Analysis of Phthalates and Alternative Plasticizers in Gloves by Gas Chromatography–Mass Spectrometry and Liquid Chromatography–UV Detection: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plasticizers
2.3. Gloves Samples
2.4. Gas Chromatography Method
2.4.1. GC–MS Instrumentation and Analysis Conditions
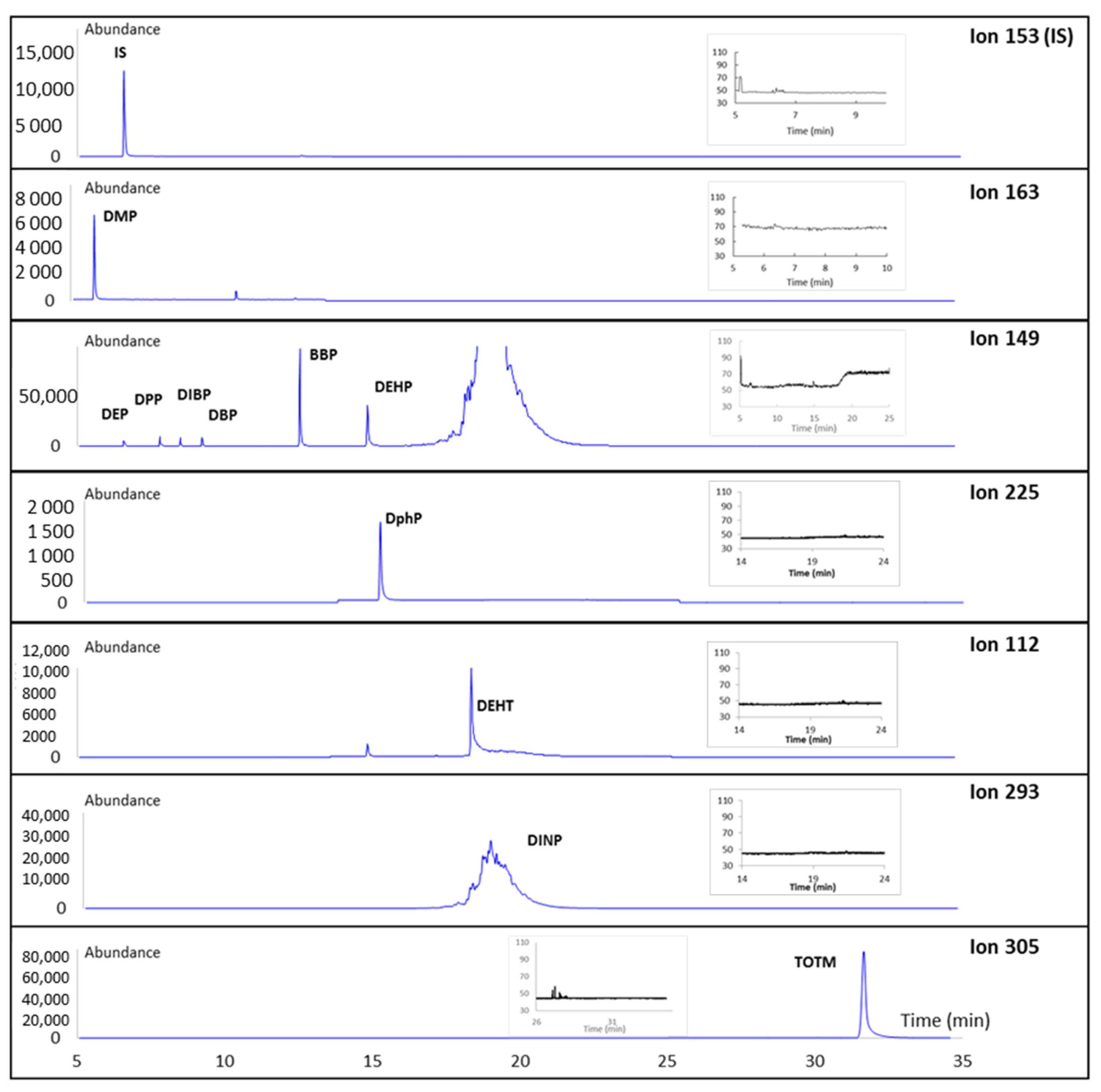
2.4.2. Choice of m/z Ions for SIM Acquisition
2.4.3. Stock and Standard Solutions
2.4.4. Extraction Protocol
2.5. High-Performance Liquid Chromatography Method
2.5.1. HPLC–DAD Instrumentation and Analysis Conditions
2.5.2. Stock and Standard Solutions
2.5.3. Extraction Protocol
2.6. Method Validation
3. Results
3.1. Gas Chromatography–Mass Spectrometry
3.1.1. Optimization of the Method
3.1.2. Validation of the Method
3.2. High Performance Liquid Chromatography–UV Detection
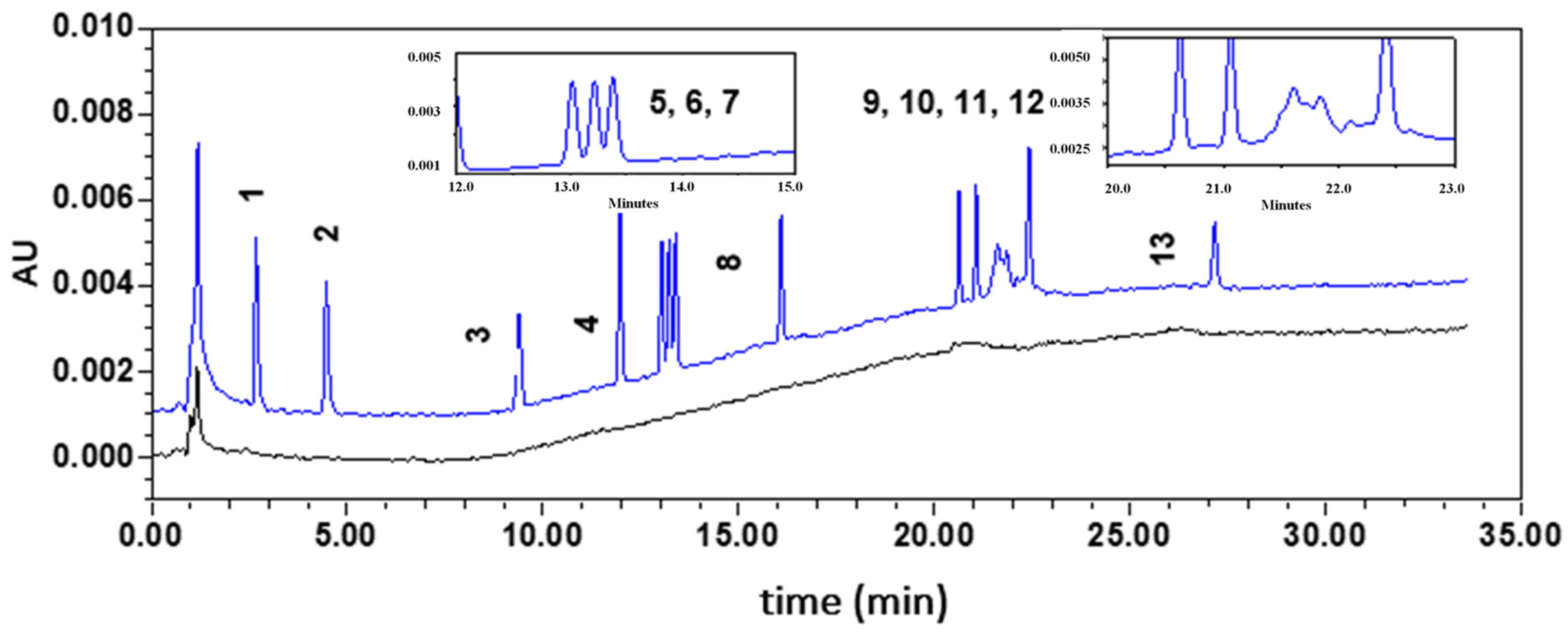
3.2.1. Optimization of the Method
Optimization of the Elution Gradient
Setting-Up a Trap Column
3.2.2. Validation of the Method
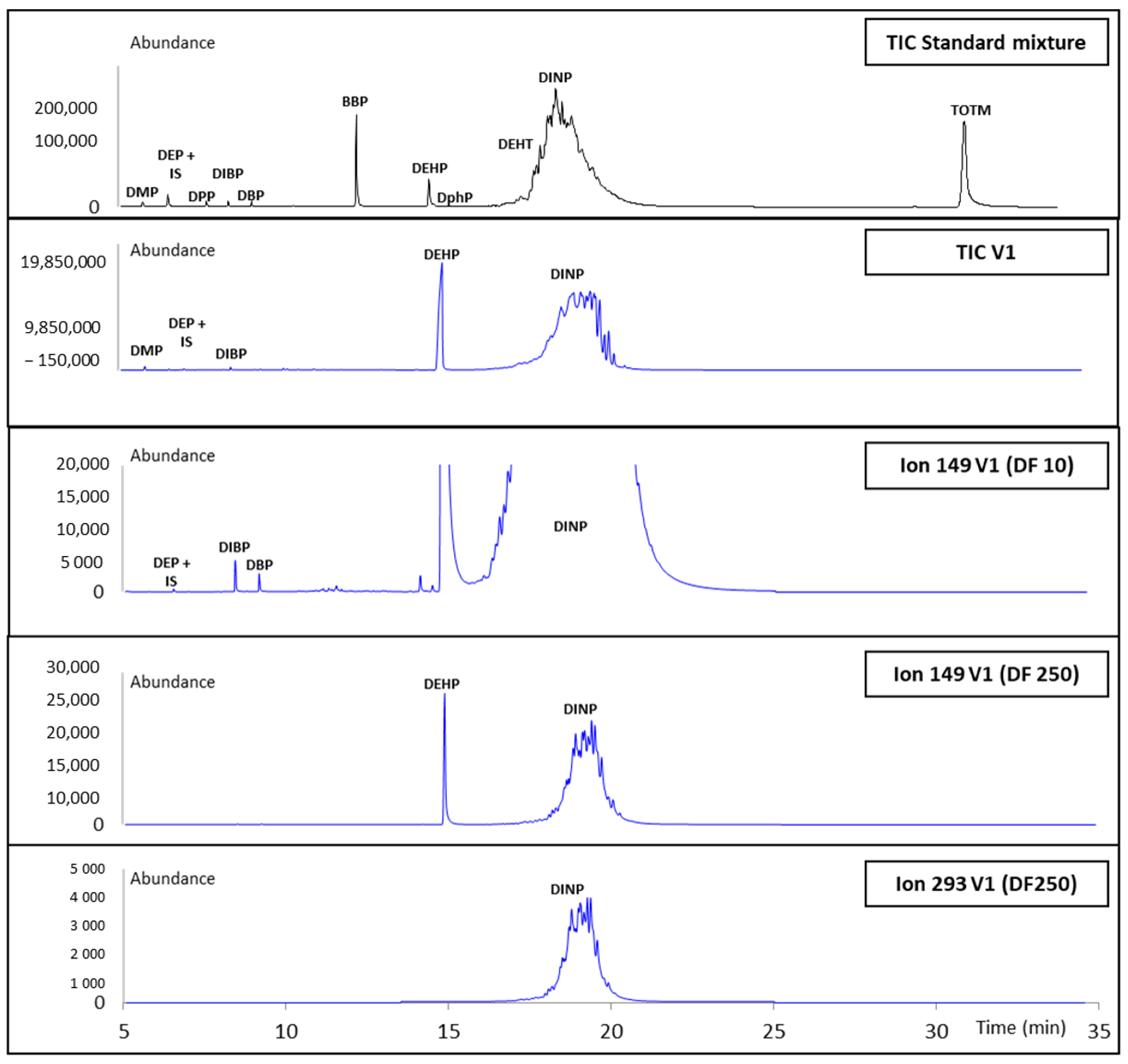
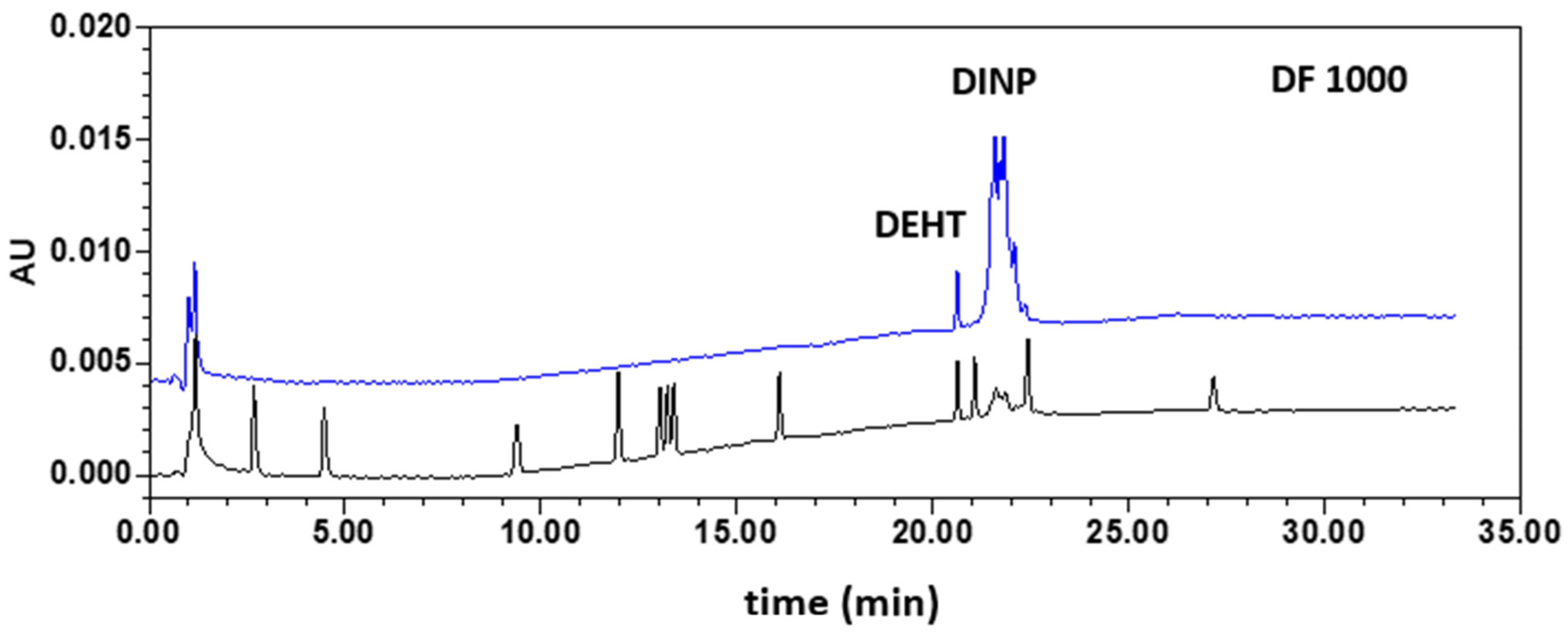
3.3. Application to Gloves Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Employer Payment for Personal Protective Equipment; Final Rule; Occupational Safety and Health Administration: Washington, DC, USA, 2007. Available online: https://www.osha.gov/laws-regs/federalregister/2007-11-15-0 (accessed on 10 April 2020).
- Ito, Y.; Yamanoshita, O.; Asaeda, N.; Tagawa, U.; Lee, C.L.; Aoyama, T.; Ichihara, G.; Furuhashi, K.; Kamijima, M.; Gonzalez, F.J.; et al. Di(2-ethylhexyl)phthalate induces hepatic tumorigenesis through a peroxisome proliferator-activated receptor alpha-independent pathway. J. Occup. Health 2007, 49, 172–182. [Google Scholar] [CrossRef]
- Johnson, K.J.; Heger, N.E.; Boekelheide, K. Of Mice and Men (and Rats): Phthalate-Induced Fetal Testis Endocrine Disruption Is Species-Dependent. Toxicol. Sci. 2012, 129, 235–248. [Google Scholar] [CrossRef]
- Mariana, M.; Feiteiro, J.; Verde, I.; Cairrao, E. The effects of phthalates in the cardiovascular and reproductive systems: A review. Environ. Int. 2016, 94, 758–776. [Google Scholar] [CrossRef]
- Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006 (Text with EEA relevance). 2008. Available online: http://data.europa.eu/eli/reg/2008/1272/oj/eng (accessed on 7 April 2020).
- Malarvannan, G.; Onghena, M.; Verstraete, S.; van Puffelen, E.; Jacobs, A.; Vanhorebeek, I.; Verbruggen, S.C.; Joosten, K.F.; Berghe, G.V.D.; Jorens, P.G.; et al. Phthalate and alternative plasticizers in indwelling medical devices in pediatric intensive care units. J. Hazard. Mater. 2018, 363, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, P.; Thomas, S.; Bousquet, C.; Maggio, A.-F.; Civade, C.; Brenier, C.; Bonnet, P.-A. Identification and quantification of 14 phthalates and 5 non-phthalate plasticizers in PVC medical devices by GC–MS. J. Chromatogr. B 2014, 949–950, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Bernard, L.; Bourdeaux, D.; Pereira, B.; Azaroual, N.; Barthelemy, C.; Breysse, C.; Chennell, P.; Cueff, R.; Dine, T.; Eljezi, T.; et al. Analysis of plasticizers in PVC medical devices: Performance comparison of eight analytical methods. Talanta 2017, 162, 604–611. [Google Scholar] [CrossRef]
- Chao, K.-P.; Huang, C.-S.; Wei, C.-Y. Health risk assessments of DEHP released from chemical protective gloves. J. Hazard. Mater. 2015, 283, 53–59. [Google Scholar] [CrossRef]
- Petersen, J.H.; Jensen, L.K. Phthalates in soft PVC products used in food production equipment and in other food contact materials on the Danish and the Nordic Market 2013-2014. Int. J. Food Contam. 2016, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Chao, K.-P.; Huang, C.-S.; Wei, C.-Y. Extraction and percolation of PAEs from chemical protective gloves. Polym. Test. 2013, 32, 1551–1557. [Google Scholar] [CrossRef]
- Tsumura, Y.; Ishimitsu, S.; Kaihara, A.; Ii, K.Y.; Nakamura, Y.; Tonogai, Y. Di(2-ethylhexyl) phthalate contamination of retail packed lunches caused by PVC gloves used in the preparation of foods. Food Addit. Contam. 2001, 18, 569–579. [Google Scholar] [CrossRef]
- Haned, Z.; Moulay, S.; Lacorte, S. Migration of plasticizers from poly(vinyl chloride) and multilayer infusion bags using selective extraction and GC–MS. J. Pharm. Biomed. Anal. 2018, 156, 80–87. [Google Scholar] [CrossRef]
- Tsai, M.-Y.; Ho, C.-H.; Chang, H.-Y.; Yang, W.-C.; Lin, C.-F.; Lin, C.-T.; Xue, Y.-J.; Lai, J.-M.; Wang, J.-H.; Chang, G.-R. Analysis of Pollution of Phthalates in Pork and Chicken in Taiwan Using Liquid Chromatography–Tandem Mass Spectrometry and Assessment of Health Risk. Molecules 2019, 24, 3817. [Google Scholar] [CrossRef] [Green Version]
- Fromme, H.; Schütze, A.; Lahrz, T.; Kraft, M.; Fembacher, L.; Siewering, S.; Burkardt, R.; Dietrich, S.; Koch, H.; Völkel, W. Non-phthalate plasticizers in German daycare centers and human biomonitoring of DINCH metabolites in children attending the centers (LUPE 3). Int. J. Hyg. Environ. Health 2015, 219, 33–39. [Google Scholar] [CrossRef]
- Larsson, K.; Lindh, C.; Jönsson, B.A.; Giovanoulis, G.; Bibi, M.; Bottai, M.; Bergström, A.; Berglund, M. Phthalates, non-phthalate plasticizers and bisphenols in Swedish preschool dust in relation to children’s exposure. Environ. Int. 2017, 102, 114–124. [Google Scholar] [CrossRef]
- Bourdeaux, D.; Yessaad, M.; Chennell, P.; Larbre, V.; Eljezi, T.; Bernard, L.; Sautou, V. Analysis of PVC plasticizers in medical devices and infused solutions by GC–MS. J. Pharm. Biomed. Anal. 2016, 118, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Wormuth, M.; Scheringer, M.; Vollenweider, M.; Hungerbuhler, K. What Are the Sources of Exposure to Eight Frequently Used Phthalic Acid Esters in Europeans? Risk Anal. 2006, 26, 803–824. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kannan, K. Challenges encountered in the analysis of phthalate esters in foodstuffs and other biological matrices. Anal. Bioanal. Chem. 2012, 404, 2539–2554. [Google Scholar] [CrossRef]
- European Chemicals Bureau. European Union Risk Assessment Report Bis(2-ethylhexyl)phthalate (DEHP). 80, 2nd Priority List. 2008. Available online: http://op.europa.eu/fr/publication-detail/-/publication/80eaeafa-5985-4481-9b837b5d39241d52 (accessed on 10 January 2021).
- Kemper, B.; Lichtblau, D.A. Extraction of plasticizers: An entire and reproducible quantification method for historical cellulose acetate material. Polym. Test. 2019, 80, 106096. [Google Scholar] [CrossRef]
- Shen, H.-Y. Simultaneous screening and determination eight phthalates in plastic products for food use by sonication-assisted extraction/GC–MS methods. Talanta 2005, 66, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Hubert, P.; Nguyen-Huu, J.-J.; Boulanger, B.; Chapuzet, E.; Cohen, N.; Compagnon, P.-A.; Dewé, W.; Feinberg, M.; Laurentie, M.; Mercier, N.; et al. Harmonization of strategies for the validation of quantitative analytical procedures: A SFSTP proposal–Part III. J. Pharm. Biomed. Anal. 2007, 45, 82–96. [Google Scholar] [CrossRef]
- Descat, A.; Lecoeur, M.; Kouach, M.; Goossens, L.; Thelliez, A.; Odou, P.; Decaudin, B.; Goossens, J.-F. Simultaneous determination of di(2-ethylhexyl) phthalate and diisononylcyclohexane-1,2-dicarboxylate and their monoester metabolites in four labile blood products by liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 2019, 181, 113063. [Google Scholar] [CrossRef] [PubMed]
- Bernard, L.; Décaudin, B.; Lecoeur, M.; Richard, D.; Bourdeaux, D.; Cueff, R.; Sautou, V. Analytical methods for the determination of DEHP plasticizer alternatives present in medical devices: A review. Talanta 2014, 129, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Bernard, L.; Cueff, R.; Breysse, C.; Décaudin, B.; Sautou, V. Migrability of PVC plasticizers from medical devices into a simulant of infused solutions. Int. J. Pharm. 2015, 485, 341–347. [Google Scholar] [CrossRef] [PubMed]

| Compound | Acronym | Molecular Formula | Molecular Mass | CAS Number |
|---|---|---|---|---|
| (g/mol) | ||||
| Phthalates | ||||
| Benzyl butyl phthalate | BBP | C19H20O4 | 312.36 | 85-68-7 |
| Dibutyl phthalate | DBP | C16H22O4 | 278.34 | 84-74-2 |
| Dicyclohexyl phthalate | DCHP | C20H26O4 | 330.42 | 84-61-7 |
| Bis-(2-ethylhexyl) phthalate | DEHP | C24H38O4 | 390.56 | 117-81-7 |
| Diethyl phthalate | DEP | C12H14O4 | 222.24 | 84-66-2 |
| Diisobutyl phthalate | DIBP | C16H22O4 | 278.34 | 84-69-5 |
| Diisononyl phthalate | DINP | C26H42O4 | 418.61 | 28553-12-0 |
| Dimethyl phthalate | DMP | C10H10O4 | 194.18 | 131-11-3 |
| Di-n-octyl phthalate | DNOP | C24H38O4 | 390.56 | 117-84-0 |
| Diphenyl phthalate | DphP | C20H14O4 | 318.32 | 84-62-8 |
| Dipropyl phthalate | DPP | C14H18O4 | 250.29 | 131-16-8 |
| Non-phthalates | ||||
| Di-(2-ethylhexyl) terephthalate | DEHT | C24H38O4 | 390.56 | 6422-86-2 |
| Tri-octyl trimellitate | TOTM | C33H54O6 | 546.78 | 3319-31-1 |
| Group | Analytes | RT (min) | Quantification Ion (m/z) | Qualification Ion (m/z) |
|---|---|---|---|---|
| 1st group from 0 to 13 min | DMP | 5.8 | 163.0 | 77.0 |
| DEP | 6.6 | 149.0 | 177.0 | |
| DEP-d4 | 6.6 | 153.0 | 181.0 | |
| DPP | 7.8 | 149.0 | 191.1 | |
| DIBP | 8.5 | 149.0 | 57.1 | |
| DBP | 9.2 | 149.0 | 223.1 | |
| BBP | 12.5 | 149.0 | 91.1 | |
| 2nd group from 13 to 20 min | DCHP | 14.6 | 249.0 | 149.0 |
| DEHP | 14.8 | 149.0 | 279.0 | |
| DphP | 15.0 | 225.0 | 77.1 | |
| DNOP | 18.2 | 149.0 | 279.1 | |
| DEHT | 18.3 | 112.1 | 261.1 | |
| DINP | 18.8 | 293.1 | 149.0 | |
| 3rd group from 20 to 35 min | TOTM | 31.7 | 305.1 | 193.0 |
| GC–MS | |||||
| Analytes | Response Function | Linearity | LLOQ (mg L−1) | ||
| Model | Equation | Equation | R2 | ||
| DMP | 1/x weighted linear | 12.90x + 1.172 × 10−2 | 0.9422x + 2.761 × 10−3 | 0.994 | 0.040 |
| DEP | 1/x weighted linear | 6.456x − 3.294 × 10−2 | 1.001x + 3.113 × 10−4 | 0.997 | 0.020 |
| DPP | 1/x weighted linear | 9.337x + 8.978 × 10−3 | 1.007x + 2.870 × 10−4 | 0.991 | 0.040 |
| DphP | quadratic | 1.315x2 + 1.377x − 4.667 × 10−3 | 1.004x + 9.521 × 10−4 | 0.98 | 0.060 |
| BBP | quadratic | 0.4008x2 + 6.262 × 10−1x + 2.761 × 10−1 | 1.018x + 8.234 × 10−2 | 0.994 | 2.4 |
| DBP | 1/x weighted linear | 8.154x + 2.770 × 10−2 | 1.022x + 7.642 × 10−4 | 0.99 | 0.020 |
| DCHP | - | - | - | - | - |
| DEHP | 1/x weighted linear | 1.950x − 2.870 × 10−1 | 1.056x − 7.081 × 10−2 | 0.98 | 0.40 |
| DNOP | - | - | - | - | - |
| DEHT | 1/x weighted linear | 0.3805x − 5.397 × 10−2 | 0.993x + 1.280 × 10−2 | 0.98 | 0.40 |
| DINP | quadratic | 0.002715x2 + 1.201 × 10−1x + 2.067 × 10−1 | 1.005x − 1.200 | 0.993 | 40 |
| TOTM | linear | 0.09951x − 1.763 | 1.050x − 1.173 | 0.98 | 25 |
| DIBP | 1/x weighted linear | 7.609x + 1.475 × 10−2 | 0.9671x + 8.764 × 10−4 | 0.97 | 0.050 |
| HPLC–DAD | |||||
| Analytes | Response Function | Linearity | LLOQ (mg L−1) | ||
| Model | Equation | Equation | R2 | ||
| DMP | linear | 55,234x − 344 | 1.007x − 5.54 × 10−4 | 0.999 | 0.050 |
| DEP | 1/x weighted linear | 50,225x − 253 | 1.004x + 3.60 × 10−3 | 0.998 | 0.050 |
| DPP | 1/x weighted linear | 44,732x − 243 | 0.9946x + 4.90 × 10−3 | 0.999 | 0.050 |
| DphP | linear | 51,953x + 7 | 0.9848x + 5.77 × 10−3 | 0.999 | 0.050 |
| BBP | linear | 39,713x + 78 | 0.9832x + 4.00 × 10−3 | 0.998 | 0.050 |
| DBP | linear | 40,661x + 274 | 0.9804x + 5.62 × 10−3 | 0.998 | 0.050 |
| DCHP | linear | 34,991x + 2.5 | 0.9922x + 2.57 × 10−3 | 0.998 | 0.050 |
| DEHP | linear | 28,433x − 161 | 0.9877x + 5.30 × 10−2 | 0.996 | 0.050 |
| DNOP | linear | 28,041x + 94 | 1.010x + 1.60 × 10−3 | 0.998 | 0.050 |
| DEHT | linear | 65,922x + 405 | 1.011x + 9.90 × 10−4 | 0.997 | 0.050 |
| DINP | 1/x weighted linear | 16,323x + 1020 | 0.9853x + 5.98 × 10−3 | 0.9994 | 0.50 |
| TOTM | linear | 27,046x − 141 | 1.024x − 5.45 × 10−3 | 0.998 | 0.050 |
| DIBP | linear | 44,105x − 1248 | 1.020x − 7.49 × 10−4 | 0.9997 | 0.050 |
| % Plasticizers (w/w) | ||||||
|---|---|---|---|---|---|---|
| Analytes | Method | V1 | V2 | V3 | V4 | V5 |
| DMP | GC–MS | (4.83 ± 0.33) × 10−4 | (4.67 ± 0.27) × 10−4 | ND | ND | ND |
| HPLC–DAD | ND | ND | ND | ND | ND | |
| DEP | GC–MS | (5.83 ± 0.67) × 10−4 | (3.00 ± 0.40) × 10−4 | ND | ND | ND |
| HPLC–DAD | ND | ND | ND | ND | ND | |
| DIBP | GC–MS | (9.68 ± 0.79) × 10−3 | (4.80 ± 0.10) × 10−3 | (8.00 ± 0.34) × 10−4 | (1.00 ± 0.34) × 10−3 | (5.20 ± 0.87) × 10−4 |
| HPLC–DAD | ND | ND | ND | ND | ND | |
| DBP | GC–MS | (4.97 ± 0.40) × 10−4 | (2.00 ± 0.07) × 10−3 | (3.33 ± 0.30) × 10−4 | (1.67 ± 0.28) × 10−3 | (1.87 ± 0.30) × 10−3 |
| HPLC–DAD | ND | ND | ND | ND | ND | |
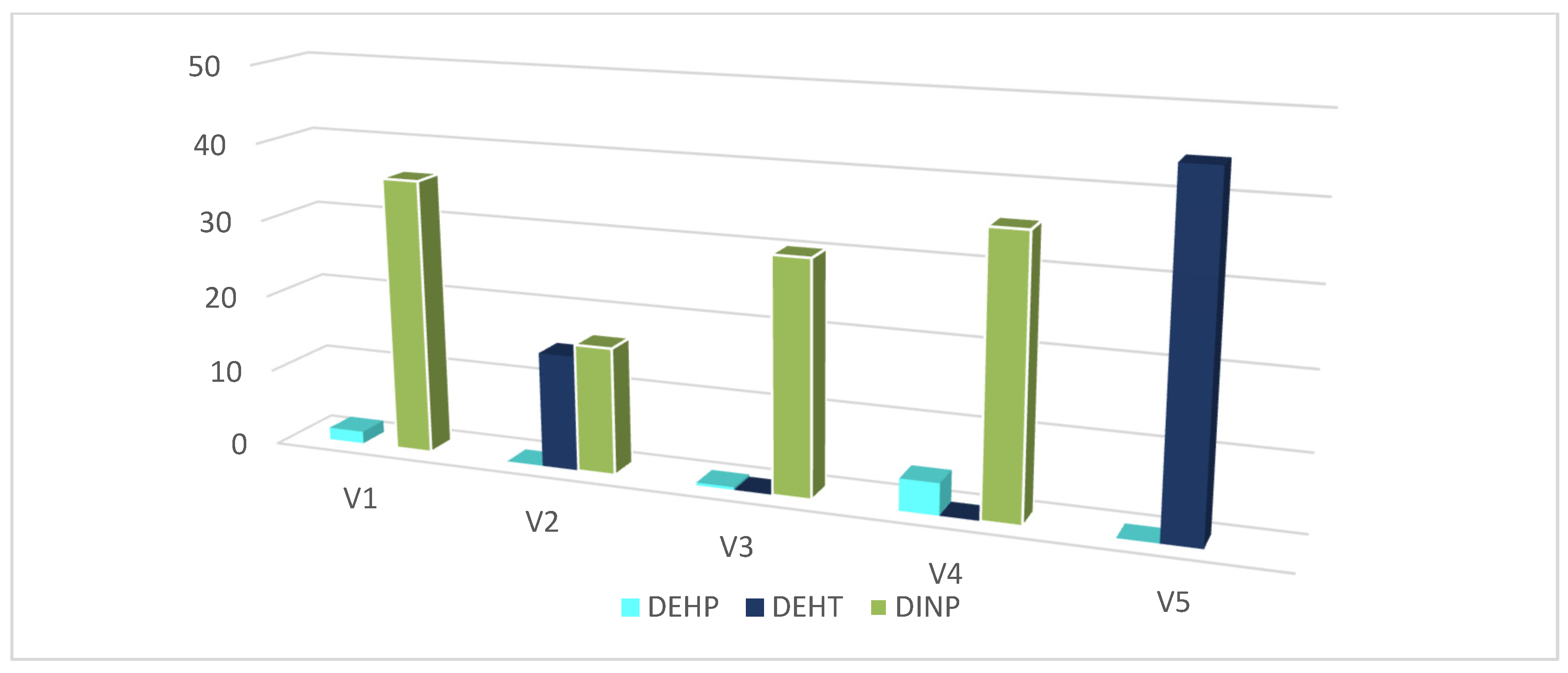
| DEHP | GC–MS | 1.58 ± 0.09 | (8.73 ± 0.03) × 10−3 | (3.39 ± 0.02) × 10−1 | 4.10 ± 0.41 | (6.20 ± 0.66) × 10−4 |
| HPLC–DAD | 1.92 ± 0.25 | ND | 0.297 ± 0.034 | 3.65 ± 0.64 | ND | |
| DEHT | GC–MS | ND | 14.9 ± 1.3 | (4.77 ± 0.13) × 10−2 | (3.87 ± 0.72) × 10−2 | 44.4 ± 2.4 |
| HPLC–DAD | ND | 15.6 ± 1.6 | ND | ND | 36.3 ± 3.7 | |
| DINP | GC–MS | 35.8 ± 4.5 | 16.5 ± 0.8 | 30.2 ± 3.1 | 35.7 ± 0.5 | ND |
| HPLC–DAD | 40.1 ± 1.2 | 16.4 ± 1.3 | 32.9 ± 1.0 | 35.3 ± 0.9 | ND | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poitou, K.; Rogez-Florent, T.; Lecoeur, M.; Danel, C.; Regnault, R.; Vérité, P.; Monteil, C.; Foulon, C. Analysis of Phthalates and Alternative Plasticizers in Gloves by Gas Chromatography–Mass Spectrometry and Liquid Chromatography–UV Detection: A Comparative Study. Toxics 2021, 9, 200. https://doi.org/10.3390/toxics9090200
Poitou K, Rogez-Florent T, Lecoeur M, Danel C, Regnault R, Vérité P, Monteil C, Foulon C. Analysis of Phthalates and Alternative Plasticizers in Gloves by Gas Chromatography–Mass Spectrometry and Liquid Chromatography–UV Detection: A Comparative Study. Toxics. 2021; 9(9):200. https://doi.org/10.3390/toxics9090200
Chicago/Turabian StylePoitou, Kelly, Tiphaine Rogez-Florent, Marie Lecoeur, Cécile Danel, Romain Regnault, Philippe Vérité, Christelle Monteil, and Catherine Foulon. 2021. "Analysis of Phthalates and Alternative Plasticizers in Gloves by Gas Chromatography–Mass Spectrometry and Liquid Chromatography–UV Detection: A Comparative Study" Toxics 9, no. 9: 200. https://doi.org/10.3390/toxics9090200
APA StylePoitou, K., Rogez-Florent, T., Lecoeur, M., Danel, C., Regnault, R., Vérité, P., Monteil, C., & Foulon, C. (2021). Analysis of Phthalates and Alternative Plasticizers in Gloves by Gas Chromatography–Mass Spectrometry and Liquid Chromatography–UV Detection: A Comparative Study. Toxics, 9(9), 200. https://doi.org/10.3390/toxics9090200






