Conditions Affecting the Release of Heavy and Rare Earth Metals from the Mine Tailings Kola Subarctic
Abstract
1. Introduction
2. Objects and Methods
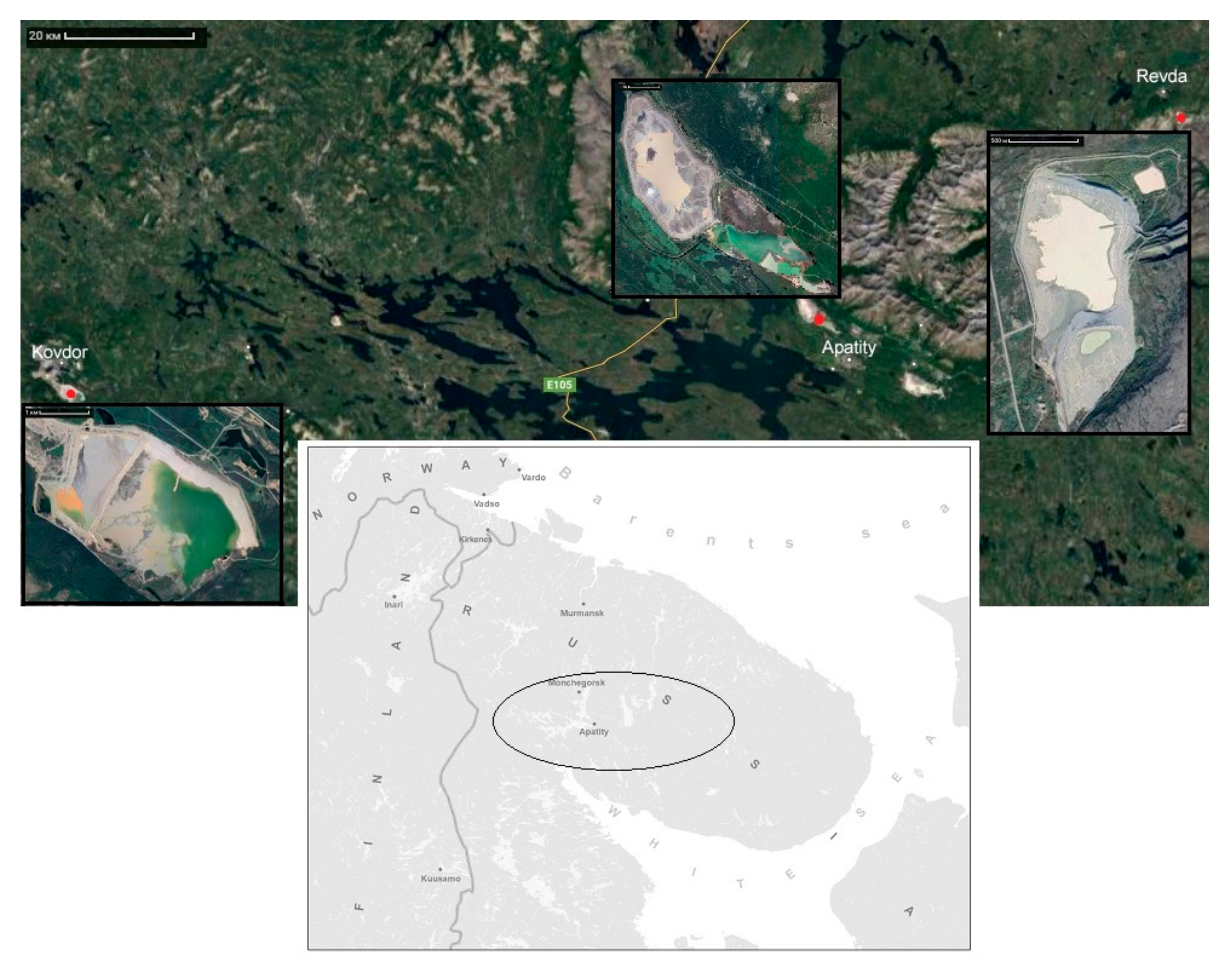
2.1. Geography and Climatic Characteristics of the Study Area
2.2. Tail Physico-Chemical Analyses
2.3. Heavy Metal Pollution and Risk Assessment
2.4. Laboratory Experiments
3. Results and Discussion
3.1. Study of Tail Material
3.2. Results of the Experiments
3.2.1. Mobilization of HM from Tailings of Concentration of Loparite, Apatite-Nepheline and Complex Ores
3.2.2. Mobilization of REE from Tailings of Concentration of Loparite, Apatite-Nepheline and Complex Ores
3.3. Condition of Environmental Components in the Zone of Influence of Tailings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Askaer, L.; Schmidt, L.B.; Elberling, B.; Asmund, G.; Jónsdóttir, I.S. Environmental Impact on an Arctic Soil–Plant System Resulting from Metals Released from Coal Mine Waste in Svalbard (78° N). Water Air Soil Pollut. 2008, 195, 99–114. [Google Scholar] [CrossRef]
- Moncur, M.; Ptacek, C.J.; Hayashi, M.; Blowes, D.W.; Birks, S.J. Seasonal cycling and mass-loading of dissolved metals and sulfate discharging from an abandoned mine site in northern Canada. Appl. Geochem. 2014, 41, 176–188. [Google Scholar] [CrossRef]
- Lindsay, M.; Moncur, M.; Bain, J.G.; Jambor, J.L.; Ptacek, C.J.; Blowes, D.W. Geochemical and mineralogical aspects of sulfide mine tailings. Appl. Geochem. 2015, 57, 157–177. [Google Scholar] [CrossRef]
- Gilyarova, O.; Churkin, A. Development of mining waste as an investment direction for the development of the mining industry of the Kola Peninsula. J. Econ. Entrep. Law 2020, 10, 905–916. (In Russian) [Google Scholar] [CrossRef]
- Makarov, D.V.; Svetlov, A.V.; Goryachev, A.A.; Konina, O.T.; Masloboev, V.A. Tailings dust emissions caused by the climate change: A case-study of a mine in Russia’s far north. Min. Inf. Anal. Bull. 2021, 5, 122–133. [Google Scholar] [CrossRef]
- Kossoff, D.; Dubbin, W.E.; Alfredsson, M.; Edwards, S.; Macklin, M.G.; Hudson-Edwards, K.A. Mine tailings dams: Characteristics, failure, environmental impacts, and remediation. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef]
- Forghani, G.; Mokhtari, A.R.; Kazemi, G.A.; Fard, M.D. Total concentration, speciation and mobility of potentially toxic elements in soils around a mining area in central Iran. Geochemistry 2015, 75, 323–334. [Google Scholar] [CrossRef]
- Ministry of Natural Resources and Environment of the Murmansk Region. Report on the State and Protection of the Environment of the Murmansk Region in 2018. Available online: https://gov-murman.ru/bitrix/components/b1team/govmurman.element.file/download.php?ID=304613&FID=317267 (accessed on 3 June 2021).
- Amosov, P.V.; Baklanov, A.A. On the assessment of the dusting intensity of tailing dumps. Math. Methods Eng. Technol. MMTT 2015, 1, 3–5. (In Russian) [Google Scholar]
- Wang, L.; Liang, T.; Zhang, Q.; Li, K. Rare earth element components in atmospheric particulates in the Bayan Obo mine region. Environ. Res. 2014, 131, 64–70. [Google Scholar] [CrossRef]
- Stille, P.; Pierret, M.-C.; Steinmann, M.; Chabaux, F.; Boutin, R.; Aubert, D.; Pourcelot, L.; Morvan, G. Impact of atmospheric deposition, biogeochemical cycling and water–mineral interaction on REE fractionation in acidic surface soils and soil water (the Strengbach case). Chem. Geol. 2009, 264, 173–186. [Google Scholar] [CrossRef]
- Mehta, U.; Dey, S.; Chowdhury, S.; Ghosh, S.; Hart, J.; Kurpad, A. The Association Between Ambient PM2.5 Exposure and Anemia Outcomes Among Children Under Five Years of Age in India. Environ. Epidemiol. 2021, 5, e125. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, I.C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.; et al. Particulate Matter Air Pollution and Cardiovascular Disease. Circ. 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Arias-Pérez, R.D.; Taborda, N.A.; Gómez, D.M.; Narvaez, J.F.; Porras, J.; Hernandez, J.C. Inflammatory effects of particulate matter air pollution. Environ. Sci. Pollut. Res. 2020, 27, 42390–42404. [Google Scholar] [CrossRef]
- Krewski, D.; Jerrett, M.; Burnett, R.T.; Ma, R.; Hughes, E.; Shi, Y.; Turner, M.C.; Pope, C.A.; Thurston, G.; Calle, E.; et al. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res. Rep. (Health Eff. Inst.) 2009, 140, 5–114, discussion 115–136. Available online: https://www.healtheffects.org/system/files/Krewski140.pdf (accessed on 9 July 2021).
- Thompson, J.E. Airborne Particulate Matter: Human Exposure and Health Effects. J. Occup. Environ. Med. 2018, 60(5), 392–423. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Van Le, Q.; Peng, W.; Yang, Y.; Yang, H.; Gu, H.; Lam, S.S.; Sonne, C. A review on phytoremediation of contaminants in air, water and soil. J. Hazard. Mater. 2021, 403, 123658. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.J.; Dinali, G.S.; Oliveira, C.; Martins, G.C.; Moreira, C.G.; Siqueira, J.O.; Guilherme, L.R.G. Rare Earth Elements in the Soil Environment. Curr. Pollut. Rep. 2016, 2, 28–50. [Google Scholar] [CrossRef]
- Mostofa, K.M.G.; Liu, C.-Q.; Feng, X.; Yoshioka, T.; Vione, D.; Pan, X.; Wu, F. Complexation of Dissolved Organic Matter with Trace Metal Ions in Natural Waters. Photobiogeochem. Org. Matter 2012, 769–849. [Google Scholar] [CrossRef]
- Powlson, D. Will soil amplify climate change? Nature 2005, 433, 204–205. [Google Scholar] [CrossRef]
- Slessarev, E.W.; Lin, Y.; Bingham, N.L.; Johnson, J.; Dai, Y.; Schimel, J.P.; Chadwick, O.A. Water balance creates a threshold in soil pH at the global scale. Nature 2016, 540, 567–569. [Google Scholar] [CrossRef]
- Zhao, F.-J.; Ma, Y.; Zhu, Y.-G.; Tang, Z.; McGrath, S.P. Soil Contamination in China: Current Status and Mitigation Strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef]
- Luo, J.; Cheng, H.; Ren, J.; Davison, W.; Zhang, H. Mechanistic Insights from DGT and Soil Solution Measurements on the Uptake of Ni and Cd by Radish. Environ. Sci. Technol. 2014, 48, 7305–7313. [Google Scholar] [CrossRef]
- Ministry of Natural Resources and Environment of the Murmansk Region. Report on the State and Protection of the Environment of the Murmansk Region in 2019. Available online: https://gov-murman.ru/bitrix/components/b1team/govmurman.element.file/download.php?ID=368407&FID=435276 (accessed on 3 June 2021).
- Tverdov, A.A. Rare metals of the Lovozero massif. Rare Earths J. 2016, 3, 164–169. (In Russian) [Google Scholar]
- Bykhovsky, L.Z.; Pikalova, V.S. Mineral resource base of rare metals in the North-West of Russia—The basis for the creation of the center of the country’s rare metal industry. Explor. Prot. Mineral Res. 2015, 1, 3–7. (In Russian) [Google Scholar]
- State Standard 5180-2015. Soils—Laboratory Methods for Determination of Physical Characteristics; Standartinform Publ.: Moscow, Russia, 2016. (In Russian) [Google Scholar]
- State Standard 12536-2014. Methods for Laboratory Determination of Granulometric (Grain) and Micro-Aggregate Composition; Standartinform Publ.: Moscow, Russia, 2019. (In Russian) [Google Scholar]
- Arkhipov, A.; Reshetnyak, S. Technogenic Deposits—Development and Shaping; Publishing House of the KSC RAS: Apatity, Russia, 2017; 175p. (In Russian) [Google Scholar]
- ICDD. PDF-2 ICDD | XRD Database | 2020. 2021. Available online: https://www.icdd.com/pdf-2/ (accessed on 24 June 2021).
- Vinogradov, A. Average contents of chemical elements in the main types of igneous rocks of the earth’s crust. Geochemistry 1962, 7, 555–571. (In Russian) [Google Scholar]
- Chen, C.-W.; Kao, C.-M.; Chen, C.-F.; Dong, C.-D. Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbor, Taiwan. Chemosphere 2007, 66, 1431–1440. [Google Scholar] [CrossRef]
- Soliman, N.F.; Nasr, S.M.; Okbah, M.A. Potential ecological risk of heavy metals in sediments from the Mediterranean coast, Egypt. J. Environ. Heal. Sci. Eng. 2015, 13, 70. [Google Scholar] [CrossRef]
- Muller, G. Index of geoaccumulation in sediments of the Rhine River. Geojournal 1969, 2, 108–118. [Google Scholar]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total. Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Loska, K.; Wiechuła, D.; Korus, I. Metal contamination of farming soils affected by industry. Environ. Int. 2004, 30, 159–165. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Chen, Z.; Wang, F.; Chen, J.; Wang, L. A systemic ecological risk assessment based on spatial distribution and source apportionment in the abandoned lead acid battery plant zone, China. J. Hazard. Mater. 2018, 354, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Hakanson, L. An ecological risk index for aquatic pollution control.a sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Ni, S.J.; Tuo, X.G.; Zhang, C.J. Calculation of Heavy Metals’ Toxicity Coefficient in the Evaluation of Potential Ecological Risk Index. Environ. Sci. Technol. 2008, 31, 112–115. [Google Scholar]
- Hou, D.; He, J.; Lü, C.; Ren, L.; Fan, Q.; Wang, J.; Xie, Z. Distribution characteristics and potential ecological risk assessment of heavy metals (Cu, Pb, Zn, Cd) in water and sediments from Lake Dalinouer, China. Ecotoxicol. Environ. Saf. 2013, 93, 135–144. [Google Scholar] [CrossRef]
- State Standard 26423-85. Soils. Methods for Determination of Specific Electric Conductivity, pH and Solid Residue of Water Extract; Standartinform Publ.: Moscow, Russia, 2011. (In Russian) [Google Scholar]
- Nikonov, V.; Lukina, N.V.; Bezel’, V.S.; Bel’sky, E.A. Scattered Elements in Boreal Forests; Nauka: Moscow, Russia, 2004. (In Russian) [Google Scholar]
- Order of the Ministry of Agriculture of Russia N 552. On Approval of Water Quality Standards for Water Bodies of Fishery Significance, Including Standards for Maximum Permissible Concentrations of Harmful Substances in Waters of Water Bodies of Fishery Significance. 13 December 2016. Available online: http://docs.cntd.ru/document/420389120 (accessed on 3 June 2021).
- Lomtadze, V.D. Engineering Geology—Engineering Petrology; Publishing House “Nedra”: Leningrad, Russia, 1984; 51p. (In Russian) [Google Scholar]
- State Standard 25100–2011. Soils—Classification; Standartinform Publ.: Moscow, Russia, 2018. (In Russian) [Google Scholar]
- Nguyen, T.; Vu, T. Assessment of environmental situation and forecast environmental fluctuations at areas of copper mining and apatite mining activities in Lao Cai province, Viet Nam. In Proceedings of the XXIII International Scientific and Practical Conference: Topical Areas of Fundamental and Applied Research, North Charleston, SC, USA, 22–23 June 2020; LuluPress, Inc.: North Charleston, SC, USA, 2020; pp. 25–41. [Google Scholar]
- Young, G.; Chen, Y.; Yang, M. Concentrations, distribution, and risk assessment of heavy metals in the iron tailings of Yeshan National Mine Park in Nanjing, China. Chemosphere 2021, 271, 129546. [Google Scholar] [CrossRef]
- Tang, H.; Wang, X.; Shuai, W.; Liu, Y. Immobilization of Rare Earth Elements of the Mine Tailings Using Phosphates and Lime. Procedia Environ. Sci. 2016, 31, 255–263. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Wei, F.; Zheng, C.; Wu, Y.; Adriano, D.C. Background concentrations of elements in soils of China. Water Air Soil Pollut. 1991, 57–58, 699–712. [Google Scholar] [CrossRef]
- Maksimova, V.V.; Krasavtseva, E.A. Study of the impact of mining waste of the Murmansk region on the growth and development of higher plants. Probl. Reg. Ecol. 2020, 4, 21–26. (In Russian) [Google Scholar]
- Federal Service for Supervision of Consumer Rights Protection and Human Welfare. Methodical recommendations 2.1.7.2297-07 Substantiation of the hazard class of production and consumption waste by phytotoxicity. Bull. Norm. Methodol. Doc. State Sanit. Epidemiol. Superv. 2007, 1, 15. [Google Scholar]
- Maksimova, V.V.; Krasavtseva, E.A. Study of organic matter influence on environmentally hazardous elements transition from the fine fraction of tailings. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Krasnoyarsk, Russia, 2021; Volume 677. [Google Scholar]
- Aznar-Sánchez, J.A.; García-Gómez, J.J.; Velasco-Muñoz, J.F.; Carretero-Gómez, A. Mining Waste and Its Sustainable Management: Advances in Worldwide Research. Minerals 2018, 8, 284. [Google Scholar] [CrossRef]
- Cobbina, S.; Myilla, M.; Michael, K. Small Scale Gold Mining and Heavy Metal Pollution: Assessment of Drinking Water Sources in Datuku in the Talensi-Nabdam District. Int. J. Sci. Technol. Res. 2013, 2, 96–100. [Google Scholar]
- Akoto, R.; Anning, A.K. Heavy metal enrichment and potential ecological risks from different solid mine wastes at a mine site in Ghana. Environ. Adv. 2021, 3, 100028. [Google Scholar] [CrossRef]
- Barsova, N.; Motuzova, G.; Kolchanova, K.; Stepanov, A.; Karpukhin, M.; Minkina, T.; Mandzhieva, S. The effect of humic substances on Cu migration in the soil profile. Chem. Ecol. 2018, 35, 86–101. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Zhang, Z.; Liu, C.; Sun, C.; Zhang, W.; Marhaba, T. pH effect on heavy metal release from a polluted sediment. J. Chem. 2018, 2018, 7597640. [Google Scholar] [CrossRef]
- van Herwijnen, R.; Laverye, T.; Poole, J.; Hodson, M.; Hutchings, T.R. The effect of organic materials on the mobility and toxicity of metals in contaminated soils. Appl. Geochem. 2007, 22, 2422–2434. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef]
- Ferraz, M.C.M.A.; Lourençlo, J.C.N. The influence of organic matter content of contaminated soils on the leaching rate of heavy metals. Environ. Prog. 2000, 19, 53–58. [Google Scholar] [CrossRef]
- Hausrath, E.M.; Neaman, A.; Brantley, S.L. Elemental release rates from dissolving basalt and granite with and without organic ligands. Am. J. Sci. 2009, 309, 633–660. [Google Scholar] [CrossRef]
- Salam, D.; El-Fadel, M. Mobility and Availability of Copper in Agricultural Soils Irrigated from Water Treated with Copper Sulfate Algaecide. Water Air Soil Pollut. 2008, 195, 3–13. [Google Scholar] [CrossRef]
- Archive.epa.gov. Persistent, Bioaccumulative and Toxic Chemicals (PBTs)—Projects—Pollution Prevention (P2) | Pacific Southwest: Waste | US EPA. 2021. Available online: https://archive.epa.gov/region9/waste/archive/web/html/pbts.html (accessed on 3 June 2021).
- Marschner, H. Relationships between Mineral Nutrition and Plant Diseases and Pests. In Marschner’s Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: Cambridge, MA, USA, 2002; pp. 436–460. [Google Scholar]
- Rout, G.R.; Das, P. Effect of Metal Toxicity on Plant Growth and Metabolism: I. Zinc. Sustain. Agric. 2009, 873–884, 873–884. [Google Scholar] [CrossRef]
- Hafeez, B.; Khanif, Y.M.; Saleem, M. Role of Zinc in Plant Nutrition–A Review. Am. J. Exp. Agric. 2013, 3, 374–391. [Google Scholar] [CrossRef]
- Dziwornu, A.K.; Shrestha, A.; Matthus, E.; Ali, B.; Wu, L.-B.; Frei, M. Responses of contrasting rice genotypes to excess manganese and their implications for lignin synthesis. Plant Physiol. Biochem. 2018, 123, 252–259. [Google Scholar] [CrossRef]
- Subrahmanyam, D.; Rathore, V. Influence of Manganese Toxicity on Photosynthesis in Ricebean (Vigna Umbellata) Seedlings. Photosynthetica 2000, 38, 449–453. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, W.; Zhou, H.; Wang, R.; Zhang, P.; Wang, H.; Pan, X.; Xu, J. Manganese Toxicity Inhibited Root Growth by Disrupting Auxin Biosynthesis and Transport in Arabidopsis. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Buxton, S.; Garman, E.; Heim, K.E.; Lyons-Darden, T.; Schlekat, C.E.; Taylor, M.D.; Oller, A.R. Concise Review of Nickel Human Health Toxicology and Ecotoxicology. Inorganics 2019, 7, 89. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Lwalaba, J.L.W.; Louis, L.T.; Zvobgo, G.; Richmond, M.E.A.; Fu, L.; Naz, S.; Mwamba, M.; Mundende, R.P.M.; Zhang, G. Physiological and molecular mechanisms of cobalt and copper interaction in causing phyto-toxicity to two barley genotypes differing in Co tolerance. Ecotoxicol. Environ. Saf. 2020, 187, 109866. [Google Scholar] [CrossRef]
- Lwalaba, J.L.W.; Louis, L.T.; Zvobgo, G.; Fu, L.; Mwamba, T.M.; Mundende, R.P.M.; Zhang, G. Copper alleviates cobalt toxicity in barley by antagonistic interaction of the two metals. Ecotoxicol. Environ. Saf. 2019, 180, 234–241. [Google Scholar] [CrossRef]
- Simonsen, L.O.; Harbak, H.; Bennekou, P. Cobalt metabolism and toxicology—A brief update. Sci. Total. Environ. 2012, 432, 210–215. [Google Scholar] [CrossRef]
- Wang, L.; Liao, C.; Yang, Y.; Xu, H.; Xiao, Y.; Yan, C. Effects of organic acids on the leaching process of ion-adsorption type rare earth ore. J. Rare Earths 2017, 35, 1233–1238. [Google Scholar] [CrossRef]
- Ji, B.; Li, Q.; Zhang, W. Leaching Recovery of Rare Earth Elements from Calcination Product of a Coal Coarse Refuse Using Organic Acids. J. Rare Earths 2020. [Google Scholar] [CrossRef]
- Evdokimova, G.A.; Pereverzev, V.N.; Zenkova, I.V.; Korneikova, M.V.; Redkina, V.V. Evolution of Technogenic Landscapes (on the Example of Waste from the Apatite Industry); Publishing House KSC RAS: Apatity, Russia, 2010; 172p. (In Russian) [Google Scholar]
- Davranche, M.; Pourret, O.; Gruau, G.; Dia, A.; Le Coz-Bouhnik, M. Adsorption of REE(III)-humate complexes onto MnO2: Experimental evidence for cerium anomaly and lanthanide tetrad effect suppression. Geochim. Cosmochim. Acta 2005, 69, 4825–4835. [Google Scholar] [CrossRef]
- Cao, X.; Chen, Y.; Wang, X.; Deng, X. Effects of redox potential and pH value on the release of rare earth elements from soil. Chemosphere 2001, 44, 655–661. [Google Scholar] [CrossRef]
- Hu, Z.; Haneklaus, S.; Sparovek, G.; Schnug, E. Rare Earth Elements in Soils. Commun. Soil Sci. Plant Anal. 2006, 37, 1381–1420. [Google Scholar] [CrossRef]
- Xiangke, W.; Wenming, D.; Xiongxin, D.; Aixia, W.; Jinzhou, D.; Zuyi, T. Sorption and desorption of Eu and Yb on alumina: Mechanisms and effect of fulvic acid. Appl. Radiat. Isot. 2000, 52, 165–173. [Google Scholar] [CrossRef]
- Pourret, O.; Davranche, M.; Gruau, G.; Dia, A. Organic complexation of rare earth elements in natural waters: Evaluating model calculations from ultrafiltration data. Geochim. Cosmochim. Acta 2007, 71, 2718–2735. [Google Scholar] [CrossRef]
- Tang, J.; Johannesson, K.H. Rare earth elements adsorption onto Carrizo sand: Influence of strong solution complexation. Chem. Geol. 2010, 279, 120–133. [Google Scholar] [CrossRef]
- Hu, Z.; Richter, H.; Sparovek, G.; Schnug, E. Physiological and Biochemical Effects of Rare Earth Elements on Plants and Their Agricultural Significance: A Review. J. Plant Nutr. 2004, 27, 183–220. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Q. The suitability of rare earth elements for geographical traceability of tea leaves. J. Sci. Food Agric. 2019, 99, 6509–6514. [Google Scholar] [CrossRef]
- Li, J.; He, E.; Romero-Freire, A.; Cao, X.; Zhao, L.; Qiu, H. Coherent toxicity prediction framework for deciphering the joint effects of rare earth metals (La and Ce) under varied levels of calcium and NTA. Chemosphere 2020, 254, 126905. [Google Scholar] [CrossRef]
- Rasoulnia, P.; Barthen, R.; Lakaniemi, A.-M. A critical review of bioleaching of rare earth elements: The mechanisms and effect of process parameters. Crit. Rev. Environ. Sci. Technol. 2021, 51, 378–427. [Google Scholar] [CrossRef]
- Cardon, P.-Y.; Triffault-Bouchet, G.; Caron, A.; Rosabal, M.; Fortin, C.; Amyot, M. Toxicity and Subcellular Fractionation of Yttrium in Three Freshwater Organisms: Daphnia magna, Chironomus riparius, and Oncorhynchus mykiss. ACS Omega 2019, 4, 13747–13755. [Google Scholar] [CrossRef]
- Herrmann, H.; Nolde, J.; Berger, S.; Heise, S. Aquatic ecotoxicity of lanthanum—A review and an attempt to derive water and sediment quality criteria. Ecotoxicol. Environ. Saf. 2016, 124, 213–238. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Guida, M.; Tommasi, F.; Oral, R. Health effects and toxicity mechanisms of rare earth elements—Knowledge gaps and research prospects. Ecotoxicol. Environ. Saf. 2015, 115, 40–48. [Google Scholar] [CrossRef]
- Kashulin, N.; Sandimirov, S.; Dauvalter, V.; Kudryavtsev, L.; Terentyev, P.; Denisov, D.; Vandysh, O.; Korolova, I.; Valkova, S.; Kashulina, T. The Annotated Ecological Catalogue of the Lakes of the Murmansk Region: The Central and Southwest Areas of the Murmansk Region (the Basins of the Barents and the White Seas and the Bothnia Gulf of the Baltic Sea). Pt. 1,2; Kola Science Center RAS: Apatity, Russia, 2013; 253p. (In Russian) [Google Scholar]
- Dauvalter, V.; Moiseenko, T.; Rodyushkin, I. Geochemistry of Rare Earth Elements in Imandra Lake, Murmansk Area. Geochem. Int. 1999, 37, 325–331. [Google Scholar]
- Krasavtseva, E.; Sandimirov, S. State of water bodies in the area of influence of mining and processing enterprises (study of Lovozersky mining and processing plant). Water Ecol. 2021, 2, 3–13. (In Russian) [Google Scholar] [CrossRef]
- Krasavtseva, E.; Maksimova, V.; Gorbacheva, T.; Makarov, D.; Alfertyev, N. Assessment of chemical contamination of soils and plants in the zone of influence of the loparite ore dressing waste storage. Mine Surv. Subsoil Use 2021, 2, 52–58. (In Russian) [Google Scholar]
- Borovichev, E.; Vronsky, N. (Eds.) Arctic Nature and Indigenous Peoples Influenced by Change Climate and Industrial Development: Murmansk Region; Publishing House “Graphite”: Moscow, Russia, 2020; 180p. (In Russian) [Google Scholar]




| Al | Ba | Cd | Ce | Co | Cr | Cu | Fe | La | Mn | Nb | Nd | Ni | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 77,000 | 830 | 0.10 | 100 | 5 | 25 | 20 | 27,000 | 60 | 600 | 20 | 46 | 8 | 20 |
| Pr | Rb | Sb | Sm | Sn | Sr | Ta | Th | Ti | U | V | W | Zn | Zr |
| 12 | 200 | 0.26 | 9 | 3 | 300 | 3.5 | 18 | 2300 | 3.5 | 40 | 1.5 | 60 | 200 |
| EFi Value | Enrichment Level | Igeo Value | Igeo Class | Pollution Level |
|---|---|---|---|---|
| <1 | no enrichment | ≤0 | 0 | unpolluted |
| 1–3 | minor enrichment | 0–1 | 1 | unpolluted to moderately polluted |
| 3–5 | moderate enrichment | 1–2 | 2 | moderately polluted |
| 5–10 | moderately severe enrichment | 2–3 | 3 | moderately polluted to highly polluted |
| 10–25 | severe enrichment | 3–4 | 4 | highly polluted |
| 25–50 | very severe enrichment | 4–5 | 5 | highly polluted to very highly polluted |
| >50 | extremely severe enrichment | >5 | 6 | very highly polluted |
| Er Value | Ecological Risk | RI Value | Ecological Risk |
|---|---|---|---|
| <40 | low ecological risk | <150 | low ecological risk |
| 40–80 | moderate ecological risk | 150–300 | moderate ecological risk |
| 80–160 | appreciable ecological risk | 300–600 | high ecological risk |
| 160–320 | high ecological risk | >600 | significantly high ecological risk |
| >320 | serious ecological risk |
| Loparite Ore Dressing Tails | Apatite-Nepheline Ore Dressing Tails | Complex Ore Dressing Tails | |
|---|---|---|---|
| Nepheline (Na,K)AlSiO4 | 59.57 | 59.51 | - |
| Feldspars (Na,K)AlSi3O8 | 16.07 | 11.10 | 1.67 |
| Apatite Ca10(PO4)6(OH,F,Cl)2 | 1.05 | 5.55 | 0.24 |
| Loparite (Na,Ce,Ca,Sr,Th)(Ti,Nb,Fe)O3 | 0.94 | - | - |
| Aegirine NaFe(Si2O6) | 20.42 | - | - |
| Sodalite 3Na₂O·3Al₂O₃·6SiO₂·2NaCl | 1.58 | - | - |
| Pyroxene SiO4 | - | 17.90 | 18.58 |
| Sphene CaTi(SiO4)O | - | 3.61 | - |
| Magnetite FeO·Fe2O3 | - | 2.26 | 1.50 |
| Calcite CaCO3 | - | 0.37 | 29.00 |
| Forsterite Mg2(SiO4) | - | - | 32.35 |
| Staffelite | - | - | 6.15 |
| Phlogopite KMg3(Si3AlO10)·(F,OH) | - | - | 9.72 |
| SiO2 | TiO2 | Al2O3 | Fe2O3 | FeO | MnO | CaO | MgO | K2O | Na2O | P2O5 | SrO | F | SO3 | LoI * | ∑ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Loparite ore dressing tails | 48.08 | 1.1 | 22.47 | 5.3 | 0.66 | 0.23 | 1.63 | 0.45 | 4.24 | 13.33 | 0.79 | 0.33 | 0.08 | 0.08 | 1.2 | 99.97 |
| Apatite-nepheline ore dressing tails | 42.56 | 2.65 | 21.35 | 4 | 2.27 | 0.16 | 5.78 | 1.31 | 6.09 | 9.4 | 2.3 | 0.27 | 0.25 | 0.14 | 1.32 | 99.85 |
| Complex ore dressing tails | 25.46 | 0.37 | 3.42 | 1.81 | 4.16 | 0.2 | 24.07 | 19.45 | 1.27 | 1.32 | 2.6 | 0.24 | 0.13 | 1.21 | 14.9 | 100.66 |
| Object | Potential Ecological Risk Factor Er | RI | Risk Grade | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cd | Cr | Cu | Mn | Ni | Pb | Zn | Co | |||
| Apatite-nepheline ore dressing tails | 14.56 | 0.78 | 16.15 | 2.85 | 6.72 | 0.40 | 2.19 | 6.99 | 50.65 | low |
| Apatite-nepheline ore dressing tails, fine fraction | 35.67 | 0.72 | 23.49 | 3.91 | 6.04 | 0.70 | 2.80 | 9.61 | 82.94 | low |
| Complex ore dressing tails | 30.29 | 4.86 | 27.56 | 3.01 | 30.33 | 0.36 | 1.04 | 23.71 | 121.15 | low |
| Complex ore dressing tails, fine fraction | 85.49 | 7.44 | 37.68 | 3.59 | 51.46 | 0.50 | 1.71 | 32.24 | 220.11 | moderate |
| Loparite ore dressing tails | 21.55 | 0.16 | 1.34 | 2.25 | 4.63 | 3.71 | 2.86 | 0.60 | 37.10 | low |
| Loparite ore dressing tails, fine fraction | 67.79 | 0.40 | 3.33 | 3.26 | 4.98 | 7.19 | 5.35 | 1.24 | 93.54 | low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasavtseva, E.; Maksimova, V.; Makarov, D. Conditions Affecting the Release of Heavy and Rare Earth Metals from the Mine Tailings Kola Subarctic. Toxics 2021, 9, 163. https://doi.org/10.3390/toxics9070163
Krasavtseva E, Maksimova V, Makarov D. Conditions Affecting the Release of Heavy and Rare Earth Metals from the Mine Tailings Kola Subarctic. Toxics. 2021; 9(7):163. https://doi.org/10.3390/toxics9070163
Chicago/Turabian StyleKrasavtseva, Eugenia, Victoria Maksimova, and Dmitry Makarov. 2021. "Conditions Affecting the Release of Heavy and Rare Earth Metals from the Mine Tailings Kola Subarctic" Toxics 9, no. 7: 163. https://doi.org/10.3390/toxics9070163
APA StyleKrasavtseva, E., Maksimova, V., & Makarov, D. (2021). Conditions Affecting the Release of Heavy and Rare Earth Metals from the Mine Tailings Kola Subarctic. Toxics, 9(7), 163. https://doi.org/10.3390/toxics9070163







