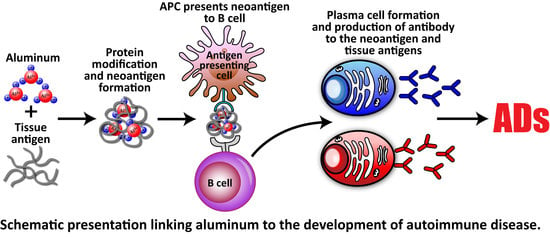
Elevated IgG Antibody to Aluminum Bound to Human Serum Albumin in Patients with Crohn’s, Celiac and Alzheimer’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Aluminum-HSA Conjugate
2.2. Measurement of IgG Antibody to Aluminum-HSA in Human Sera by ELISA
2.3. Specificity of Antigen-Antibody Reaction
2.3.1. Serial Dilution
2.3.2. Inhibition Assay
2.4. Statistical Analysis
3. Results
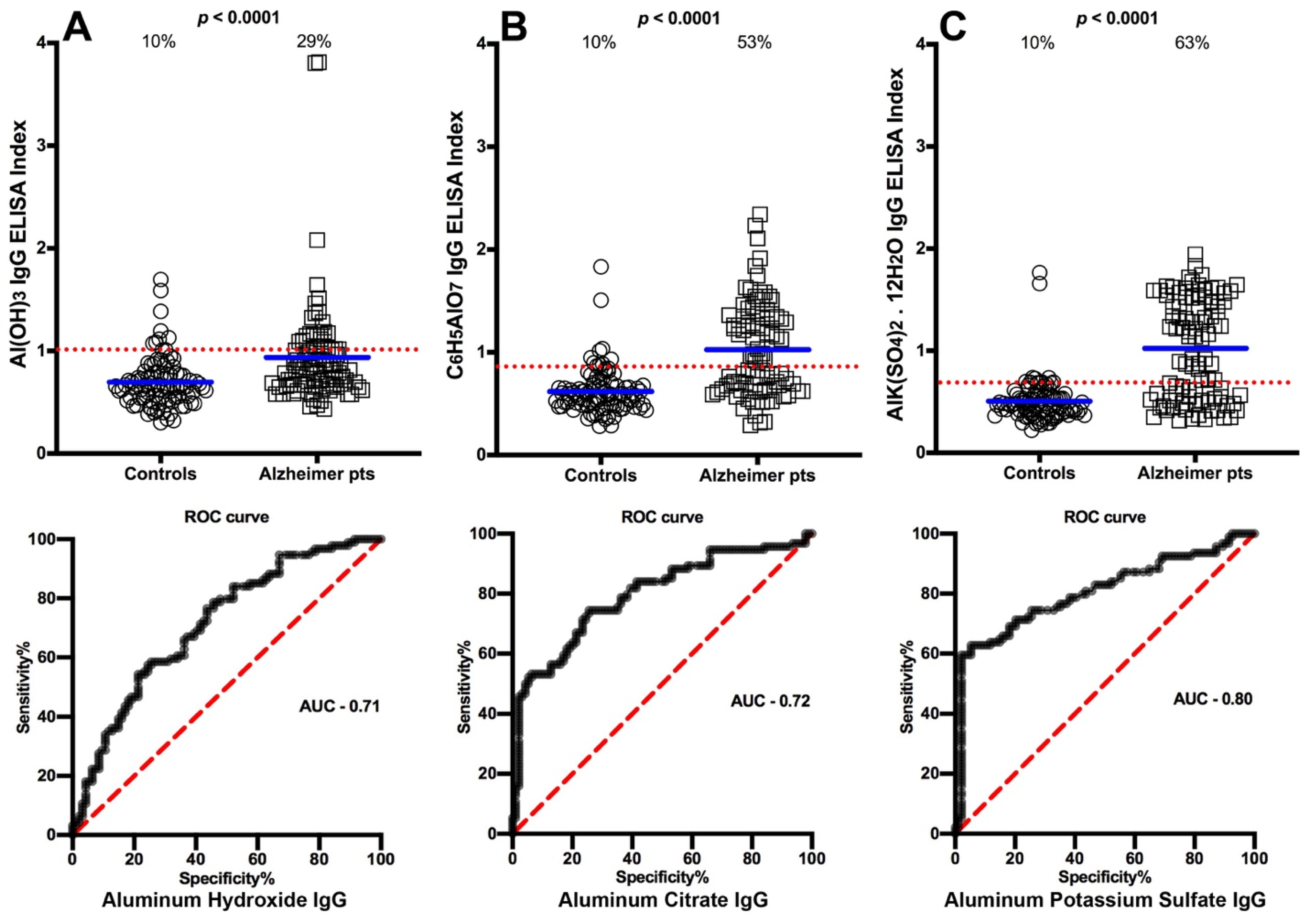
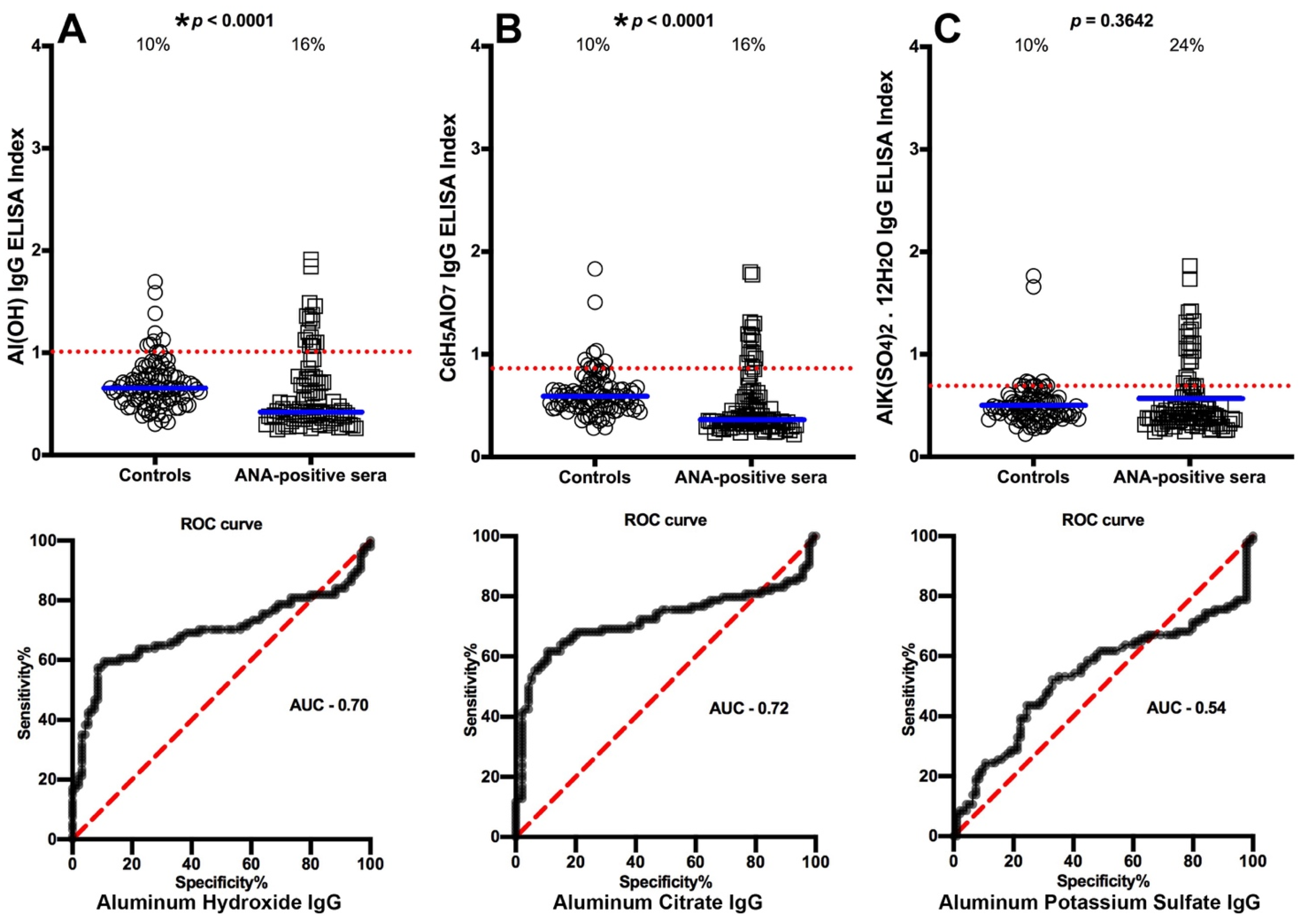
3.1. Detection of Aluminum Antibody in Blood Donors and in Patients with Different Diseases
3.2. Specificity of Anti-Aluminum Antibody
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keith, S.; Jones, D.; Rosemond, Z.; Ingerman, L.; Chappell, L. Toxicological Profile for Aluminum. US Department of Health and Human Services. Public Health Service. Agency for Toxic Substances and Disease Registry. September 2008. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp22.pdf (accessed on 21 July 2021).
- Greger, J.L.; Sutherland, J.E. Aluminum exposure and metabolism. Crit. Rev. Clin. Lab. Sci. 1997, 34, 439–474. [Google Scholar] [CrossRef]
- Stahl, T.; Taschan, H.; Brunn, H. Aluminum content of selected foods and food products. Environ. Sci. Eur. 2011, 23, 37. [Google Scholar] [CrossRef] [Green Version]
- Burrell, S.A.M.; Exley, C. There is (still) too much aluminium in infant formulas. BMC Pediatr. 2010, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- McKee, A.S.; Munks, M.W.; Macleod, M.K.; Fleenor, C.J.; Van Rooijen, N.; Kappler, J.W.; Marrack, P. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J. Immunol. 2009, 183, 4403–4414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrack, P.; McKee, A.S.; Munks, M.W. Towards an understanding of the adjuvant action of aluminum. Nat. Rev. Immunol. 2009, 9, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Nido, J.; Avila, J. Aluminum induces the in vitro aggregation of bovine brain cytoskeletal proteins. Neurosci. Lett. 1990, 110, 221–226. [Google Scholar] [CrossRef]
- Kawahara, M.; Kato-Negishi, M. Link between aluminum and the pathogenesis of Alzheimer’s disease: The integration of the aluminum and amyloid cascade hypothesis. Int. J. Alzheimer’s Dis. 2011, 2011, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Vojdani, A.; Kharrazian, D.; Mukherjee, P.S. Elevated levels of antibodies against xenobiotics in a subgroup of healthy subjects. J. Appl. Toxicol. 2015, 35, 383–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morefield, G.L.; Sokolovska, A.; Jiang, D.; Hogenesch, H.; Robinson, J.P.; Hem, S.L. Role of aluminum containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine 2005, 23, 1588–1595. [Google Scholar] [CrossRef]
- Gupta, R.K.; Rost, B.E.; Relyveld, E.; Siber, G.R. Adjuvant properties of aluminum and calcium compounds. Pharm. Biotechnol. 1995, 6, 229–248. [Google Scholar]
- Centers for Disease Control and Prevention. Vaccine Safety. Available online: https://www.cdc.gov/vaccinesafety/index.html (accessed on 22 July 2021).
- Centers for Disease Control and Prevention. Immunization Schedules. Available online: https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html (accessed on 22 July 2021).
- Centre for Food Safety. Risk Assessment Studies. Report No. 35. Chemical Hazard Evaluation. Aluminium in Food. Food and Environmental Hygiene Department. The Government of the Hong Kong Special Administrative Region. May 2009. Available online: https://www.cfs.gov.hk/english/programme/programme_rafs/files/RA35_Aluminium_in_Food_e.pdf (accessed on 22 July 2021).
- De Chambrun, G.P.; Body-Malapel, M.; Frey-Wagner, I.; Djouina, M.; Deknuydt, F.; Atrott, K.; Esquerre, N.; Altare, F.; Neut, C.; Arrieta, M.C.; et al. Aluminum enhances inflammation and decreases mucosal healing in experimental colitis in mice. Mucosal Immunol. 2014, 7, 589–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizvi, S.H.M.; Parveen, A.; Verma, A.K.; Ahmad, I.; Arshad, M.; Mahdi, A.A. Aluminium induced endoplasmic reticulum stress mediated cell death in SH-SY5Y neuroblastoma cell line is independent of p53. PLoS ONE 2014, 9, e98409. [Google Scholar] [CrossRef] [Green Version]
- Hamdi, H. Testicular dysfunction induced by aluminum oxide nanoparticle administration in albino rats and the possible protective role of the pumpkin seed oil. J. Basic Appl. Zool. 2020, 81, 42. [Google Scholar] [CrossRef]
- Mold, M.; Linhart, C.; Gomez-Ramirez, J.; Villegas-Lanau, A.; Exley, C. Aluminum and amyloid-β in familial Alzheimer’s disease. J. Alzheimer’s Dis. 2020, 73, 1627–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunat, L.; Lanners, M.C.; Joyeux, M.; Burnel, D. Bioavailability and intestinal absorption of aluminum in rats: Effects of aluminum compounds and some dietary constituents. Biol. Trace Elem. Res. 2000, 76, 31–55. [Google Scholar] [CrossRef]
- Malaki, M. Acute encephalopathy following the use of aluminum hydroxide in a boy affected with chronic kidney disease. J. Pediatr. Neurosci. 2013, 8, 81–82. [Google Scholar] [CrossRef] [Green Version]
- Altschulier, E. Aluminum-containing antacids as a cause of idiopathic Parkinson’s disease. Med. Hypothesis 1999, 53, 22–23. [Google Scholar] [CrossRef]
- Demers, P.; Arrandale, V.; DeBono, N.; Berriault, C.; Macleod, J.; Zeng, X.; Harris, A. Investigation of McIntyre Powder Exposure and Neurological Outcomes in the Mining Master File Cohort. Occupational Cancer Research Centre. 2020. Available online: https://www.occupationalcancer.ca/2020/mcintyre-powder-study (accessed on 27 August 2021).
- Youmoto, S.; Kakimi, S.; Ishikawa, A. Colonization of aluminum and iron in nuclei of nerve cells in brains of patients with Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 65, 1267–1281. [Google Scholar] [CrossRef] [Green Version]
- Vojdani, A.; Vojdani, E.; Saidara, E.; Kharrazian, D. Reaction of amyloid-β peptide antibody with different infectious agents involved in Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 63, 847–860. [Google Scholar] [CrossRef]
- Jensen-Jarolim, E. Aluminum in allergies and allergen immunotherapy. World Allergy Organ. J. 2015, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, F.; Boutselis, I.; Borch, R.F.; Hogenesch, H. Control of antigen-binding to aluminum adjuvants and the immune response with a novel phosphonate linker. Vaccine 2013, 31, 4362–4367. [Google Scholar] [CrossRef]
- Mirza, A.; King, A.; Troakes, C.; Exley, C. The identification of aluminum in human brain tissue using lumogallion and fluorescence microsopy. J. Alzheimer’s Dis. 2016, 54, 1333–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gherardi, R.K.; Eidi, H.; Crepeaux, G.; Authier, F.J.; Cadusseau, J. Biopersistence and brain translocation of aluminum adjuvants of vaccines. Front. Neurol. 2015, 6, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomljenovic, L.; Shaw, C.A. Mechanisms of aluminum adjuvant toxicity and autoimmunity in pediatric populations. Lupus 2012, 21, 223–230. [Google Scholar] [CrossRef]
- Mold, M.; Umar, D.; King, A.; Exley, C. Aluminum in brain tissue in autism. J. Trace Elem. Med. Biol. 2018, 46, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Tietz, T.; Lenzner, A.; Kolbaum, A.E.; Zellmer, S.; Riebeling, C.; Gurtler, R.; Jung, C.; Kappenstein, O.; Tentschert, J.; Giulbudagian, M.; et al. Aggregated aluminum exposure: Risk assessment for the general population. Arch. Toxicol. 2019, 93, 3503–3521. [Google Scholar] [CrossRef] [Green Version]
- Fatemi, S.J.A.; Kadir, F.H.A.; Moore, G.R. Aluminum transport in blood serum. Binding of aluminum by human transferrin in the presence of human albumin and citrate. Biochem. J. 1991, 280, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Powell, J.J.; Ainley, C.C.; Evans, R.; Thompson, R.P. Intestinal perfusion of dietary levels of aluminum: Association with the mucosa. Gut 1994, 35, 1053–1057. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, N.A.; Crocker, R.R.; Smith, A.P.; Levison, D.A. Exogenous pigment in Peyer’s patches. Hum. Pathol. 1987, 18, 50–54. [Google Scholar] [CrossRef]
- Urbanski, S.J.; Arsenault, A.L.; Grenn, F.H.; Haber, G. Pigment resembling atmospheric dust in Peyer’s patches. Mod. Pathol. 1989, 2, 222–226. [Google Scholar]
- Powell, J.J.; Ainley, C.C.; Harvey, R.S.; Mason, I.M.; Kendall, M.D.; Sankey, E.A.; Dhillion, A.P.; Thompson, R.P. Characterisation of inorganic microparticles in pigment cells of human gut associated lymphoid tissue. Gut 1996, 38, 390–395. [Google Scholar] [CrossRef] [Green Version]
- Lerner, A.; Eril, S.; Peri, D.; Moalem, S.; Sachar, D.; Tonkonogy, S.; Sartor, R. The role of aluminum in bacterial-induced colitis in IL-10 deficient mice. J. Ped. Gastroenterol. Nutr. 2006, 43, S13–S14. [Google Scholar] [CrossRef]
- Meyer-Baron, M.; Schraper, M.; Knapp, G.; van Thriel, C. Occupational aluminum exposure evidence in support of its neurobehavioral impact. Neurotoxicology 2007, 28, 1068–1078. [Google Scholar] [CrossRef]
- Mold, M.J.; O’Farrell, A.; Morris, B.; Exley, C. Aluminum and tau in neurofibrillary tangles in familial Alzheimer’s disease. J. Alzheimer’s Dis. Rep. 2021, 5, 283–294. [Google Scholar] [CrossRef]
- Zhao, Y.; Hill, J.M.; Bhattacharjee, S.; Percy, M.E.; Pogue, A.I.D.; Lukiw, W.J. Aluminum-induced amyloidogenesis and impairment in the clearance of amyloid peptides from the central nervous system in Alzheimer’s disease. Front. Neurol. 2014, 5, 167. [Google Scholar] [CrossRef] [Green Version]
- Exley, C.; Vickers, T. Elevated brain aluminium and early onset Alzheimer’s disease in an individual occupationally exposed to aluminium: A case report. J. Med. Case Rep. 2014, 8, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulgenzi, A.; De Guiseppe, R.; Bamonti, F.; Vietti, D.; Ferrero, M.E. Efficacy of chelation therapy to remove aluminum intoxication. J. Inorg. Biochem. 2015, 152, 214–218. [Google Scholar] [CrossRef] [PubMed]


| Diseases | Biomarkers | Acronyms |
|---|---|---|
| Crohn’s disease | Anti-Saccharomyces cerevisiae antibodies | ASCA |
| Celiac disease | Transglutaminase | tTG |
| Alzheimer’s disease | Amyloid-β-peptide | Aβ-peptide |
| Mixed connective tissue disease | Antinuclear antibody | ANA |
| Antigens | 1:200 | 1:400 | 1:800 | 1:1600 |
|---|---|---|---|---|
| HSA | 0.231 | 0.186 | 0.178 | 0.156 |
| Alum hydroxide | 1.84 | 1.19 | 0.76 | 0.31 |
| Alum citrate | 1.97 | 1.240 | 0.86 | 0.322 |
| Alum sulfate | 2.26 | 1.45 | 0.92 | 0.43 |
| Antigens | HSA[0] μg/mL | 1 μg/mL | 10 μg/mL | 50 μg/mL | 100 μg/mL |
|---|---|---|---|---|---|
| Aluminum hydroxide | 1.97 | 1.92 | 1.37 | 0.77 | 0.46 |
| % inhibition | – | 2.5 | 31 | 61 | 77 |
| Aluminum citrate | 2.17 | 2.11 | 1.63 | 0.94 | 0.43 |
| % inhibition | – | 3 | 25 | 57 | 80 |
| Aluminum sulfate | 2.38 | 2.06 | 1.45 | 0.83 | 0.37 |
| % inhibition | – | 13 | 39 | 65 | 85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vojdani, A. Elevated IgG Antibody to Aluminum Bound to Human Serum Albumin in Patients with Crohn’s, Celiac and Alzheimer’s Disease. Toxics 2021, 9, 212. https://doi.org/10.3390/toxics9090212
Vojdani A. Elevated IgG Antibody to Aluminum Bound to Human Serum Albumin in Patients with Crohn’s, Celiac and Alzheimer’s Disease. Toxics. 2021; 9(9):212. https://doi.org/10.3390/toxics9090212
Chicago/Turabian StyleVojdani, Aristo. 2021. "Elevated IgG Antibody to Aluminum Bound to Human Serum Albumin in Patients with Crohn’s, Celiac and Alzheimer’s Disease" Toxics 9, no. 9: 212. https://doi.org/10.3390/toxics9090212
APA StyleVojdani, A. (2021). Elevated IgG Antibody to Aluminum Bound to Human Serum Albumin in Patients with Crohn’s, Celiac and Alzheimer’s Disease. Toxics, 9(9), 212. https://doi.org/10.3390/toxics9090212