working | 0.655 | *** | Internal control anger | −0.549 | *** |
| NAS of Pain | 0.551 | ** | Alexithymia | 0.578 | ** |
| Sensory Pain | 0.508 | ** | Alexithymia ID | 0.556 | ** |
| Pain Minimum | - | Alexithymia Expression | 0.496 | * | |
| Mean | 0.795 | *** | Suppression | 0.417 | * |
| Current | 0.943 | *** | Depression | ||
| Relief | 0.535 | ** | Interference Enjoy | 0.454 | * |
| Interference Affective State | 0.632 | *** | PTSD | 0.750 | *** |
| Interference Walking | 0.652 | *** | Loneliness | 0.342 | * |
| Interference Working | 0.692 | *** | State of anger | 0.453 | ** |
| Interference Slipping | 0.507 | ** | Temperament anger | 0.345 | * |
| NAS of Pain | 0.672 | *** | Internal control anger | −0.611 | *** |
| Sensory Pain | 0.590 | *** | PTSD | ||
| Internal control of Anger | 0.438 | * | Loneliness | 0.510 | ** |
| Pain Current | State of anger | 0.768 | *** | ||
| Relief | 0.614 | *** | Anger State | ||
| Interference General Activity | 0.715 | *** | Loneliness | 0.392 | * |
| Interference Affective State | 0.659 | *** | Internal Control anger | −0.455 | ** |
| Interference Walking | 0.580 | ** | External Control Anger | ||
| Interference Working | 0.660 | *** | Internal Control anger | 0.482 | ** |
| Interference sleeping | 0.681 | *** | Suppression | −0.462 | * |
| Interference Enjoy | 0.430 | * | Internal Control Anger | ||
| NAS of Pain | 0.686 | *** | Loneliness | −0.390 | * |
| Sensory Pain | 0.689 | ** | Alexithymia | −0.423 | * |
| Relief | -Alexithymia Expression | −0.474 | * | ||
| Interference sleeping | 0.524 | ** | Suppression | −0.666 | *** |
| Interference General Activity | Alexithymia | ||||
| Interference Affective State | 0.875 | *** | Interference General Activity | 0.662 | * |
| Interference Walking | 0.778 | *** | Suppression | 0.501 | * |
| Interference Working | 0.832 | *** | Alexithymia ID | ||
| Interference sleeping | 0.464 | * | Interference General Activity | 0.784 | ** |
| Interference Enjoy | 0.570 | ** | Interference Affective State | 0.635 | * |
| NAS of Pain | 0.711 | *** | NAS of Pain | 0.652 | * |
| Sensory Pain | 0.626 | *** | -Alexithymia Expression | 0.732 | *** |
| Interference Affective State | Alexithymia Expression | ||||
| Interference Walking | 0.816 | *** | Interference General Activity | 0.611 | * |
| Interference Working | 0.894 | *** | Internal Control anger | −0.474 | * |
| Interference in Relationship | 0.481 | * | Suppression | 0.566 | ** |
| Interference sleeping | 0.596 | * | Suppression | ||
| Interference Enjoy | 0.693 | Sensory Pain | 0.650 | ** | |
| NAS of Pain | 0.609 | *** | Affective Pain | 0.748 | ** |
| Sensory Pain | 0.569 | ** | Loneliness | ||
| Interference Working | Interference Affective State | 0.392 | * | ||
| Interference sleeping | 0.461 | * | Interference sleeping | 0.479 | * |
| Interference Enjoy | 0.545 | ** | Interference Enjoy | 0.476 | * |
| NAS of Pain | 0.609 | *** | Alexithymia Expression | 0.416 | * |
| Sensory Pain | 0.579 | ** | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez Medina, D.A.; Domínguez Trejo, B.; Cortés Esteban, P.; Cruz Albarrán, I.A.; Morales Hernández, L.A.; Leija Alva, G. Biopsychosocial Assessment of Pain with Thermal Imaging of Emotional Facial Expression in Breast Cancer Survivors. Medicines 2018, 5, 30. https://doi.org/10.3390/medicines5020030
Rodríguez Medina DA, Domínguez Trejo B, Cortés Esteban P, Cruz Albarrán IA, Morales Hernández LA, Leija Alva G. Biopsychosocial Assessment of Pain with Thermal Imaging of Emotional Facial Expression in Breast Cancer Survivors. Medicines. 2018; 5(2):30. https://doi.org/10.3390/medicines5020030
Chicago/Turabian StyleRodríguez Medina, David Alberto, Benjamín Domínguez Trejo, Patricia Cortés Esteban, Irving Armando Cruz Albarrán, Luis Alberto Morales Hernández, and Gerardo Leija Alva. 2018. "Biopsychosocial Assessment of Pain with Thermal Imaging of Emotional Facial Expression in Breast Cancer Survivors" Medicines 5, no. 2: 30. https://doi.org/10.3390/medicines5020030
APA StyleRodríguez Medina, D. A., Domínguez Trejo, B., Cortés Esteban, P., Cruz Albarrán, I. A., Morales Hernández, L. A., & Leija Alva, G. (2018). Biopsychosocial Assessment of Pain with Thermal Imaging of Emotional Facial Expression in Breast Cancer Survivors. Medicines, 5(2), 30. https://doi.org/10.3390/medicines5020030






