Design of Bio-Absorbent Systems for the Removal of Hydrocarbons from Industrial Wastewater: Pilot-Plant Scale
Abstract
:1. Introduction
2. Materials and Methods
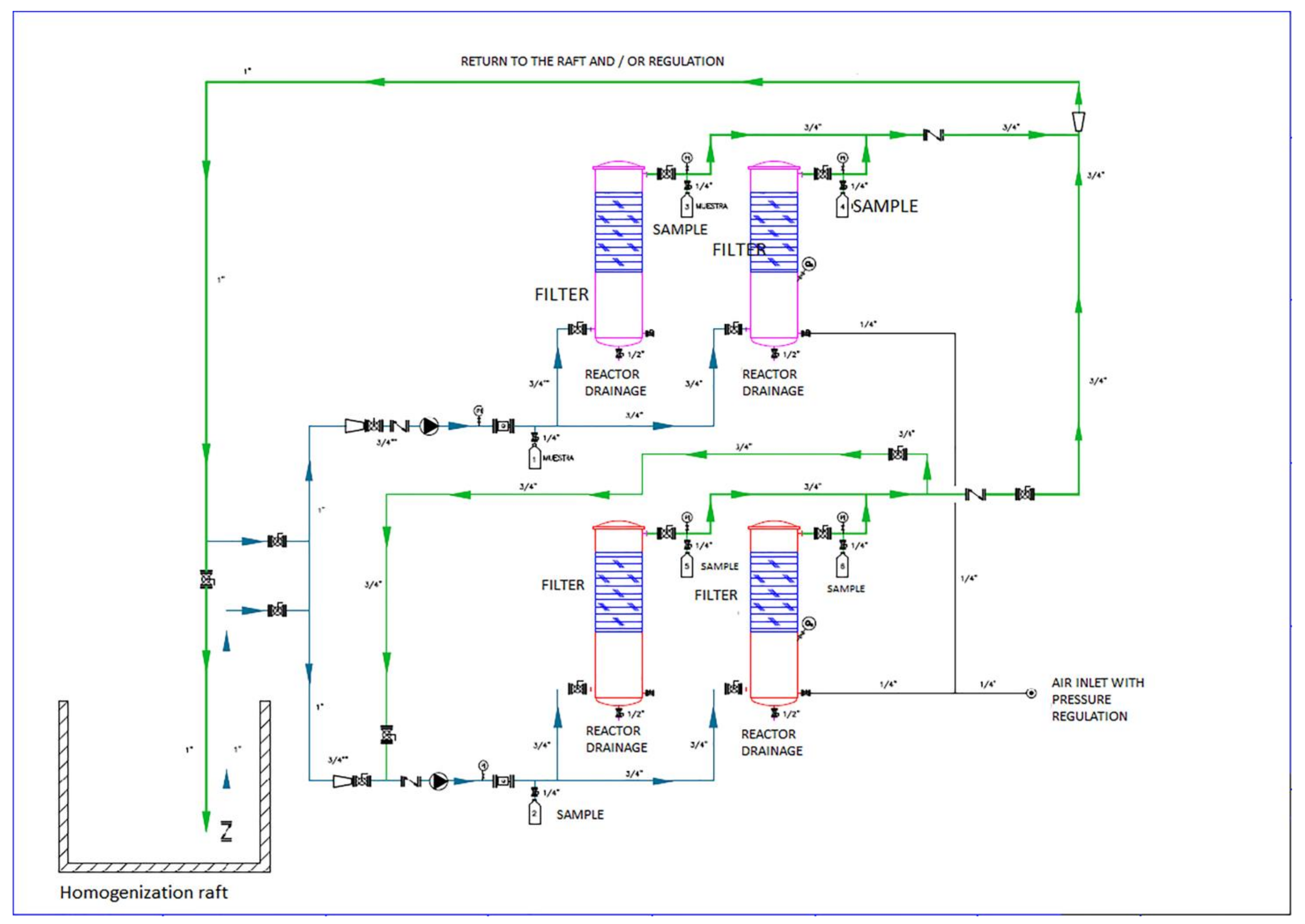
2.1. Design and Construction of the Pilot Plant
2.2. Hydrocarbon Analyses
2.3. Scanning Electron Microscopy (SEM)
2.4. Microbial Diversity
2.5. Processing of Sequencing Data
2.6. Statistical Analyses
3. Results and Discussion
3.1. Bioreactor Experiments
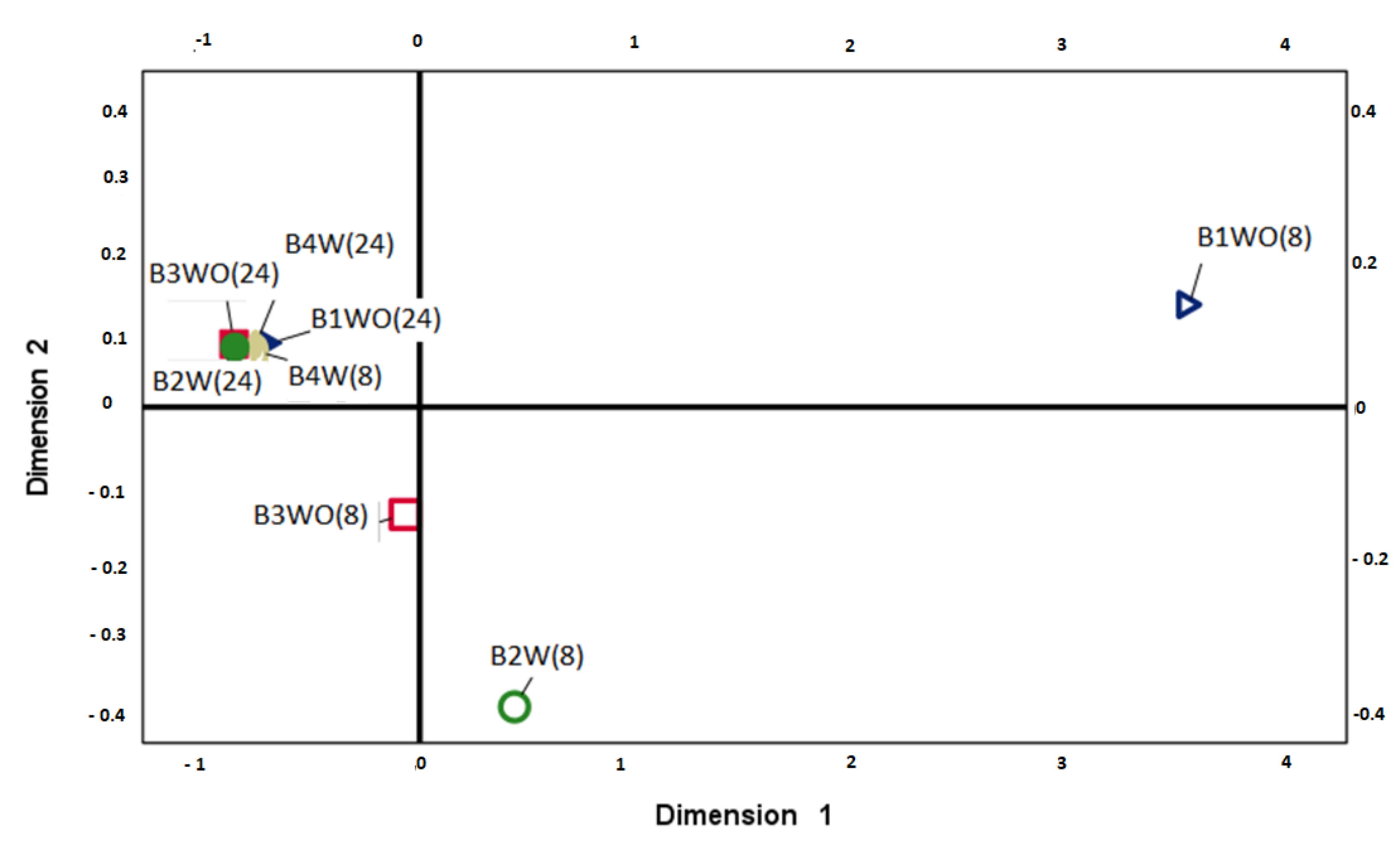
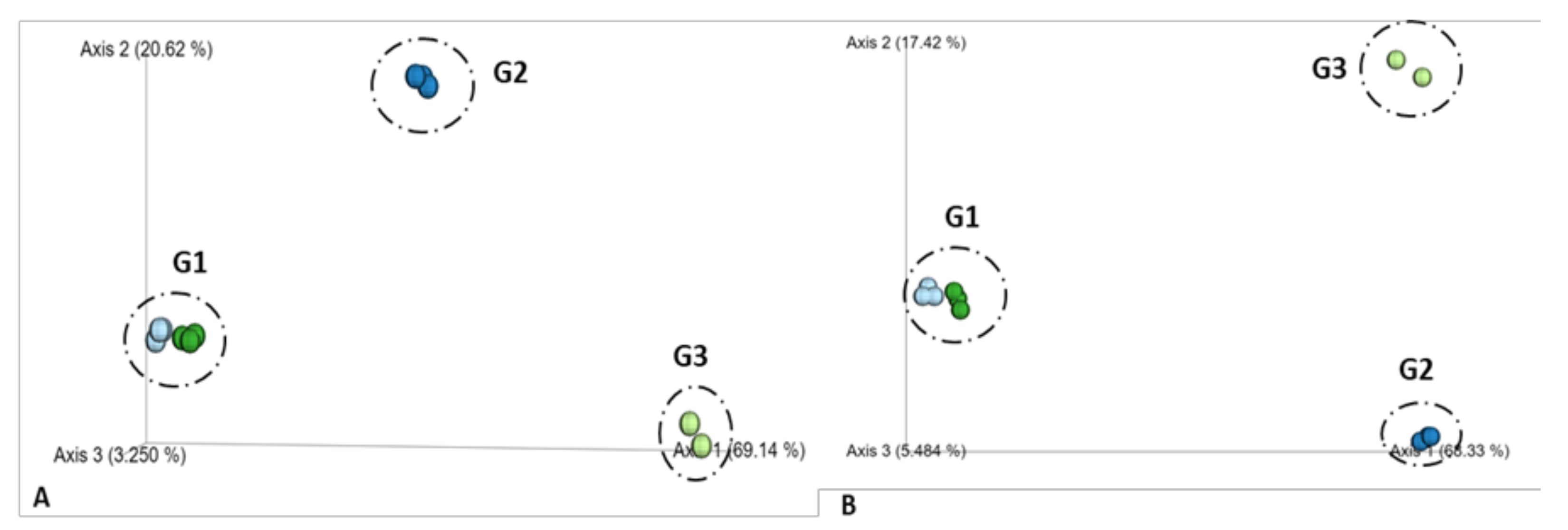
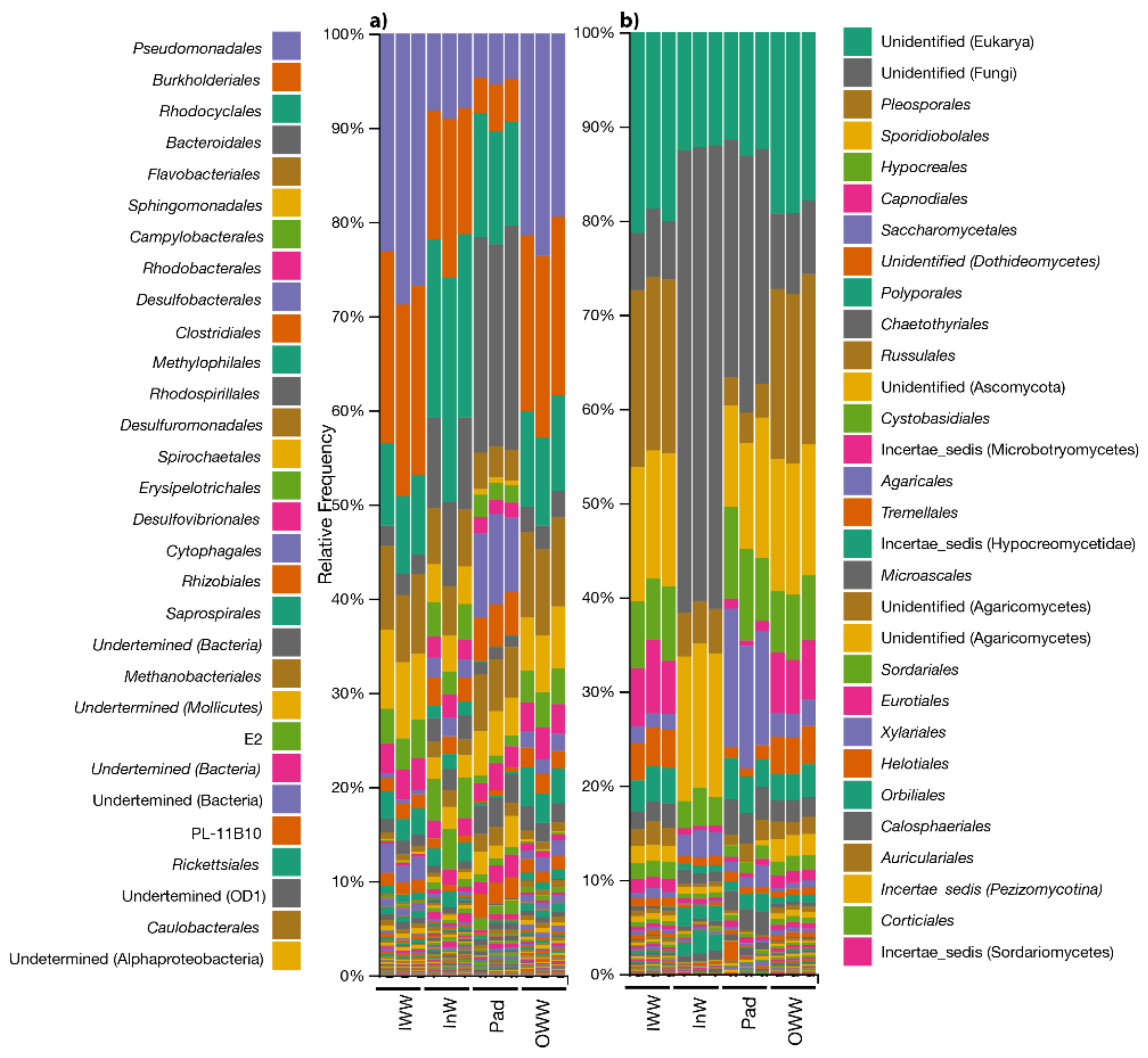
3.2. Microbial Diversity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rengarajan, T.; Rajendran, P.; Nandakumar, N.; Lokeshkumar, B.; Rajendran, P.; Nishigaki, I. Exposure to Polycyclic Aromatic Hydrocarbons with Special Focus on Cancer. Asian Pac. J. Trop. Biomed. 2015, 5, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Mangwani, N.; Kumari, S.; Das, S. Marine Bacterial Biofilms in Bioremediation of Polycyclic Aromatic Hydrocarbons (PAHs) Under Terrestrial Condition in a Soil Microcosm. Pedosphere 2017, 27, 548–558. [Google Scholar] [CrossRef]
- Tang, J.; Wang, M.; Wang, F.; Sun, Q.; Zhou, Q. Eco-Toxicity of Petroleum Hydrocarbon Contaminated Soil. J. Environ. Sci. 2011, 23, 845–851. [Google Scholar] [CrossRef]
- Bacosa, H.P.; Thyng, K.M.; Plunkett, S.; Erdner, D.L.; Liu, Z. The Tarballs on Texas Beaches Following the 2014 Texas City “Y” Spill: Modeling, Chemical, and Microbiological Studies. Mar. Pollut. Bull. 2016, 109, 236–244. [Google Scholar] [CrossRef]
- Uad, I.; Silva-Castro, G.A.; Pozo, C.; González-López, J.; Calvo, C. Biodegradative Potential and Characterization of Bioemulsifiers of Marine Bacteria Isolated from Samples of Seawater, Sediment and Fuel Extracted at 4000 m of Depth (Prestige Wreck). Int. Biodeterior. Biodegrad. 2010, 64, 511–518. [Google Scholar] [CrossRef]
- Yin, F.; John, G.F.; Hayworth, J.S.; Clement, T.P. Long-Term Monitoring Data to Describe the Fate of Polycyclic Aromatic Hydrocarbons in Deepwater Horizon Oil Submerged off Alabama’s Beaches. Sci. Total. Environ. 2015, 508, 46–56. [Google Scholar] [CrossRef]
- Gargouri, B.; Karray, F.; Mhiri, N.; Aloui, F.; Sayadi, S. Application of a Continuously Stirred Tank Bioreactor (CSTR) for Bioremediation of Hydrocarbon-Rich Industrial Wastewater Effluents. J. Hazard. Mater. 2011, 189, 427–434. [Google Scholar] [CrossRef]
- Rodríguez-Calvo, A.; Silva-Castro, G.A.; Robledo-Mahón, T.; González-López, J.; Calvo, C. Capacity of Hydrophobic Carriers to Form Biofilm for Removing Hydrocarbons from Polluted Industrial Wastewater: Assay in Microcosms. Water Air Soil Pollut. 2018, 229, 175. [Google Scholar] [CrossRef]
- Dellagnezze, B.M.; de Sousa, G.V.; Martins, L.L.; Domingos, D.F.; Limache, E.E.G.; de Vasconcellos, S.P.; da Cruz, G.F.; de Oliveira, V.M. Bioremediation Potential of Microorganisms Derived from Petroleum Reservoirs. Mar. Pollut. Bull. 2014, 89, 191–200. [Google Scholar] [CrossRef]
- Silva-Castro, G.A.; Rodriguez-Calvo, A.; Laguna, J.; González-López, J.; Calvo, C. Autochthonous Microbial Responses and Hydrocarbons Degradation in Polluted Soil during Biostimulating Treatments under Different Soil Moisture. Assay in Pilot Plant. Int. Biodeterior. Biodegrad. 2016, 108, 91–98. [Google Scholar] [CrossRef]
- Gieg, L.M.; Fowler, S.J.; Berdugo-Clavijo, C. Syntrophic Biodegradation of Hydrocarbon Contaminants. Curr. Opin. Biotechnol. 2014, 27, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Mukhopadhyay, S.; Mitra, A.; Mukhopadhyay, S. Biofilm Mediated Decontamination of Pollutants from the Environment. AIMS Bioeng. 2016, 3, 44–59. [Google Scholar] [CrossRef]
- Sutherland, I.W. The Biofilm Matrix—An Immobilized but Dynamic Microbial Environment. Trends Microbiol. 2001, 9, 222–227. [Google Scholar] [CrossRef]
- Leyva-Díaz, J.C.; González-Martínez, A.; González-López, J.; Muñío, M.M.; Poyatos, J.M. Kinetic Modeling and Microbiological Study of Two-Step Nitrification in a Membrane Bioreactor and Hybrid Moving Bed Biofilm Reactor–Membrane Bioreactor for Wastewater Treatment. Chem. Eng. J. 2015, 259, 692–702. [Google Scholar] [CrossRef]
- El-Borai, A.M.; Eltayeb, K.M.; Mostafa, A.R.; El-Assar, S.A. Biodegradation of Industrial Oil-Polluted Wastewater in Egypt by Bacterial Consortium Immobilized in Different Types of Carriers. Pol. J. Environ. Stud. 2016, 25, 1901–1909. [Google Scholar] [CrossRef]
- Jou, C.-J.G.; Huang, G.-C. A Pilot Study for Oil Refinery Wastewater Treatment Using a Fixed-Film Bioreactor. Adv. Environ. Res. 2003, 7, 463–469. [Google Scholar] [CrossRef]
- Rodríguez-Calvo, A.; Silva-Castro, G.A.; Olicón-Hernández, D.R.; González-López, J.; Calvo, C. Biodegradation and Absorption Technology for Hydrocarbon-Polluted Water Treatment. Appl. Sci. 2020, 10, 841. [Google Scholar] [CrossRef] [Green Version]
- Robledo-Mahón; Gómez-Silván, C.; Andersen, G.L.; Calvo, C.; Aranda, E. Assessment of Bacterial and Fungal Communities in a Full-Scale Thermophilic Sewage Sludge Composting Pile under a Semipermeable Cover. Bioresour. Technol. 2020, 298, 122550. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global Patterns of 16S RRNA Diversity at a Depth of Millions of Sequences per Sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-High-Throughput Microbial Community Analysis on the Illumina HiSeq and MiSeq Platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [Green Version]
- Apprill, A.; McNally, S.; Parsons, R.; Weber, L. Minor Revision to V4 Region SSU RRNA 806R Gene Primer Greatly Increases Detection of SAR11 Bacterioplankton. Aquat. Microb. Ecol. 2015, 75, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every Base Matters: Assessing Small Subunit RRNA Primers for Marine Microbiomes with Mock Communities, Time Series and Global Field Samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. 38—Amplification and direct sequencing of fungal ribosomal rna genes for phylogenetics. In PCR Protocols; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. ISBN 978-0-12-372180-8. [Google Scholar]
- Lozupone, C.A.; Knight, R. Session: OOS 31—From Microbial to Conservation Biology: Exploring Phylogenetic Beta Diversity as a Theoretical Tool Uniting Disciplines. Available online: https://eco.confex.com/eco/2010/techprogram/S5605.HTM (accessed on 13 May 2021).
- Vázquez-Baeza, Y.; Pirrung, M.; Gonzalez, A.; Knight, R. EMPeror: A Tool for Visualizing High-Throughput Microbial Community Data. GigaScience 2013, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayat, A.; Aghamiri, S.F.; Moheb, A.; Vakili-Nezhaad, G.R. Oil Spill Cleanup from Sea Water by Sorbent Materials. Chem. Eng. Technol. 2005, 28, 1525–1528. [Google Scholar] [CrossRef]
- Gómez, M.A.; González-López, J.; Hontoria-García, E. Influence of Carbon Source on Nitrate Removal of Contaminated Groundwater in a Denitrifying Submerged Filter. J. Hazard. Mater. 2000, 80, 69–80. [Google Scholar] [CrossRef]
- Bazargan, A.; Hui, C.W.; Mckay, G. Marine Residual Fuel Sorption and Desorption Kinetics by Alkali Treated Rice Husks. Cellulose 2014, 21, 1997–2006. [Google Scholar] [CrossRef]
- Ebadi, A.; Khoshkholgh Sima, N.A.; Olamaee, M.; Hashemi, M.; Ghorbani Nasrabadi, R. Effective Bioremediation of a Petroleum-Polluted Saline Soil by a Surfactant-Producing Pseudomonas Aeruginosa Consortium. J. Adv. Res. 2017, 8, 627–633. [Google Scholar] [CrossRef]
- Patowary, K.; Patowary, R.; Kalita, M.C.; Deka, S. Characterization of Biosurfactant Produced during Degradation of Hydrocarbons Using Crude Oil As Sole Source of Carbon. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neethu, C.S.; Saravanakumar, C.; Purvaja, R.; Robin, R.S.; Ramesh, R. Oil-Spill Triggered Shift in Indigenous Microbial Structure and Functional Dynamics in Different Marine Environmental Matrices. Sci. Rep. 2019, 9, 1354. [Google Scholar] [CrossRef] [Green Version]
- Ławniczak, Ł.; Woźniak-Karczewska, M.; Loibner, A.P.; Heipieper, H.J.; Chrzanowski, Ł. Microbial Degradation of Hydrocarbons-Basic Principles for Bioremediation: A Review. Molecules 2020, 25, 856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruberto, L.; Dias, R.; Lo Balbo, A.; Vazquez, S.C.; Hernandez, E.A.; Mac Cormack, W.P. Influence of Nutrients Addition and Bioaugmentation on the Hydrocarbon Biodegradation of a Chronically Contaminated Antarctic Soil. J. Appl. Microbiol. 2009, 106, 1101–1110. [Google Scholar] [CrossRef]
- Aranda, E. Promising Approaches towards Biotransformation of Polycyclic Aromatic Hydrocarbons with Ascomycota Fungi. Curr. Opin. Biotechnol. 2016, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mawad, A.M.M.; Hesham, A.E.-L.; Khan, S.; Nawab, J. The Role of Fungi and Genes for the Removal of Environmental Contaminants from Water/Wastewater Treatment Plants. In Fungal Biotechnology and Bioengineering; Hesham, A.E.-L., Upadhyay, R.S., Sharma, G.D., Manoharachary, C., Gupta, V.K., Eds.; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2020; pp. 349–370. ISBN 978-3-030-41870-0. [Google Scholar]
- Zhou, H.; Huang, X.; Bu, K.; Wen, F.; Zhang, D.; Zhang, C. Fungal Proliferation and Hydrocarbon Removal during Biostimulation of Oily Sludge with High Total Petroleum Hydrocarbon. Environ. Sci. Pollut. Res. Int. 2019, 26, 33192–33201. [Google Scholar] [CrossRef]
- Grujić, S.; Vasić, S.; Radojević, I.; Čomić, L.; Ostojić, A. Comparison of the Rhodotorula Mucilaginosa Biofilm and Planktonic Culture on Heavy Metal Susceptibility and Removal Potential. Water Air Soil Pollut. 2017, 228, 73. [Google Scholar] [CrossRef]
- Nhi Cong, L.T.; Ngoc Mai, C.T.; Thanh, V.T.; Nga, L.P.; Minh, N.N. Application of a Biofilm Formed by a Mixture of Yeasts Isolated in Vietnam to Degrade Aromatic Hydrocarbon Polluted Wastewater Collected from Petroleum Storage. Water Sci. Technol. 2014, 70, 329–336. [Google Scholar] [CrossRef]


| Hydrocarbon Concentration in mg L−1 | % of Removal | ||||
|---|---|---|---|---|---|
| IWW | OWW 8 Cycles | OWW 24 h | 8 Cycles | 24 h | |
| Bioreactor 1 | |||||
| TPH | 86,530 ± 8565 | 25,933 ± 4397 | 1813 ± 95 | 70.0 | 97.9 |
| c10–c20 | 10,619 ± 6135 | 8596 ± 453 | 673 ± 127 | 19.1 | 93.7 |
| c20–c40 | 4399 ± 768 | 1383 ± 1669 | 241 ± 78 | 68.6 | 94.5 |
| Branched/Cyclic alkanes | 800 ± 504 | 599 ± 120 | 25.1 ± 16.5 | 25.1 | 96.9 |
| Alkenes | ND 1 | ND | ND | ND | ND |
| Aromatics | 468.96 ± 185.42 | 19.5 ± 4.8 | ND | 95.8 | 100 |
| Pregnanes | 6484 ± 2491 | 438.7 ± 146.7 | 29.2 ± 11.5 | 93.2 | 99.6 |
| Bioreactor 2 | |||||
| TPH | 86,530 ± 38,565 | 9196 ± 2338 | 950.0 ± 85.4 | 83.4 | 98.9 |
| c10–c20 | 10,619 ± 6135 | 7.15 ± 1.27 | 374 ± 125 | 99.9 | 96.5 |
| c20–c40 | 4399 ± 768 | 156.1 ± 24.4 | 140.2 ± 26.1 | 96.5 | 96.8 |
| Branched/Cyclic alkanes | 800 ± 504 | 3.6 ± 2.9 | 6.7 ± 8.2 | 99.6 | 99.2 |
| Alkenes | ND | ND | ND | ND | ND |
| Aromatics | 468 ± 185 | 1.22 ± 0.21 | ND | 99.7 | 100 |
| Pregnanes | 6484 ± 2491 | 507 ± 108 | 7.2 ± 5.7 | 92.2 | 99.9 |
| Bioreactor 3 | |||||
| TPH | 68,077 ± 24,599 | 5227 ± 1072 | 920 ± 173 | 92.32 | 98.7 |
| c10–c20 | 9073 ± 1317 | 754 ± 394 | 395.7 ± 21.8 | 91.69 | 95.6 |
| c20–c40 | 1301 ± 404 | 223 ± 87 | 102.2 ± 11.3 | 82.88 | 92.2 |
| Branched/Cyclic alkanes | 792 ± 71 | 65.2 ± 12.4 | 7.0 ± 1.1 | 91.76 | 99.1 |
| Alkenes | 1075 ± 161 | ND | ND | 100 | 100 |
| Aromatics | 158 ± 26 | 9.4 ± 2.5 | 4.1 ± 0.2 | 94.03 | 97.4 |
| Pregnanes | 1675 ± 151 | 370 ± 167 | 14.7 ± 1.9 | 77.92 | 99.1 |
| Bioreactor 4 | |||||
| TPH | 68,077 ± 24,599 | 1607 ± 307 | 1293 ± 349 | 97.64 | 98.1 |
| c10–c20 | 9073 ± 1317 | 545 ± 126 | 570 ± 5 | 93.99 | 93.7 |
| c20–c40 | 1301 ± 404 | 140.4 ± 34.6 | 170 ± 19 | 89.21 | 86.9 |
| Branched/Cyclic alkanes | 792 ± 70 | 11.77 ± 4.37 | 4.19 ± 5.92 | 98.52 | 99.5 |
| Alkenes | 1075 ± 161 | ND | ND | 100 | 100 |
| Aromatics | 158 ± 26 | 4.6 ± 0.1 | 4.7 ± 1.0 | 97.11 | 97.0 |
| Pregnanes | 1675.34 ± 151.30 | 72.9 ± 10.5 | 46 ± 3 | 95.65 | 97.3 |
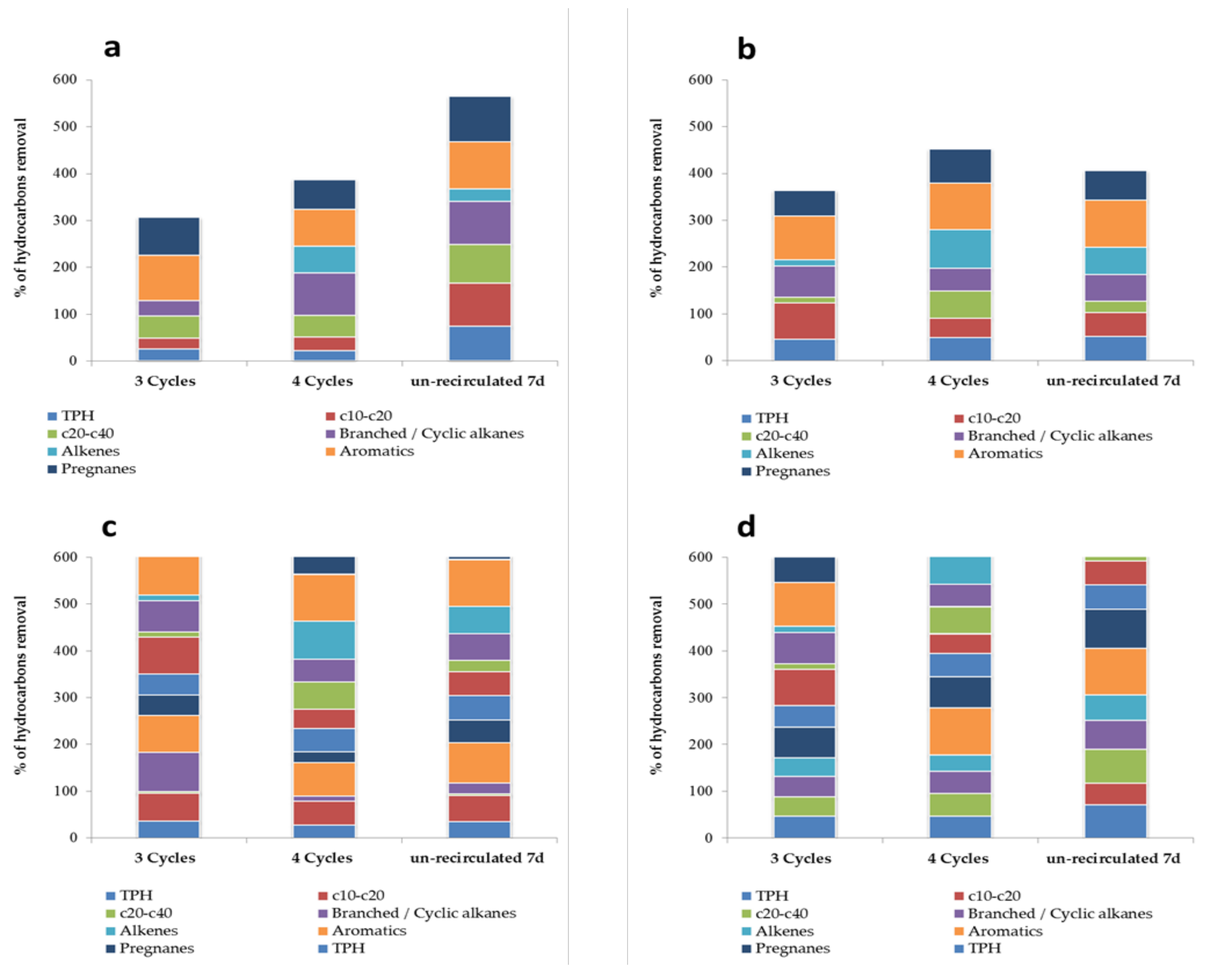
| Hydrocarbon Concentration in mg L−1 | ||||
|---|---|---|---|---|
| IWW | OWW 3 Cycles | OWW 4 Cycles | OWW Unrecirculated 7 d | |
| Trial 1 | ||||
| TPH | 124.0 ± 28.3 | 93.0 ± 18.6 | 97.3 ± 24.1 | 33.0 ± 4.2 |
| c10–c20 | 24.5 ± 3.7 | 19.0 ± 4.7 | 17.5 ± 0.4 | 2.0 ± 0.3 |
| c20–c40 | 27.3 ± 4.1 | 14.4 ± 2.9 | 14.5 ± 8.3 | 4.7 ± 0.01 |
| Branched/Cyclic alkanes | 6.6 ± 0.4 | 4.4 ± 0.9 | 0.6 ± 0.3 | 0.5 ± 2.8 |
| Alkenes | 13.4 ± 0.5 | 13.8 ± 2.7 | 5.8 ± 2.1 | 9.8 ± 3.5 |
| Aromatics | 2.9 ± 0.4 | 0.10 ± 0.02 | 0.6 ± 0.3 | ND |
| Pregnanes | 20.3 ± 3.0 | 3.8 ± 0.7 | 7.5 ± 1.4 | 0.6 ± 0.2 |
| Trial 2 | ||||
| TPH | 135.0 ± 2.8 | 73.3 ± 11.5 | 68.3 ± 10.4 | 65.0 ± 7.0 |
| c10–c20 | 11.3 ± 0.3 | 2.5 ± 1.2 | 6.6 ± 3.6 | 5.6 ± 1.3 |
| c20–c40 | 14.0 ± 3.1 | 12.4 ± 1.0 | 5.9 ± 0.6 | 10.6 ± 2.3 |
| Branched/Cyclic alkanes | 20.1 ± 3.1 | 6.6 ± 2.7 | 10.4 ± 0.7 | 8.5 ± 1.1 |
| Alkenes | 29.3 ± 15.2 | 25.5 ± 10.0 | 5.2 ± 1.7 | 12.2 ± 2.8 |
| Aromatics | 1.7 ± 0.3 | 0.1 ± 0.05 | ND | ND |
| Pregnanes | 6.5 ± 2.8 | 3.0 ± 1.6 | 1.8 ± 0.4 | 2.4 ± 3.8 |
| Trial 3 | ||||
| TPH | 89.5 ± 14.1 | 57.7 ± 10.4 | 65.0 ± 10.0 | 58.8 ± 4.7 |
| c10–c20 | 12.5 ± 3.9 | 5.0 ± 0.4 | 6.2 ± 2.0 | 5.4 ± 1.0 |
| c20–c40 | 6.3 ± 1.2 | 6.1 ± 0.7 | 6.8 ± 3.0 | 6.1 ± 1.0 |
| Branched/Cyclic alkanes | 2.5 ± 0.7 | 0.4 ± 0.2 | 2.3 ± 1.5 | 2.0 ± 1.0 |
| Alkenes | 3.3 ± 1.3 | 5.1 ± 0.5 | 4.1 ± 0.5 | 10.5 ± 3.0 |
| Aromatics | 1.4 ± 0.01 | 0.3 ± 0.2 | 0.4 ± 0.1 | 0.2 ± 0.1 |
| Pregnanes | 19.6 ± 0.7 | 11.2 ± 1.1 | 15.0 ± 2.2 | 10.0 ± 2.2 |
| Trial 4 | ||||
| TPH | 75.0 ± 7.1 | 40.0 ± 10.0 | 40.0 ± 5.0 | 21.67 ± 2.9 |
| c10–c20 | 2.8 ± 0.4 | 3.8 ± 1.4 | 4.1 ± 0.3 | 1.5 ± 0.1 |
| c20–c40 | 6.8 ± 0.2 | 4.0 ± 0.1 | 3.5 ± 0.1 | 1.9 ± 0.1 |
| Branched/Cyclic alkanes | 7.6 ± 0.3 | 4.3 ± 0.1 | 4.0 ± 0.4 | 2.9 ± 0.2 |
| Alkenes | 13.4 ± 0.1 | 8.1 ± 0.1 | 8.5 ± 0.9 | 6.1 ± 0.1 |
| Aromatics | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Pregnanes | 17.2 ± 6.3 | 5.8 ± 3.5 | 5.6 ± 0.9 | 2.8 ± 1.5 |
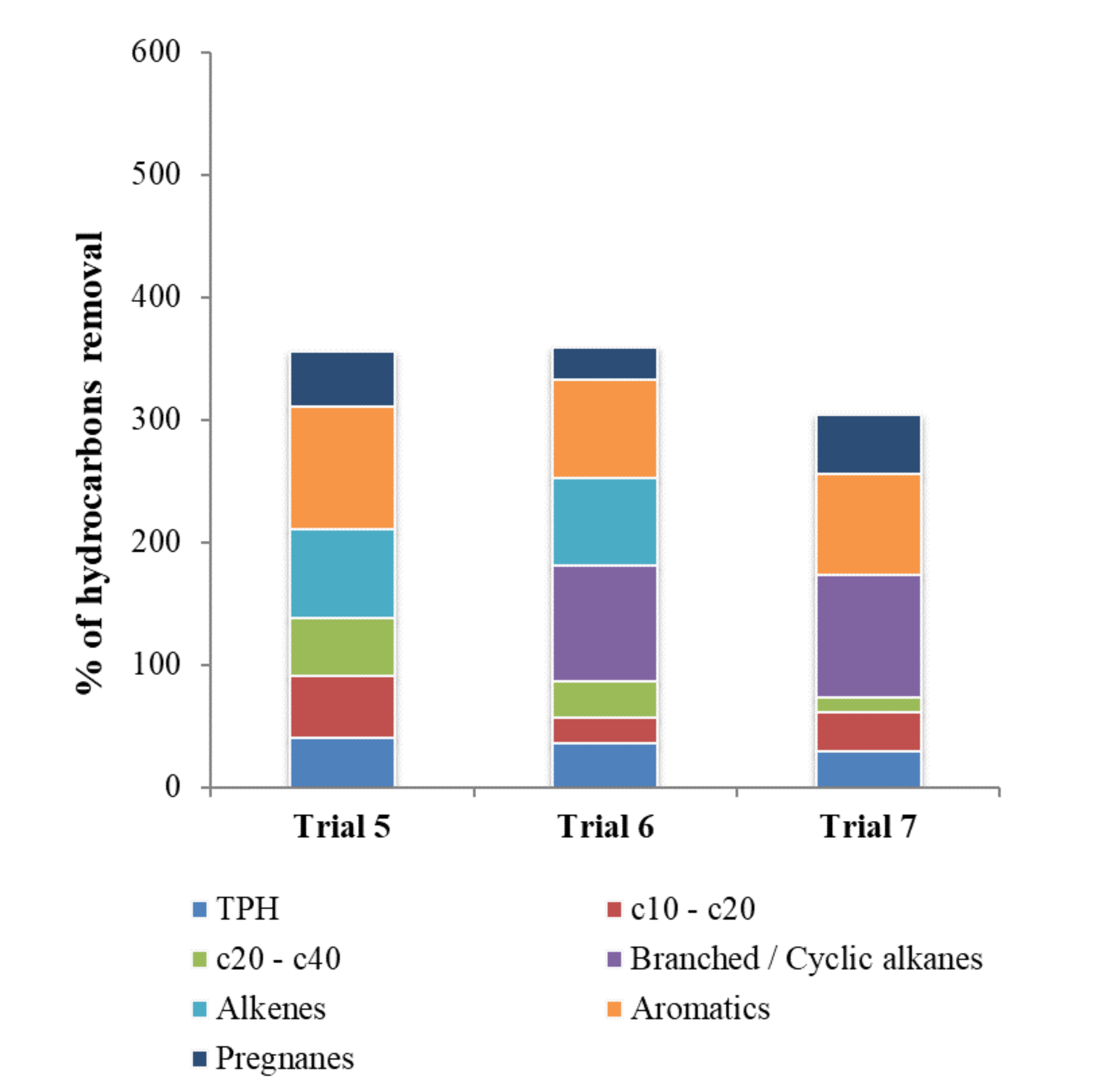
| Trial 5 | Trial 6 | Trial 7 | ||||
|---|---|---|---|---|---|---|
| Influent | Effluent | Influent | Effluent | Influent | Effluent | |
| TPH | 81.7 ± 7.6 | 48.3 ± 2.9 | 51.7 ± 2.9 | 33.3 ± 7.6 | 102 ± 18 | 71.7 ± 5.8 |
| c10–c20 | 3.9 ± 0.7 | 1.9 ± 0.2 | 3.8 ± 0.6 | 3.0 ± 0.7 | 7.8 ± 1.2 | 5.3 ± 0.7 |
| c20–c40 | 5.4 ± 1.4 | 2.8 ± 0.4 | 3.4 + 0.9 | 2.4 ± 0.7 | 5.8 ± 1.4 | 5.1 ± 0.7 |
| Branched/Cyclic alkanes | ND | ND | 2.1 ± 0.5 | 0.1 ± 0.0 | 0.9 ± 0.3 | 0.0 ± 0.0 |
| Alkenes | 1.1 ± 0.4 | 0.30 ± 0.05 | 1.4 ± 0.2 | 0.40 ± 0.05 | 0.8 ± 0.3 | 1.6 ± 0.5 |
| Aromatics | 0.4 ± 0.1 | ND | 0.5 ± 0.2 | 0.10 ± 0.05 | 3.5 ± 0.1 | 0.6 ± 0.2 |
| Pregnanes | 14.4 ± 1.8 | 8.1 ± 0.7 | 8.4 ± 0.1 | 6.2 ± 1.0 | 27.6 ± 6.9 | 14.3 ± 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Castro, G.A.; Rodríguez-Calvo, A.; Robledo-Mahón, T.; Aranda, E.; González-López, J.; Calvo, C. Design of Bio-Absorbent Systems for the Removal of Hydrocarbons from Industrial Wastewater: Pilot-Plant Scale. Toxics 2021, 9, 162. https://doi.org/10.3390/toxics9070162
Silva-Castro GA, Rodríguez-Calvo A, Robledo-Mahón T, Aranda E, González-López J, Calvo C. Design of Bio-Absorbent Systems for the Removal of Hydrocarbons from Industrial Wastewater: Pilot-Plant Scale. Toxics. 2021; 9(7):162. https://doi.org/10.3390/toxics9070162
Chicago/Turabian StyleSilva-Castro, Gloria Andrea, Alfonso Rodríguez-Calvo, Tatiana Robledo-Mahón, Elisabet Aranda, Jesús González-López, and Concepción Calvo. 2021. "Design of Bio-Absorbent Systems for the Removal of Hydrocarbons from Industrial Wastewater: Pilot-Plant Scale" Toxics 9, no. 7: 162. https://doi.org/10.3390/toxics9070162
APA StyleSilva-Castro, G. A., Rodríguez-Calvo, A., Robledo-Mahón, T., Aranda, E., González-López, J., & Calvo, C. (2021). Design of Bio-Absorbent Systems for the Removal of Hydrocarbons from Industrial Wastewater: Pilot-Plant Scale. Toxics, 9(7), 162. https://doi.org/10.3390/toxics9070162









