Comprehensive Insight from Phthalates Occurrence: From Health Outcomes to Emerging Analytical Approaches
Abstract
:1. Introduction
2. Phthalates Background
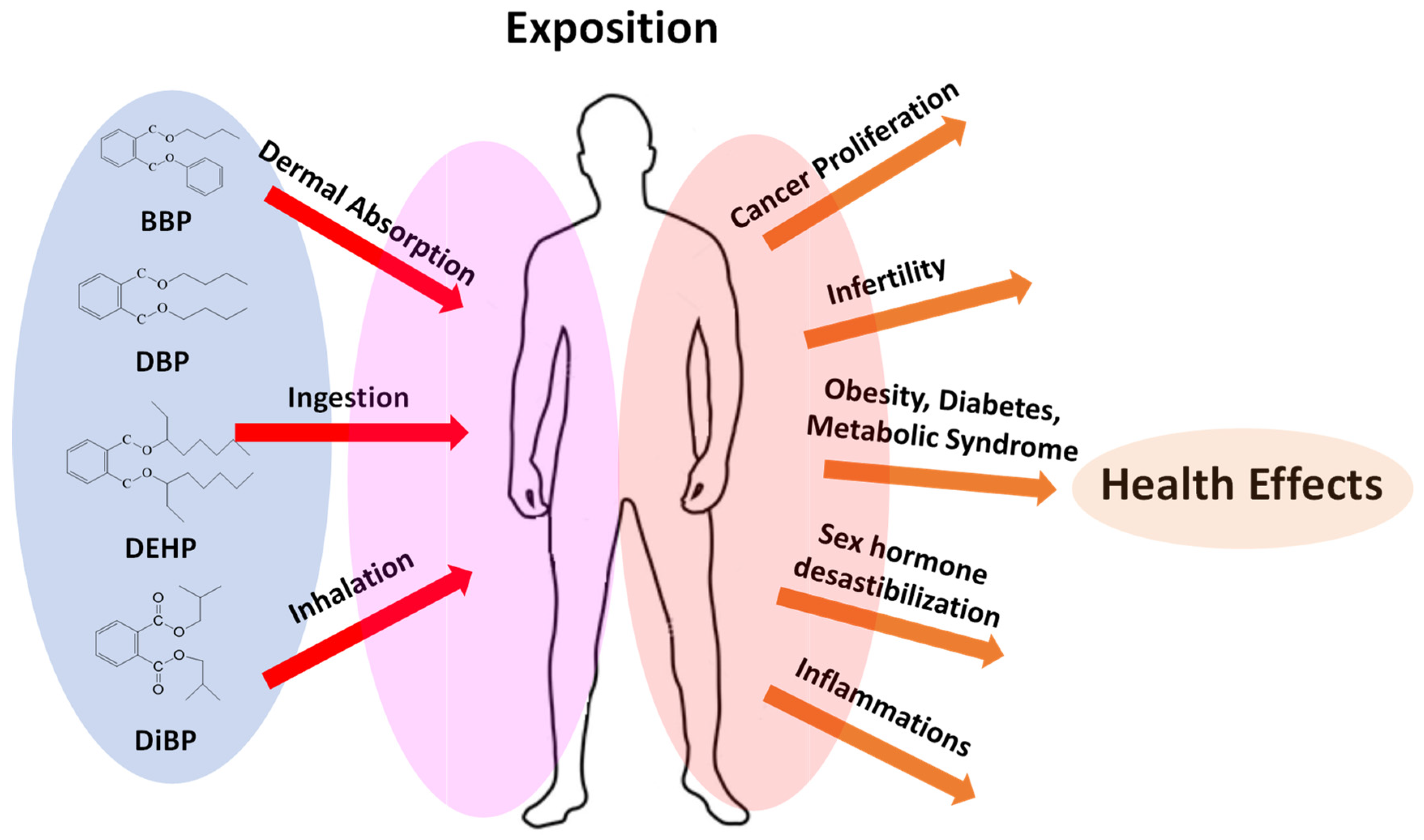
2.1. Human Exposure Routes
2.2. Healthy Risks
2.3. Phthalates Regulation
3. Occurrence of Phthalates
3.1. Environmental
3.2. Foods
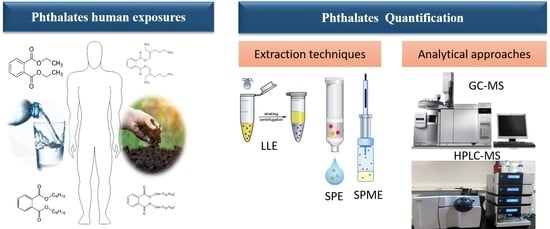
4. Analytical Approaches
4.1. Sample Preparation and Extraction Techniques
4.2. Extraction Techniques
4.3. Analytical Approaches
4.3.1. Gas Chromatography
4.3.2. Liquid Chromatography
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement:
Data Availa bility Statement
Conflicts of Interest
References
- Sicińska, P. Di-n-butyl phthalate, butylbenzyl phthalate, and their metabolites exhibit different apoptotic potential in human peripheral blood mononuclear cells. Food Chem. Toxicol. 2019, 133. [Google Scholar] [CrossRef]
- Čtveráčková, L.; Jančula, D.; Raška, J.; Babica, P.; Sovadinová, I. Structure-dependent effects of phthalates on intercellular and intracellular communication in liver oval cells. Int. J. Mol. Sci. 2020, 21, 6069. [Google Scholar] [CrossRef]
- Mariana, M.; Cairrao, E. Phthalates Implications in the Cardiovascular System. J. Cardiovasc. Dev. Dis. 2020, 7, 26. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, Z.H.; Yin, H.; Dang, Z.; Wu, P.X.; Zhu, N.W.; Lin, Z.; Liu, Y. Global review of phthalates in edible oil: An emerging and nonnegligible exposure source to human. Sci. Total Environ. 2020, 704, 135369. [Google Scholar] [CrossRef]
- Kim, D.Y.; Chun, S.H.; Jung, Y.; Mohamed, D.F.M.S.; Kim, H.S.; Kang, D.Y.; An, J.W.; Park, S.Y.; Kwon, H.W.; Kwon, J.H. Phthalate plasticizers in children’s products and estimation of exposure: Importance of migration rate. Int. J. Environ. Res. Public Health 2020, 17, 8582. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical review on the presence of phthalates in food and evidence of their biological impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef]
- Zaki, G.; Shoeib, T. Concentrations of several phthalates contaminants in Egyptian bottled water: Effects of storage conditions and estimate of human exposure. Sci. Total Environ. 2018, 618, 142–150. [Google Scholar] [CrossRef]
- Heinemeyer, G.; Sommerfeld, C.; Springer, A.; Heiland, A.; Lindtner, O.; Greiner, M.; Heuer, T.; Krems, C.; Conrad, A. Estimation of dietary intake of bis(2-ethylhexyl)phthalate (DEHP) by consumption of food in the German population. Int. J. Hyg. Environ. Health 2013, 216, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Fierens, T.; Servaes, K.; Van Holderbeke, M.; Geerts, L.; De Henauw, S.; Sioen, I.; Vanermen, G. Analysis of phthalates in food products and packaging materials sold on the Belgian market. Food Chem. Toxicol. 2012, 50, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Guart, A.; Bono-Blay, F.; Borrell, A.; Lacorte, S. Effect of bottling and storage on the migration of plastic constituents in Spanish bottled waters. Food Chem. 2014, 156, 73–80. [Google Scholar] [CrossRef]
- Guo, Y.; Kannan, K. Challenges encountered in the analysis of phthalate esters in foodstuffs and other biological matrices. Anal. Bioanal. Chem. 2012, 404, 2539–2554. [Google Scholar] [CrossRef] [PubMed]
- Jeddi, M.Z.; Rastkari, N.; Ahmadkhaniha, R.; Yunesian, M. Concentrations of phthalates in bottled water under common storage conditions: Do they pose a health risk to children? Food Res. Int. 2015, 69, 256–265. [Google Scholar] [CrossRef]
- Bennett, D.; Bellinger, D.C.; Birnbaum, L.S.; Bradman, A.; Chen, A.; Cory-Slechta, D.A.; Engel, S.M.; Fallin, M.D.; Halladay, A.; Hauser, R.; et al. Project TENDR: Targeting environmental neuro-developmental risks. the TENDR consensus statement. Environ. Health Perspect. 2016, 124, A118–A122. [Google Scholar] [CrossRef] [PubMed]
- Phthalates in Food—EU Regulatory Overview: Food Contact Materials and Food Law, European Commission. Available online: https://ec.europa.eu/food/food/chemical-safety/food-contact-materials/legislation_en (accessed on 14 May 2021).
- Gao, D.; Li, Z.; Wang, H.; Liang, H. An overview of phthalate acid ester pollution in China over the last decade: Environmental occurrence and human exposure. Sci. Total Environ. 2018, 645, 1400–1409. [Google Scholar] [CrossRef]
- Llompart, M.; Celeiro, M.; Pablo Lamas, J.; Sanchez-Prado, L.; Lores, M.; Garcia-Jares, C. Analysis of plasticizers and synthetic musks in cosmetic and personal care products by matrix solid-phase dispersion gas chromatography-mass spectrometry. J. Chromatogr. A 2013, 1293, 10–19. [Google Scholar] [CrossRef]
- Ashworth, M.J.; Chappell, A.; Ashmore, E.; Fowles, J. Analysis and assessment of exposure to selected phthalates found in children’s toys in christchurch, New Zealand. Int. J. Environ. Res. Public Health 2018, 15, 200. [Google Scholar] [CrossRef] [Green Version]
- Bekö, G.; Weschler, C.J.; Langer, S.; Callesen, M.; Toftum, J.; Clausen, G. Children’s phthalate intakes and resultant cumulative exposures estimated from urine compared with estimates from dust ingestion, inhalation and dermal absorption in their homes and daycare centers. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Meeker, J.D.; Sathyanarayana, S.; Swan, S.H. Phthalates and other additives in plastics: Human exposure and associated health outcomes. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2097–2113. [Google Scholar] [CrossRef] [Green Version]
- Sugeng, E.J.; Symeonides, C.; O’Hely, M.; Vuillermin, P.; Sly, P.D.; Vijayasarathy, S.; Thompson, K.; Pezic, A.; Mueller, J.F.; Ponsonby, A.L. Predictors with regard to ingestion, inhalation and dermal absorption of estimated phthalate daily intakes in pregnant women: The Barwon infant study. Environ. Int. 2020, 139. [Google Scholar] [CrossRef] [PubMed]
- González-Castro, M.I.; Olea-Serrano, M.F.; Rivas-Velasco, A.M.; Medina-Rivero, E.; Ordoñez-Acevedo, L.G.; De León-Rodríguez, A. Phthalates and bisphenols migration in Mexican food cans and plastic food containers. Bull. Environ. Contam. Toxicol. 2011, 86, 627–631. [Google Scholar] [CrossRef]
- Bouma, K.; Schakel, D.J. Migration of phthalates from PVC toys into saliva simulant by dynamic extraction. Food Addit. Contam. 2002, 19, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.H.; Lin-Tan, D.T.; Lin, J.L. Food safety involving ingestion of foods and beverages prepared with phthalate-plasticizer-containing clouding agents. J. Formos. Med. Assoc. 2011, 110, 671–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wu, Z.; Gong, M.; Xu, Y.; Zhang, Y. Non-dietary exposure to phthalates for pre-school children in kindergarten in Beijing, China. Build. Environ. 2020, 167, 106438. [Google Scholar] [CrossRef]
- Bi, C.; Liang, Y.; Xu, Y. Fate and Transport of Phthalates in Indoor Environments and the Influence of Temperature: A Case Study in a Test House. Environ. Sci. Technol. 2015, 49, 9674–9681. [Google Scholar] [CrossRef]
- Wu, Y.; Eichler, C.M.A.; Cao, J.; Benning, J.; Olson, A.; Chen, S.; Liu, C.; Vejerano, E.P.; Marr, L.C.; Little, J.C. Particle/Gas Partitioning of Phthalates to Organic and Inorganic Airborne Particles in the Indoor Environment. Environ. Sci. Technol. 2018, 52, 3583–3590. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Wang, X.; Li, H.; Li, X.; Xu, Y. Direct Transfer of Phthalate and Alternative Plasticizers from Indoor Source Products to Dust: Laboratory Measurements and Predictive Modeling. Environ. Sci. Technol. 2021, 55, 341–351. [Google Scholar] [CrossRef]
- Bourdeaux, D.; Yessaad, M.; Chennell, P.; Larbre, V.; Eljezi, T.; Bernard, L.; Sautou, V.; Azaroual, N.; Barthelémy, C.; Décaudin, B.; et al. Analysis of PVC plasticizers in medical devices and infused solutions by GC-MS. J. Pharm. Biomed. Anal. 2016, 118, 206–213. [Google Scholar] [CrossRef]
- Malarvannan, G.; Onghena, M.; Verstraete, S.; van Puffelen, E.; Jacobs, A.; Vanhorebeek, I.; Verbruggen, S.C.A.T.; Joosten, K.F.M.; Van den Berghe, G.; Jorens, P.G.; et al. Phthalate and alternative plasticizers in indwelling medical devices in pediatric intensive care units. J. Hazard. Mater. 2019, 363, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, H.; Kannan, K. A review of biomonitoring of phthalate exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, S.; Masai, E.; Kamimura, N.; Takahashi, K.; Anderson, R.C.; Faisal, P.A. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard. Mater. 2017, 340, 360–383. [Google Scholar] [CrossRef] [PubMed]
- Katsikantami, I.; Sifakis, S.; Tzatzarakis, M.N.; Vakonaki, E.; Kalantzi, O.I.; Tsatsakis, A.M.; Rizos, A.K. A global assessment of phthalates burden and related links to health effects. Environ. Int. 2016, 97, 212–236. [Google Scholar] [CrossRef]
- Hidalgo-Serrano, M.; Borrull, F.; Pocurull, E.; Marcé, R.M. Pressurised Liquid Extraction and Liquid Chromatography–High Resolution Mass Spectrometry for the Simultaneous Determination of Phthalate Diesters and Their Metabolites in Seafood Species. Food Anal. Methods 2020, 13, 1442–1453. [Google Scholar] [CrossRef]
- Woodward, M.J.; Obsekov, V.; Jacobson, M.H.; Kahn, L.G.; Trasande, L. Phthalates and sex steroid hormones among men from nhanes, 2013–2016. J. Clin. Endocrinol. Metab. 2020, 105, E1225–E1234. [Google Scholar] [CrossRef] [Green Version]
- Wen, H.J.; Chen, C.C.; Wu, M.T.; Chen, M.L.; Sun, C.W.; Wu, W.C.; Huang, I.W.; Huang, P.C.; Yu, T.Y.; Hsiung, C.A.; et al. Phthalate exposure and reproductive hormones and sex-hormone binding globulin before puberty—Phthalate contaminated-foodstuff episode in Taiwan. PLoS ONE 2017, 12, e0175536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.Y.; Hwang, J.S.; Sung, F.C.; Lin, C.Y.; Hsieh, C.J.; Chen, P.C.; Su, T.C. Mono-2-ethylhexyl phthalate associated with insulin resistance and lower testosterone levels in a young population. Environ. Pollut. 2017, 225, 112–117. [Google Scholar] [CrossRef]
- Rehman, S.; Usman, Z.; Rehman, S.; AlDraihem, M.; Rehman, N.; Rehman, I.; Ahmad, G. Endocrine disrupting chemicals and impact on male reproductive health. Transl. Androl. Urol. 2018, 7, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Milošević, N.; Milanović, M.; Sudji, J.; Bosić Živanović, D.; Stojanoski, S.; Vuković, B.; Milić, N.; Medić Stojanoska, M. Could phthalates exposure contribute to the development of metabolic syndrome and liver disease in humans? Environ. Sci. Pollut. Res. 2020, 27, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Dales, R.E.; Kauri, L.M.; Cakmak, S. The associations between phthalate exposure and insulin resistance, β-cell function and blood glucose control in a population-based sample. Sci. Total Environ. 2018, 612, 1287–1292. [Google Scholar] [CrossRef]
- Zhai, W.; Huang, Z.; Chen, L.; Feng, C.; Li, B.; Li, T. Thyroid endocrine disruption in zebrafish larvae after exposure to mono-(2-ethylhexyl) phthalate (MEHP). PLoS ONE 2014, 9, e92465. [Google Scholar] [CrossRef]
- Asghari, M.H.; Saeidnia, S.; Abdollahi, M. A review on the biochemical and molecular mechanisms of phthalate-induced toxicity in various organs with a focus on the reproductive system. Int. J. Pharmacol. 2015, 11, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Zuccarello, P.; Oliveri Conti, G.; Cavallaro, F.; Copat, C.; Cristaldi, A.; Fiore, M.; Ferrante, M. Implication of dietary phthalates in breast cancer. A systematic review. Food Chem. Toxicol. 2018, 118, 667–674. [Google Scholar] [CrossRef]
- Ahern, T.P.; Broe, A.; Lash, T.L.; Cronin-Fenton, D.P.; Ulrichsen, S.P.; Christiansen, P.M.; Cole, B.F.; Tamimi, R.M.; Sørensen, H.T.; Damkier, P. Phthalate exposure and breast cancer incidence: A danish nationwide cohort study. J. Clin. Oncol. 2019, 37, 1800–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Li, J.; Wang, Y.; Xu, S.; Li, Y.; Liu, H.; Zhou, Y.; Zhao, H.; Fang, J.; Cai, Z.; et al. Association between phthalate exposure and blood pressure during pregnancy. Ecotoxicol. Environ. Saf. 2020, 189, 109944. [Google Scholar] [CrossRef]
- Ferguson, K.K.; McElrath, T.F.; Cantonwine, D.E.; Mukherjee, B.; Meeker, J.D. Phthalate metabolites and bisphenol-A in association with circulating angiogenic biomarkers across pregnancy. Placenta 2015, 36, 699–703. [Google Scholar] [CrossRef] [Green Version]
- Van’T Erve, T.J.; Rosen, E.M.; Barrett, E.S.; Nguyen, R.H.N.; Sathyanarayana, S.; Milne, G.L.; Calafat, A.M.; Swan, S.H.; Ferguson, K.K. Phthalates and Phthalate Alternatives Have Diverse Associations with Oxidative Stress and Inflammation in Pregnant Women. Environ. Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Harmon, A.C.; Cornelius, D.C.; Amaral, L.M.; Faulkner, J.L.; Cunningham, M.W.; Wallace, K.; LaMarca, B. The role of inflammation in the pathology of preeclampsia. Clin. Sci. 2016, 130, 409–419. [Google Scholar] [CrossRef] [Green Version]
- Su, T.C.; Hwang, J.S.; Torng, P.L.; Wu, C.; Lin, C.Y.; Sung, F.C. Phthalate exposure increases subclinical atherosclerosis in young population. Environ. Pollut. 2019, 250, 586–593. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Phthalates Action Plan; U.S. Environmental Protection Agency: Washington, DC, USA, 2012. [Google Scholar]
- Commission Regulation (EU) 2018/2005—of 17 December 2018—Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards bis(2-ethylhexyl) phthalate (DEHP), dibutyl phthalate (DBP), benzyl butyl phthalate (BBP) and diisobutyl phthalate (DIBP). Off. J. Eur. Union 2018, 322, 14–19.
- Silano, V.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mortensen, A.; et al. Update of the risk assessment of di-butylphthalate (DBP), butyl-benzyl-phthalate (BBP), bis(2-ethylhexyl)phthalate (DEHP), di-isononylphthalate (DINP) and di-isodecylphthalate (DIDP) for use in food contact materials. Eur. Food Saf. Auth. J. 2019, 17, 5838–5923. [Google Scholar] [CrossRef] [Green Version]
- Directive 2011/65/eu of the european parliament and of the council of 8 June 2011 on the restriction of the use of certain hazardous substances in electrical and electronic equipment (recast) (Text with EEA relevance). O. J. Eur. Union 2011, 174, 88.
- Commission Delegated Directive (EU) 2015/ 863—of 31 March 2015—amending Annex II to Directive 2011/ 65/ EU of the European Parliament and of the Council as regards the list of restricted substances. O. J. Eur. Union 2015, 137, 10–12.
- Wang, W.; Leung, A.O.W.; Chu, L.H.; Wong, M.H. Phthalates contamination in China: Status, trends and human exposure-with an emphasis on oral intake. Environ. Pollut. 2018, 238, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Canada Consumer Products Safety Act: Phthalates Regulations (SOR/2016-188); Government of Canada: Ottawa, ON, Canada, 2016.
- Scheduling Delegate’s Final Decisions, October 2017: 1.6 Butyl Benzyl Phthalate|Therapeutic Goods Administration (TGA). Available online: https://www.tga.gov.au/book-page/16-butyl-benzyl-phthalate (accessed on 18 June 2021).
- Net, S.; Delmont, A.; Sempéré, R.; Paluselli, A.; Ouddane, B. Reliable quantification of phthalates in environmental matrices (air, water, sludge, sediment and soil): A review. Sci. Total Environ. 2015, 515–516, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Net, S.; Sempéré, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, fate, behavior and ecotoxicological state of phthalates in different environmental matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, M.; Dobaradaran, S.; Torabbeigi, M.; Jorfi, S.; Gholamnia, R.; Koolivand, A.; Darabi, H.; Kavousi, A.; Saeedi, R. Health risk of phthalates in water environment: Occurrence in water resources, bottled water, and tap water, and burden of disease from exposure through drinking water in tehran, Iran. Environ. Res. 2019, 173, 469–479. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.L.; Algarra, M.; Câmara, J.S. Evaluation of the occurrence of phthalates in plastic materials used in food packaging. Appl. Sci. 2021, 11, 2130. [Google Scholar] [CrossRef]
- Notardonato, I.; Protano, C.; Vitali, M.; Avino, P. Phthalates and Bisphenol-A Determination and Release from Different Beverage Plastic Containers by Dispersive Liquid-Liquid Microextraction and GC-IT/MS Analysis. Food Anal. Methods 2019, 12, 2562–2571. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lim, J.E.; Lee, S.; Moon, H.B. Phthalates and non-phthalate plasticizers in sediment from Korean coastal waters: Occurrence, spatial distribution, and ecological risks. Mar. Pollut. Bull. 2020, 154, 111119–111125. [Google Scholar] [CrossRef]
- Jebara, A.; Albergamo, A.; Rando, R.; Potortì, A.G.; Lo Turco, V.; Mansour, H.B.; Di Bella, G. Phthalates and non-phthalate plasticizers in Tunisian marine samples: Occurrence, spatial distribution and seasonal variation. Mar. Pollut. Bull. 2021, 163, 111967–111978. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, J.; Li, X.; Peng, Q.; Yuan, H.; Li, N.; Duan, L.; Ma, J. Concentrations and distribution of phthalate esters in the seamount area of the Tropical Western Pacific Ocean. Mar. Pollut. Bull. 2019, 140, 107–115. [Google Scholar] [CrossRef]
- Borges Ramirez, M.M.; Dzul Caamal, R.; Rendón von Osten, J. Occurrence and seasonal distribution of microplastics and phthalates in sediments from the urban channel of the Ria and coast of Campeche, Mexico. Sci. Total Environ. 2019, 672, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, C.; Huang, Z.; Yi, X.H.; Zeng, H.; Zhang, M.; Huang, M. Residue levels and spatial distribution of phthalate acid esters in water and sediment from urban lakes of Guangzhou, China. J. Environ. Sci. Heal. Part A Toxic Hazardous Subst. Environ. Eng. 2019, 54, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Min, N.; Yao, J.; Amde, M.; Wu, L.; Sunahara, G.; Li, H.; Chen, Y.; Liu, J.; Mihucz, V.G. Accelerated solvent extraction combined with GC–MS: A convenient technique for the determination and compound-specific stable isotope analysis of phthalates in mine tailings. Microchem. J. 2020, 153, 104366–104373. [Google Scholar] [CrossRef]
- Chen, H.; Mao, W.; Shen, Y.; Feng, W.; Mao, G.; Zhao, T.; Yang, L.; Yang, L.; Meng, C.; Li, Y.; et al. Distribution, source, and environmental risk assessment of phthalate esters (PAEs) in water, suspended particulate matter, and sediment of a typical Yangtze River Delta City, China. Environ. Sci. Pollut. Res. 2019, 26, 24609–24619. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Silva, C.L.; Algarra, M.; Câmara, J.S. Monitoring Phthalates in Table and Fortified Wines by Headspace Solid-Phase Microextraction Combined with Gas Chromatography-Mass Spectrometry Analysis. J. Agric. Food Chem. 2020, 68, 8431–8437. [Google Scholar] [CrossRef]
- García Ibarra, V.; de Quirós, A.R.B.; Paseiro Losada, P.; Sendón, R. Identification of intentionally and non-intentionally added substances in plastic packaging materials and their migration into food products. Anal. Bioanal. Chem. 2018, 410, 3789–3803. [Google Scholar] [CrossRef]
- Hu, H.; Fang, S.; Zhao, M.; Jin, H. Occurrence of phthalic acid esters in sediment samples from East China Sea. Sci. Total Environ. 2020, 722, 137997–138004. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramos, R.; Socas-Rodríguez, B.; Santana-Mayor, Á.; Rodríguez-Delgado, M.Á. Nanomaterials as alternative dispersants for the multiresidue analysis of phthalates in soil samples using matrix solid phase dispersion prior to ultra-high performance liquid chromatography tandem mass spectrometry. Chemosphere 2019, 236, 124377–124386. [Google Scholar] [CrossRef]
- Hu, A.; Qiu, M.; Liu, H.; Xu, Y.; Tao, Y.; Yang, G.; He, Y.; Xu, J.; Lu, Z. Simultaneous determination of phthalate diesters and monoesters in soil using accelerated solvent extraction and ultra-performance liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 2020, 1626, 461347–461367. [Google Scholar] [CrossRef]
- Wei, L.; Li, Z.; Sun, J.; Zhu, L. Pollution characteristics and health risk assessment of phthalate esters in agricultural soil and vegetables in the Yangtze River Delta of China. Sci. Total Environ. 2020, 726, 137978–137986. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Jalón-Rojas, I.; Wang, X.H.; Fredj, E.; Zhang, D.; Feng, L.; Li, X. Assessing the potential risk and relationship between microplastics and phthalates in surface seawater of a heavily human-impacted metropolitan bay in northern China. Ecotoxicol. Environ. Saf. 2020, 204, 111067–111077. [Google Scholar] [CrossRef] [PubMed]
- Nagorka, R.; Koschorreck, J. Trends for plasticizers in German freshwater environments—Evidence for the substitution of DEHP with emerging phthalate and non-phthalate alternatives. Environ. Pollut. 2020, 262, 114237–114246. [Google Scholar] [CrossRef] [PubMed]
- Arfaeinia, L.; Dobaradaran, S.; Nasrzadeh, F.; Shamsi, S.; Poureshgh, Y.; Arfaeinia, H. Phthalate acid esters (PAEs) in highly acidic juice packaged in polyethylene terephthalate (PET) container: Occurrence, migration and estrogenic activity-associated risk assessment. Microchem. J. 2020, 155, 104719–104726. [Google Scholar] [CrossRef]
- Notardonato, I.; Salimei, E.; Russo, M.V.; Avino, P. Simultaneous determination of organophosphorus pesticides and phthalates in baby food samples by ultrasound–vortex-assisted liquid–liquid microextraction and GC–IT/MS. Anal. Bioanal. Chem. 2018, 410, 3285–3296. [Google Scholar] [CrossRef] [PubMed]
- Socas-Rodríguez, B.; González-Sálamo, J.; Herrera-Herrera, A.V.; Santana-Mayor, Á.; Hernández-Borges, J. Determination of phthalic acid esters in different baby food samples by gas chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 5617–5628. [Google Scholar] [CrossRef]
- Pang, Y.H.; Yue, Q.; Huang, Y.Y.; Yang, C.; Shen, X.F. Facile magnetization of covalent organic framework for solid-phase extraction of 15 phthalate esters in beverage samples. Talanta 2020, 206, 120194–120204. [Google Scholar] [CrossRef]
- Huang, Z.; Tu, C.; Liu, H.; Wang, L.; Zhu, Z.; Watanabe, I. Hollow fiber-solid phase microextraction of phthalate esters from bottled water followed by flash evaporation gas chromatography-flame ionization detection. J. Chromatogr. A 2020, 1619, 460953–460961. [Google Scholar] [CrossRef]
- Panio, A.; Fabbri Corsarini, S.; Bruno, A.; Lasagni, M.; Labra, M.; Saliu, F. Determination of phthalates in fish fillets by liquid chromatography tandem mass spectrometry (LC-MS/MS): A comparison of direct immersion solid phase microextraction (SPME) versus ultrasonic assisted solvent extraction (UASE). Chemosphere 2020, 255, 127034–127042. [Google Scholar] [CrossRef]
- Li, T.; Song, Y.; Li, J.; Zhang, M.; Shi, Y.; Fan, J. New low viscous hydrophobic deep eutectic solvents in vortex-assisted liquid-liquid microextraction for the determination of phthalate esters from food-contacted plastics. Food Chem. 2020, 309, 125752. [Google Scholar] [CrossRef] [PubMed]
- Diamantidou, D.; Begou, O.; Theodoridis, G.; Gika, H.; Tsochatzis, E.; Kalogiannis, S.; Kataiftsi, N.; Soufleros, E.; Zotou, A. Development and validation of an ultra high performance liquid chromatography-tandem mass spectrometry method for the determination of phthalate esters in Greek grape marc spirits. J. Chromatogr. A 2019, 1603, 165–178. [Google Scholar] [CrossRef]
- Otoukesh, M.; Es’haghi, Z.; Feizy, J.; Nerin, C. Graphene oxide/ layered double hydroxides@ sulfonated polyaniline: A sorbent for ultrasonic assisted dispersive solid phase extraction of phthalates in distilled herbal beverages. J. Chromatogr. A 2020, 1625, 461307–461318. [Google Scholar] [CrossRef]
- Notardonato, I.; Passarella, S.; Ianiri, G.; Di Fiore, C.; Russo, M.V.; Avino, P. Analytical scheme for simultaneous determination of phthalates and bisphenol a in honey samples based on dispersive liquid–liquid microextraction followed by GC-IT/MS. Effect of the thermal stress on PAE/BP-A levels. Methods Protoc. 2020, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Notardonato, I.; Passarella, S.; Ianiri, G.; Di Fiore, C.; Russo, M.V.; Avino, P. Analytical method development and chemometric approach for evidencing presence of plasticizer residues in nectar honey samples. Int. J. Environ. Res. Public Health 2020, 17, 1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobaradaran, S.; Akhbarizadeh, R.; Javad Mohammadi, M.; Izadi, A.; Keshtkar, M.; Tangestani, M.; Moazzen, M.; Shariatifar, N.; Mahmoodi, M. Determination of phthalates in bottled milk by a modified nano adsorbent: Presence, effects of fat and storage time, and implications for human health. Microchem. J. 2020, 159, 105516–105525. [Google Scholar] [CrossRef]
- Korkmaz, S.D.; Küplülü, Ö. Determination of phthalates in some milk products by liquid chromatography/tandem mass spectrometry. Ankara Univ. Vet. Fak. Derg. 2019, 66, 231–236. [Google Scholar] [CrossRef]
- Pereira, J.; Selbourne, M.d.C.; Poças, F. Determination of phthalates in olive oil from European market. Food Control 2019, 98, 54–60. [Google Scholar] [CrossRef]
- Kıralan, S.S.; Toptancı, İ.; Öncül Abacıgil, T.; Ramadan, M.F. Phthalates levels in olive oils and olive pomace oils marketed in Turkey. Food Addit. Contam. Part A 2020, 37, 1332–1338. [Google Scholar] [CrossRef]
- Aghaziarati, M.; Yamini, Y.; Shamsayei, M. An electrodeposited terephthalic acid-layered double hydroxide (Cu-Cr) nanosheet coating for in-tube solid-phase microextraction of phthalate esters. Microchim. Acta 2020, 187, 1–10. [Google Scholar] [CrossRef]
- Mirzajani, R.; Kardani, F.; Ramezani, Z. Fabrication of UMCM-1 based monolithic and hollow fiber—Metal-organic framework deep eutectic solvents/molecularly imprinted polymers and their use in solid phase microextraction of phthalate esters in yogurt, water and edible oil by GC-FID. Food Chem. 2020, 314, 126179. [Google Scholar] [CrossRef]
- Kingsley, O.; Witthayawirasak, B. Deterministic assessment of the risk of phthalate esters in sediments of U-Tapao Canal, Southern Thailand. Toxics 2020, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.H.; Lü, H.; Li, H.; Li, Y.W.; Mo, C.H.; Cai, Q.Y. Persistent contamination of polycyclic aromatic hydrocarbons (PAHs) and phthalates linked to the shift of microbial function in urban river sediments. J. Hazard. Mater. 2021, 414, 125416–125428. [Google Scholar] [CrossRef] [PubMed]
- Diepenheim, G.; Gift, S.C.; Harb, C.; Wallace, M.; Layshock, J. Survey of Phthalate Mitigation and Distribution in Water, Sediment, and Typha in a Fully Operational Constructed Wetland: A Pilot Study. Bull. Environ. Contam. Toxicol. 2020, 105, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhao, D.; Chi, J. Impact of different environmental particles on degradation of dibutyl phthalate in coastal sediments with and without Cylindrotheca closterium. Environ. Pollut. 2020, 261, 114228–114237. [Google Scholar] [CrossRef]
- Zhao, X.; Jin, H.; Li, D.; Kaw, H.Y.; Cui, M.; Ji, Z. Simple and rapid analysis of phthalate esters in marine sediment using ultrasound-assisted extraction combined with gas purge microsyringe extraction followed by GC–MS. Mar. Pollut. Bull. 2020, 160, 111667–111674. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, S.; Lim, J.E.; Moon, H.B. Occurrence and emission of phthalates and non-phthalate plasticizers in sludge from wastewater treatment plants in Korea. Sci. Total Environ. 2019, 692, 354–360. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Q.; He, W.; Xu, F. The occurrence, composition and partitioning of phthalate esters (PAEs) in the water-suspended particulate matter (SPM) system of Lake Chaohu, China. Sci. Total Environ. 2019, 661, 285–293. [Google Scholar] [CrossRef]
- He, M.-J.; Lu, J.-F.; Wang, J.; Wei, S.-Q.; Hageman, K.J. Phthalate esters in biota, air and water in an agricultural area of western China, with emphasis on bioaccumulation and human exposure. Sci. Total Environ. 2019, 698, 134264–134273. [Google Scholar] [CrossRef]
- Rodríguez-Ramos, R.; Socas-Rodríguez, B.; Santana-Mayor, Á.; Rodríguez-Delgado, M.Á. A simple, fast and easy methodology for the monitoring of plastic migrants in alcoholic and non-alcoholic beverages using the QuEChERS method prior to gas chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2020, 412, 1551–1561. [Google Scholar] [CrossRef]
- Dong, W.; Guo, R.; Sun, X.; Li, H.; Zhao, M.; Zheng, F.; Sun, J.; Huang, M.; Wu, J. Assessment of phthalate ester residues and distribution patterns in Baijiu raw materials and Baijiu. Food Chem. 2019, 283, 508–516. [Google Scholar] [CrossRef]
- Xiang, W.; Gong, Q.; Xu, J.; Li, K.; Yu, F.; Chen, T.; Qin, S.; Li, C.; Wang, F. Cumulative risk assessment of phthalates in edible vegetable oil consumed by Chinese residents. J. Sci. Food Agric. 2020, 100, 1124–1131. [Google Scholar] [CrossRef]
- Notardonato, I.; Protano, C.; Vitali, M.; Bhattacharya, B.; Avino, P. A method validation for simultaneous determination of phthalates and bisphenol A released from plastic water containers. Appl. Sci. 2019, 9, 2945. [Google Scholar] [CrossRef] [Green Version]
- Ibarra, V.G.; de Quirós, A.R.B.; Losada, P.P.; Sendón, R. Non-target analysis of intentionally and non intentionally added substances from plastic packaging materials and their migration into food simulants. Food Packag. Shelf Life 2019, 21, 100325. [Google Scholar] [CrossRef]
- He, M.; Yang, C.; Geng, R.; Zhao, X.; Hong, L.; Piao, X.; Chen, T.; Quinto, M.; Li, D. Monitoring of phthalates in foodstuffs using gas purge microsyringe extraction coupled with GC-MS. Anal. Chim. Acta 2015, 879, 63–68. [Google Scholar] [CrossRef]
- Rodríguez-Ramos, R.; Socas-Rodríguez, B.; Santana-Mayor, Á.; Salazar-Carballo, P.Á.; Rodríguez-Delgado, M.Á. Sustainable polypyrrole-based magnetic-microextraction of phthalates from jellies and apple-based beverages prior to tandem mass spectrometry analysis. J. Chromatogr. A 2021, 1637, 461858–461872. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-Y.; Ho, C.-H.; Chang, H.-Y.; Yang, W.-C.; Lin, C.-F.; Lin, C.-T.; Xue, Y.-J.; Lai, J.-M.; Wang, J.-H.; Chang, G.-R. Analysis of pollution of phthalates in pork and chicken in Taiwan using liquid chromatography–tandem mass spectrometry and assessment of health risk. Molecules 2019, 24, 3817. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Song, Z.; Liu, Y.; Gao, M. Polystyrene particles combined with di-butyl phthalate cause significant decrease in photosynthesis and red lettuce quality. Environ. Pollut. 2021, 278, 116871–116880. [Google Scholar] [CrossRef]
- Hidalgo-Serrano, M.; Borrull, F.; Marcé, R.M.; Pocurull, E. Simple method for determining phthalate diesters and their metabolites in seafood species using QuEChERS extraction and liquid chromatography-high resolution mass spectrometry. Food Chem. 2021, 336, 127722. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Chen, T.; Xu, X. Simultaneous Determination of 16 Phthalate Esters in Suet Oil by GC–EIMS Coupled with Refrigerant Centrifugation and Ethylenediamine-N-propylsilane Depuration. Chromatographia 2019, 82, 1721–1732. [Google Scholar] [CrossRef]
- Santana-Mayor, Á.; Herrera-Herrera, A.V.; Rodríguez-Ramos, R.; Socas-Rodríguez, B.; Rodríguez-Delgado, M.Á. Development of a Green Alternative Vortex-Assisted Dispersive Liquid-Liquid Microextraction Based on Natural Hydrophobic Deep Eutectic Solvents for the Analysis of Phthalate Esters in Soft Drinks. ACS Sustain. Chem. Eng. 2021, 9, 2161–2170. [Google Scholar] [CrossRef]
- Li, Y.; Huang, G.; Zhang, L.; Gu, H.; Lou, C.; Zhang, H.; Liu, H. Phthalate esters (PAEs) in soil and vegetables in solar greenhouses irrigated with reclaimed water. Environ. Sci. Pollut. Res. 2020, 27, 22658–22669. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Song, N.; Wang, D.; Ma, J.; Jia, Q. A modulation approach for covalent organic frameworks: Application to solid phase microextraction of phthalate esters. Talanta 2019, 198, 277–283. [Google Scholar] [CrossRef]
- Xue, S.; Wang, Y.; Wu, D.; Shen, J.; Wei, Y.; Wang, C. Core-shell structured magnetic mesoporous carbon nanospheres derived from metal-polyphenol coordination polymer-coated Fe 3 O 4 and its application in the enrichment of phthalates from water samples. J. Sep. Sci. 2019, 42, 3512–3520. [Google Scholar] [CrossRef] [PubMed]
- Notardonato, I.; Russo, M.V.; Avino, P. Phthalates and bisphenol-A residues in water samples: An innovative analytical approach. Rend. Lincei 2018, 29, 831–840. [Google Scholar] [CrossRef]
- Haji Harunarashid, N.Z.I.; Lim, L.H.; Harunsani, M.H. Phthalate Sample Preparation Methods and Analysis in Food and Food Packaging: A Review. Food Anal. Methods 2017, 10, 3790–3814. [Google Scholar] [CrossRef]
- Reid, A.M.; Brougham, C.A.; Fogarty, A.M.; Roche, J.J. An investigation into possible sources of phthalate contamination in the environmental analytical laboratory. Int. J. Environ. Anal. Chem. 2007, 87, 125–133. [Google Scholar] [CrossRef]
- Tankiewicz, M.; Olkowska, E.; Berg, A.; Wolska, L. Advancement in determination of phthalate metabolites by gas chromatography eliminating derivatization step. Front. Chem. 2020, 7, 928–941. [Google Scholar] [CrossRef] [Green Version]
- Fernández-González, V.; Moscoso-Pérez, C.; Muniategui-Lorenzo, S.; López-Mahía, P.; Prada-Rodríguez, D. Reliable, rapid and simple method for the analysis of phthalates in sediments by ultrasonic solvent extraction followed by head space-solid phase microextraction gas chromatography mass spectrometry determination. Talanta 2017, 162, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Tienpont, B. Determination of Phthalates in Environmental, Food and Biomatrices-An Analytical Challenge. Ph.D. Thesis, Ghent University, Faculty of Sciences, Ghent, Belgium, 2004. [Google Scholar]
- Fankhauser-Noti, A.; Grob, K. Blank problems in trace analysis of diethylhexyl and dibutyl phthalate: Investigation of the sources, tips and tricks. Anal. Chim. Acta 2007, 582, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Fernandes, J.O.; Cunha, S.C. A novel dispersive liquid-liquid microextraction (DLLME) gas chromatography-mass spectrometry (GC-MS) method for the determination of eighteen biogenic amines in beer. Food Control 2012, 25, 380–388. [Google Scholar] [CrossRef]
- González-Sálamo, J.; Socas-Rodríguez, B.; Hernández-Borges, J. Analytical methods for the determination of phthalates in food. Curr. Opin. Food Sci. 2018, 22, 122–136. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.M.J.A.M.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS—Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Carlos, K.S.; de Jager, L.S.; Begley, T.H. Determination of phthalate concentrations in paper-based fast food packaging available on the U.S. market. Food Addit. Contam. Part A 2021, 38, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Dubocq, F.; Kärrman, A.; Gustavsson, J.; Wang, T. Comprehensive chemical characterization of indoor dust by target, suspect screening and nontarget analysis using LC-HRMS and GC-HRMS. Environ. Pollut. 2021, 276, 116701. [Google Scholar] [CrossRef] [PubMed]


| Phthalate | Chemical Structure | CF a/MW b | Common Uses | Effects | Metabolites c |
|---|---|---|---|---|---|
| Butyl benzyl phthalate (BBP) | 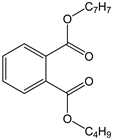 | C19H20O4/ 312.4 g/mol (LMW) d | As a plasticizer for vinyl foams, often used as floor tiles. Traffic cones, food conveyor belts, and artificial leather. | Long-term occupational exposure to BBP increase the risk of multiple myeloma, teratogenicity, and reproductive effects. | Mono benzyl phthalate (MBzP) |
| Di-n-butyl phthalate (DBP) | 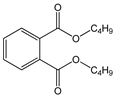 | C16H22O4/ 278.3 g/mol (LMW) | As a plasticizer. Most common phthalate added to nail polish. | Suspected teratogenic and endocrine disruptor | Mono-n-butyl phthalate (MBP); Mono-isobutyl phthalate (MiBP) |
| Di-(2-ethylhexyl) phthalate (DEHP) | 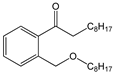 | C24H38O4/ 390.6 g/mol (HMW) e | As plasticizers in medical devices, such as intravenous tubing and bags. | Endocrine disruption in males, through its action as an androgen antagonist. Associated with lower levels of reproductive function in adolescent males. | Mono-(2-ethylhexyl) phthalate (MEHP) |
| Diethyl phthalate (DEP) | 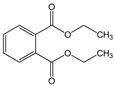 | C12H14O4/ 222.2 g/mol (LMW) | Personal care products to enhance fragrances. | Repeated administration of DEP results in loss of germ cell populations in the testis. | Monoethyl phthalate (MEP) |
| Di-isodecyl phthalate (DiDP) | 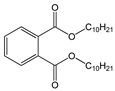 | C28H46O4/ 446.7 g/mol (HMW) | Production of plastic and plastic coating. | Reproductive toxicity. | - |
| Di-isononyl phthalate (DiNP) | 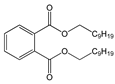 | C26H42O4/ 418.6 g/mol (HMW) | Plasticizer. Added as a softener in the manufacture of toys and childcare products. | High concentrations of DiNP in zebrafish disrupt the endocannabinoid system (ECS) and affect reproduction. Upregulates orexigenic signals and causes hepatosteatosis together with deregulation of the peripheral ECS and lipid metabolism. | - |
| Target Analytes | Matrices (Amount) | Extraction Technique (Conditions) | Analytical Tool/Column | Method Performance | Ref. | |
|---|---|---|---|---|---|---|
| Environmental | ||||||
| DMP, DEP, DBP, and DEHP | Mine tailings (5 g) | ASE (2 × DCM) | GC-MS/HP-5MS (30 m × 0.25 mm i.d. × 0.25 µm) | LOD (µg/kg) | 1.2–2 | [67] |
| LOQ (µg/kg) | 3.0–4.6 | |||||
| RSD (%) | <7 | |||||
| Rec. (%) | 71–115 | |||||
| DMP, DEP, DiPrP, DnPrP, DiBP, DBP, DPP, DiHP, BBP, DCHP, DPhP, DEHP, DOP, and DDP | Seawater (2 L) | LLE (2 × 40 mL DCM) | GC-MS/HP-5MS (30 m × 0.25 mm i.d. × 0.25 µm) | LOD (ng/mL) | 0.07–0.32 | [64] |
| LOQ | - | |||||
| RSD (%) | <10 | |||||
| Rec. (%) | 93–97 | |||||
| DMP, DEP, DiPrP, DBP, DiBP, BBP, DPhP, DCHP, DHepP, and DEHP | Seawater (500 mL), Sediments (5 g), Seagrass (0.2 g), and Fish (0.2 g) | LLE (30 mL HEX:ACET, 1:1 v/v), SPE (5 g Floridil, and 60 mL Et2O:HEX, 1:1 v/v) | GC-MS/SPB-5MS (30 m × 0.25 mm i.d. × 0.25 µm) | LOD (ng/Kg) | 5–763 | [63] |
| LOQ | - | |||||
| RSD (%) | <10 | |||||
| Rec. (%) | 79–110 | |||||
| DMP, DEP, DAIP, DiPrP, DnPrP, DiBP, DnBP, DnPeP, BBzP, DCHP, DnHxP, DiHpP, DEHP, DnOP, DiNP, and DiDP | Sediments (5 g) | LLE (3 × DCM), SPE (clean-up, EtAc) | GC-MS/DB-5MS (-) | LOD | - | [62] |
| LOQ (ng/g) | 0.002–3.92 | |||||
| RSD (%) | - | |||||
| Rec. (%) | 74–98 | |||||
| DMP, DEP, DiBP, DBP, BMPP, DMEP, DNPP, DEEP, DNHP, BBP, DEHP, DBEP, DCHP, DnOP, and DNP | Sediments (2 g) | LLE (10 mL HEX:EtAc, 1:1 v/v) | GC-MS/MS/HP-35MS (30 m × 0.25 mm i.d. × 0.25 μm) | LOD (ng/mL) | 0.14–0.88 | [71] |
| LOQ | - | |||||
| RSD (%) | <15 | |||||
| Rec. (%) | 71–102 | |||||
| DBP, BBP, DEHP, DnOP, DiNP, and DiDP | Sediments (5.0 g) | LLE (2 × 10 mL ACET:HEX) | GC-MS/HP-5MS (30 m × 0.25 mm i.d. × 0.25 µm) | LOD (ng/mL) | 0.12–1.04 | [94] |
| LOQ (ng/mL) | 1.78–2.98 | |||||
| RSD (%) | <9 | |||||
| Rec. (%) | 81–105 | |||||
| DMP, DEP, DBP, BBP, DEHP, and DOP | Sediments (2 g) | LLE (DCM:ACET, 7:3 v/v) | GC-MS/HP-5MS (30 m × 0.25 mm i.d. × 0.25 µm) | LOD (µg/L) | 1.25–9.43 | [95] |
| LOQ (µg/L) | 4.17–31.4 | |||||
| RSD (%) | - | |||||
| Rec. (%) | 72–99 | |||||
| DMP, DEP, DiBP, DBP, DMEP, DNPP, DeoEP, DNHP, DBEP, BBzP, DMPP, DEHP, DCHO, Dnop, and DnNP | Sediments (5 g) and plants (5 g) | LLE (2 × HEX:ACET, 1:1 v/v) clean-up SPE (500 mg Florisil, ACET:HEX 1:4 v/v) | GC-MS/SHR5XLB (30 m × 0.25 mm i.d. × 0.25 µm) | LOD (ppb) | - | [96] |
| LOQ (ppb) | 2.8–21.2 | |||||
| RSD (%) | - | |||||
| Rec. (%) | 79–137 | |||||
| DBP | Sediments (2 g) | UAE (3 × 45 mL DCM) | GC-FID/HP-5 (30 m × 0.25 mm i.d. × 0.25 µm) | LOD | - | [97] |
| LOQ (ng/g) | - | |||||
| RSD (%) | - | |||||
| Rec. (%) | - | |||||
| DMP, DEP, DiBP, DBP, DMEP, BMPP, DEEP, DPP, DnHP, BBP, DBEP, DCHP, DEHP, DPhP, DnOP, and DiNP | Sediments (0.5 g) | UAE (1 × 2 mL DCM) | GC-MS/DB-5 (30 m × 0.25 mm i.d. × 0.25 µm) | LOD (pg/g) | 3–5 | [98] |
| LOQ | - | |||||
| RSD (%) | <10 | |||||
| Rec. (%) | 84–119 | |||||
| DMP, DEP, DBP, DEHP, and DnOP | Sediments (3 g) | Microwave (110 °C), clean-up (2 × 5 mL HEX:TOL 4:1 v/v and 5 mL EtAC) | GC-FID/DB-5 (30 m × 0.32 mm × 0.25 µm) | LOD (µg/g) | 0.015 | [65] |
| LOQ | - | |||||
| RSD (%) | - | |||||
| Rec. (%) | 85–103 | |||||
| DMP, DEP, DBP, BBP, DEHP, and DnOP | Sediments (20 g) and Water (500 mL) | LLE (30 mL DCM) | GC-MS/DB-5MS (30 m × 0.25 mm × 0.25 µm) | LOD (ng/mL) | [66] | |
| LOQ (ng/mL) | 0.60–0.80 | |||||
| RSD (%) | <10 | |||||
| Rec. (%) | 77–110 | |||||
| DMP, DEP, DAIP, DiPrP, DnPrP, DiBP, DBP, DnPeP, BBzP, DCHP, DnHxP, DiHpP, DEHP, DnOP, DiNP, and DiDP | Sludge (0.1 g) | LLE (10 mL DCM), clean-up (SPE, 8 mL EtAC) | GC-MS/MS/DB-5MS (30 m × 0.25 mm i.d. × 0.25 μm) | LOD (ng/g) | - | [99] |
| LOQ (ng/g) | 0.093–196 | |||||
| RSD (%) | <21 | |||||
| Rec. (%) | 68–103 | |||||
| DMEP, DPP, DBP, DCHP, DnOP, DiNP, and DiDP | Soil (1 g) | MSPD (30 mg MOF, 5 mL MeCN) | UHPLC-MS/MS/BEH C18 (50 mm × 2.1 mm i.d. × 1.7 µm) | LOD (µg/kg) | 0.042–0.80 | [72] |
| LOQ (µg/kg) | 0.14–2.7 | |||||
| RSD (%) | <20 | |||||
| Rec. (%) | 70–115 | |||||
| DMP, DEP, DiBP, DBP, DMGP, DEEP, DCHP, DMPP, BBP, DNHP, HEHP, DBEP, DEHP, and DnOP | Soil (1 g) | ASE-in-line clean-up (MeOH 0.01% FA) | UHPLC-MS/MS/BEH Phenyl (100 mm × 2.1 mm i.d. × 1.7 µm) | LOD (ng/g) | 0.59–10.08 | [73] |
| LOQ (ng/g) | 0.93–17.20 | |||||
| RSD (%) | <15 | |||||
| Rec. (%) | 69–131 | |||||
| DMP, DEP, DBP, BBP, DEHP, and DnOP | Soil (5 g) and Vegetables (1 g) | LLE (20 mL HEX:DCM, 1:1 v/v) | GC-MS/DB-5 MS (30 m × 0.25 mm i.d. × 0.25 μm) | LOD (ng/g) | 0.1–0.5 | [74] |
| LOQ | - | |||||
| RSD (%) | - | |||||
| Rec. (%) | 73–105 | |||||
| DMP, DEP, DBP, BBP, DEHP, and DnOP | Surface water (500 mL) | SPE (2 mL MeOH, 5 mL EtAC) | GC-MS/DB-5MS (30 m × 0.25 mm i.d. × 0.25 μm) | LOD (ng/L) | 0.61–2.96 | [75] |
| LOQ | - | |||||
| RSD (%) | <9 | |||||
| Rec. (%) | 84–101 | |||||
| DMP, DEP, DAP, DMEP, BBP, DIBP, DBP, DBEP, DPP, DcHP, DHP, DHpP, DEHP, DiNP, DiDPP, DPHP, and DiUP | SPM (1 g) | LLE (15 mL ACET:DCM:HEX, 20:20:60 v/v/v; 15 mL HEX/ACET 30/70 v/v) | LC-MS/HSS T3 (75 mm × 2.1 mm i.d. × 1.7 μm) | LOD (ng/g) | 0.33–43 | [76] |
| LOQ (ng/g) | 1–130 | |||||
| RSD (%) | <20 | |||||
| Rec. (%) | 91–117 | |||||
| DMP, DEP, DiBP, DBP, BBP, and DEHP | Water (1L) and SPM (2 L) | Soxhlet (40 mL HEX:ACE, 8:2 v/v) | GC-MS/DB-5MS (30 m × 0.25 mm i.d. × 0.25 μm) | LOD (µg/g) | 0.1–0.5 | [100] |
| LOQ (µg/g) | - | |||||
| RSD (%) | - | |||||
| Rec. (%) | 71–106 | |||||
| DMP, DEP, DiBP, DBP, DEHP, and DOP | Water (1 mL), SPM (1 g), and Sediments (1 g) | SPE (500 mg C18, 10 mL MeOH/DCM) | GC-MS/DB-5MS (30 m × 0.25 mm i.d. × 0.25 µm) | LOD (ng/L) | 0.54–12.36 | [68] |
| LOQ (ng/L) | - | |||||
| RSD (%) | <11 | |||||
| Rec. (%) | 81–112 | |||||
| Foods | ||||||
| DMP, DEP, DBP, BBP, DEHP, and DnOP | Acidic juice (5 mL) | LLE (20 mL ACET:HEX, 1:1 v/v) | GC-MS (-) | LOD (ng/L) | 0.001–0.002 | [77] |
| LOQ (ng/L) | 0.004–0.008 | |||||
| RSD (%) | - | |||||
| Rec. (%) | 72–111 | |||||
| DEP, DMP, BBP, DBP, DiBP, DnOP, and DEHP | Animal tissue (1 g), Vegetable powders (5 g), and Water (0.5 L) | Soxhlet (ACET:HEX, 1:1 v/v) and SPE (15 mL EtAC) | UPLC-TOF-MS/BEH C18 column (100 mm × 2.1 mm i.d. × 1.7 μm) | LOD (ng/mL) | 0.03–0.14 | [101] |
| LOQ (ng/mL) | 0.1–0.50 | |||||
| RSD (%) | - | |||||
| Rec. (%) | 60–120 | |||||
| DMP, DEP, DBP, iBcEP, BBP, and DEHP | Baby foods (0.1–0.2 g) | UVA-DLLME (250 µL heptane, 0.1 g NaCl) | GC-MS/SE-54 (30 m × 0.25 mm i.d. × 0.25 μm) | LOD (ng/g) | 0.4–4.4 | [78] |
| LOQ (ng/g) | 2.3–7.5 | |||||
| RSD (%) | <10 | |||||
| Rec. (%) | 91–110 | |||||
| BBP, DBEP, DBP, DCHP, DEEP, DEP, DiDP, DiNP, DiPP, DMEP, DMP, DnOP, DnPP, DPP, and DEHA | Baby foods (10 g) | QuEChERS-dSPE (4 g MgSO4, 1 g NaCl, 10 mL MeCN) clean-up dSPE (1.2 g MgSO4, 200 mg PSA) | GC-MS/MS/HP-5MS (15 m × 0.25 mm i.d. × 0.25 μm) | LOD (µg/kg) | [79] | |
| LOQ (µg/kg) | 0.03–1.11 | |||||
| RSD (%) | <19 | |||||
| Rec. (%) | 70–120 | |||||
| DMP, DEP, DiBP, DBP, DMEP, BMPP, DEEP, DPP, DHXP, BBP, DBEP, DCHP, DEHP, DPhP, and DnOP | Beverages (30 mL) | MSPE (COF-(TpBD)/Fe3O4) | GC-MS/MS/Rxi-5MS (30m × 0.25 μm i.d. × 0.5 μm) | LOD (µg /L) | 0.005–2.748 | [80] |
| LOQ (µg /L) | 0.018–9.15 | |||||
| RSD (%) | <10 | |||||
| Rec. (%) | 80–120 | |||||
| DPP, DMEP, DCHP, DnOP, DiNP, DiDP, DiPP, DEEP, DnPP, BBP, DEHA, and DBEP | Beverages (10 mL) | QuEChERS (4 g MgSO4, 1 g NaCl, 10 mL MeCN) and clean-up dSPE (1.2 g MgSO4, 200 mg PSA) | GC-MS/MS/HP-5MS (15 m × 0.25 mm i.d. × 0.25 μm) | LOD (µg /mL) | - | [102] |
| LOQ (µg /mL) | 0.034–1.415 | |||||
| RSD (%) | <20 | |||||
| Rec. (%) | 75–120 | |||||
| DMP, DEP, DiBP, DBP, DEHP, and DOP | Beverages plastic containers (10 mL) | DLLME (40 µL HEX) | GC-MS/SE-54 (30 m × 0.25 mm i.d. × 0.25 μm) | LOD (ng/mL) | 0.1–1.2 | [61] |
| LOQ (ng/mL) | 2.1–4.9 | |||||
| RSD (%) | <13 | |||||
| Rec. (%) | 76–102 | |||||
| DPRP, DEP, DBP, DiBP, DPP, DMEP, BBP, DnHP, DEHP, and DnOP | Bottled water (4 mL) | HF-SPME (PSF fiber) | FE-GC-FID /DB-5 (30 m × 0.25 mm i.d. × 0.25 µm) | LOD (µg/L) | 0.001–0.130 | [81] |
| LOQ | - | |||||
| RSD (%) | <10 | |||||
| Rec. (%) | 87–118 | |||||
| DEHP, BBP, DBP, DEP, DMP, and DnOP | Bottled water (2 L) and Tap water (2 L) | MSPE (C18, 3 mL MeOH:DCM, 1:1 v/v) | GC-FID/CP-Sil 8 CB (30 m × 0.32 mm i.d. × 0.25 µm) | LOD (ng/L) | 17–31 | [59] |
| LOQ | - | |||||
| RSD (%) | <20 | |||||
| Rec. (%) | 98–102 | |||||
| DMP, DEP, DPrP, DiBP, DBP, DMEP, BMPP, DEEP, DPP, DHP, BBP, DCHP, DEHP, and DnOP | Brands (5 mL), Rice (0.5 g), Wheat (0.5 g), and Sorghum (0.5 g) | VSLLME (500 µL C2Cl4, 125 µL Tween-20) QuEChERS-dSPE (0.32 g NaCl, 0.70g MgSO4, 2 mL MeCN) | GC-MS/TG-5MS (30m × 0.25 μm × 0.25 μm) | LOD (µg/L) | 0.05–2.50 | [103] |
| LOQ (µg/L) | 0.125–5.00 | |||||
| RSD (%) | <10 | |||||
| Rec. (%) | 85–121 | |||||
| DEHP and DBP | Edible vegetable oil (0.5 g) | LLE (2 × 2 mL MeCN + 100 µL Hex) and clean-up SPE (5 mL MeCN) | GC-MS/Rtx-5MS (30 m × 0.25 mm i.d. × 0.25 μm) | LOD (ng/mL) | - | [104] |
| LOQ | - | |||||
| RSD (%) | - | |||||
| Rec. (%) | - | |||||
| DMP, DEP, DBP, BBzP, and DEHP | Fish fillets (2 g) | SPME (C18 fibers)/UASE (Acet:HEX 1:1, v/v) | LC-MS/MS/Accucore C-18 aQ (100 mm × 2.1 mm i.d. × 2.6 mm) | LOD (µg/kg) | 0.1–0.5 | [82] |
| LOQ (µg/kg) | 0.3–1.5 | |||||
| RSD (%) | <24 | |||||
| Rec. (%) | - | |||||
| DMP, DEP, DiBP, DBP, DEHP, and DnOP | Food contacted plastics (1 L) | DLLME (200 µL HEX) | GC-MS/TRB-Meta X5 (30 m × 0.25 mm i.d. × 0.25 μm) | LOD (ng/mL) | 1.0–8.0 | [105] |
| LOQ (ng/mL) | 5.0–14 | |||||
| RSD (%) | <10 | |||||
| Rec. (%) | 93–104 | |||||
| DEHP, DEP, DiBP, and DBP | Food contact plastics (2 g) | LLE (20 mL MeCN) | GC-MS/ZB-5MS (30 m × 0.25 mm i.d. × 0.25 μm) | LOD (ng/mL) | 1–13.3 | [106] |
| LOQ (ng/mL) | 2.5–36.3 | |||||
| RSD (%) | <16 | |||||
| Rec. (%) | 83–116 | |||||
| DnPP, DAP, BBP, and DOP | Food contact plastics (0.8 g) | VALLME (80 µL DES) | GC-MS/HP-5MS (30 m × 0.25 mm i.d. × 0.25 µm) | LOD (µg/L) | 1 | [83] |
| LOQ (µg/L) | 5 | |||||
| RSD (%) | <6 | |||||
| Rec. (%) | 86–103 | |||||
| DBP, BBP, BDE, and DOP | Food contact plastics (2 mL) | SPME (0.2 g NaCl, PDMS/DVB) | GC-MS/HP-5 (60 m × 0.25 mm i.d. × 0.25 μm) | LOD (µg/L) | 0.03–0.08 | [60] |
| LOQ (µg/L) | 0.10–0.24 | |||||
| RSD (%) | <13 | |||||
| Rec. (%) | 90–111 | |||||
| DMP, DEP, DBP, BBP, DEHP, and DnOP | Foodstuffs (1 g for solids, 200 mL liquids) | UAE (DCM, 30 min), clean-up with GP-MSE (10 µL DCM, 2 min, 280 °C) | GC-MS/DB-5 (30 m × 0.25 mm i.d. × 0.25 μm) | LOD (ng/g) solid | 0.14–0.38 | [107] |
| LOD (ng/L) liquid | 2.1–9.6 | |||||
| RSD (%) | <10 | |||||
| Rec. (%) | 86–103 | |||||
| DMP, DBP, DEP, DPeP, DPP, DEHP, DiPP, DnOP, DPhP, DiNP, BBP, and DiDP | Grape marc spirit (-) | UHPLC-MS/MS/U-VDSpher PUR 100 C18-E (100 mm × 2.0 mm i.d. × 1.8 µm) | LOD (µg/L) | 0.3–33.3 | [84] | |
| LOQ (µg/L) | 1.0–100 | |||||
| RSD (%) | <10 | |||||
| Rec. (%) | 82–110 | |||||
| DMP, DBP, BBP, and DEHP | Herbal beverages (10 mL) and Water (10 mL) | UA-D-SPE (5 mg hybrid nanocomposite) | GC-MS/HP-5MS (30 m × 0.25 mm i.d. × 0.25 µm) | LOD (ng/mL) | 0.06–0.3 | [85] |
| LOQ (ng/mL) | 0.20–1.00 | |||||
| RSD (%) | <12 | |||||
| Rec. (%) | 55–113 | |||||
| DMP, DEP, DiBP, DBP, DEHP, and DNOP | Honey (2.5 g) | UVA-DLLME (75 µL benzene, NaCl 10 g/L) | GC-MS/TRB-5MS (30 m × 0.25 mm i.d. × 0.25 μm) | LOD (ng/g) | 3.0–13 | [86] |
| LOQ (ng/g) | 7.0–22 | |||||
| RSD (%) | <10 | |||||
| Rec. (%) | 71–10 | |||||
| DMP, DEP, DiBP, DBP, DEHP, and DnOP | Honey (2.5 g) | UVA-DLLME (150 µL TOL, and NaCl 10 g/L) | GC-MS/ SE-54 (30 m × 0.25 mm i.d. × 0.25 μm) | LOD (ng/g) | 2.0–6.0 | [87] |
| LOQ (ng/g) | 7.0–11 | |||||
| RSD (%) | <4 | |||||
| Rec. (%) | 86–117 | |||||
| BBP, DAP, DBEP, DCHP, DEEP, DiDP, DiNP, DiPP, DNOP, DNPP, and DPP | Jellies (25 mL) and Apple-based beverages (25 mL) | m-µ-dSPE (40 mg Fe3O4@PPy, 2 mL MeCN) | UHPLC-MS/MS/BEH C18 (50 mm × 2.0 mm i.d. × 1.7 µm) | LOD (µg/L) | - | [108] |
| LOQ (µg/L) | 0.15–0.42 | |||||
| RSD (%) | <20 | |||||
| Rec. (%) | 60–114 | |||||
| DMP, DEP, DBP, DEHP, and DnOP | Milk (10 mL) | QuEChERS-dSPE (0.01 g MWCNT-Fe3O4 and 0.5 g NaCl, 5 mL MeCN) | GC-MS/HP-5MS (30 m × 0.25 mm i.d. × 0.25 µm) | LOD (ng/L) | 1.2–19 | [88] |
| LOQ (ng/L) | 3.3–63 | |||||
| RSD (%) | <7 | |||||
| Rec. (%) | 82–112 | |||||
| DBP, DEHP, BBP, DiNP, DNOP, and DiDP | Milk products (2 g) | LLE (2mL MeOH, 2 mL HEX, 2 mL TBME) | LC-MS/MS/Zorbax SB-C18 (50 m × 2.1 mm i.d. × 1.8 µm) | LOD (µg/kg) | 6.0–9.0 | [89] |
| LOQ (µg/kg) | 20–30 | |||||
| RSD (%) | <20 | |||||
| Rec. (%) | 84–96 | |||||
| DBP, BBP, DEHP, DiNP, and DiDP | Olive oil (1 g) | LLE (10 mL MeCN) | GC-MS/MS/Rxi-5MS (30 m × 0.25 μm i.d. × 0.25 μm) | LOD (ng/mL) | 7–130 | [90] |
| LOQ | 23–420 | |||||
| RSD (%) | <4 | |||||
| Rec. (%) | 90–108 | |||||
| DBP, BBP, DEHP, DiDP, and DiNP | Olive oil (1 g) | LLE (10 mL MeCN) | GC-MS/HP-5MS (30 m × 0.25 mm i.d. × 0.25 μm) | LOD (mg/kg) | 0.06–1.97 | [91] |
| LOQ (mg/kg) | 0.09–2.28 | |||||
| RSD (%) | <12 | |||||
| Rec. (%) | 87–100 | |||||
| DEHP, BBP, DiDP, DBP, and DiNP | Pork (0.5 g) and Chicken (0.5 g) | LLE (3 mL PENT:MeOH 1:4 v/v) | LC-MS/MS/BEH C18 (100 m × 2.1 mm i.d. × 1.7 μm) | LOD (ng/g) | - | [109] |
| LOQ (ng/g) | 40 | |||||
| RSD (%) | <10 | |||||
| Rec. (%) | 96–103 | |||||
| DBP | Red lettuce (-) | LLE (20 mL DCM) | HPLC-UV/Venusil C18 (250 mm × 4.6 mm i.d. × 5 µm) | LOD | - | [110] |
| LOQ | - | |||||
| RSD (%) | - | |||||
| Rec. (%) | - | |||||
| MMP, MEP, MBP, MBzP, MEHP, MOP, DMP, DEP, BzBP, DBP, DEHP, and DnOP | Seafood species (1 g) | QuEChERS (4 g MgSO4, 1 g NaCl, 0.5 g SCDE, 1 g SCTD, 10 mL MeCN), and clean-up dSPE (200 mg C18) | LC-HRMS/Ascentis Express C18 (100 mm × 2.1 mm i.d. × 2.7 µm) | LOD (ng/g) | 1–100 | [111] |
| LOQ (ng/g) | 5–250 | |||||
| RSD (%) | <15 | |||||
| Rec. (%) | 13–79 | |||||
| MMP, MEP, DMP, MBP, MBzP, DEP, MEHP, MOP, BzBP, DBP, DEHP, and DOP | Seafood species (1 g) | ASE (MeOH, 80 °C, 10 min, 1500 psi), clean-up SPE (200 mg bond elut plexa and 5 mL MeOH) | LC-HRMS/Ascentis Express C18 (100 mm × 2.1 mm i.d. × 2.7 µm) | LOD (µg/L) | 0.5–25 | [33] |
| LOQ (µg/L) | 1–50 | |||||
| RSD (%) | <25 | |||||
| Rec. (%) | 6–76 | |||||
| DMP, DEP, DiBP, DBP, DMEP, BMPP, DEEP, DPP, DHXP, BBP, DBEP, DCHP, DEHP, DPhP, DnOP, and DNP | Suet Oil (1 g) | LLE (2 × 5 mL MeCN (HEX saturared)) | GC-MS/CD-5MD (30 m × 0.25 mm i.d. × 0.25 μm) | LOD (ng/mL) | 0.10–0.70 | [112] |
| LOQ | 0.33–2.31 | |||||
| RSD (%) | <10 | |||||
| Rec. (%) | 83–106 | |||||
| BBP, DiBP, DnPP, DnOP, DiNP, and DiDP | Tea (10 mL), Apple juice (10 mL), and Pineapple juice (10 mL) | VA-EDLLME (440 µL DES ChCl:phenol 1:2) | LC-DAD-MS/MS/X-BridgeC18 (100 m × 4.6 mm i.d. × 3.5 µm) | LOD (µg/L) | 5.1–17.8 | [113] |
| LOQ (µg/L) | 17.2–59.4 | |||||
| RSD (%) | <20 | |||||
| Rec. (%) | 84–120 | |||||
| DMP, DBP, BBP, DEHP, DnOP, and DEP | Vegetables (2 g) and soil (10 g) | Soxhlet (220 mL MeOH:ACET, 1:1 v/v) | GC-MS/DB-5MS (30 m × 0.25 mm i.d. × 0.25 μm) | LOD (µg/kg) | 0.032–0.191 | [114] |
| LOQ | - | |||||
| RSD (%) | <11 | |||||
| Rec. (%) | 70–120 | |||||
| DEP, DPP, DAP, DBP, BBP, and DEHP | Water (-) | SPME (OH50%-TPB-COF fiber) | GC-FID/HP-5 (50 m × 0.32 mm i.d. × 0.52 µm) | LOD (µg/L) | 0.032–0.451 | [115] |
| LOQ | - | |||||
| RSD (%) | <10 | |||||
| Rec. (%) | 79–100 | |||||
| DEP, DPrP, DiBP, and DCHP | Water (20 mL) | MSPE (20 mg MagC-TA, 500 µL MeCN) | HPLC-UV/InertSustain-C18 (250 m × 4.6 μm i.d. × 5 μm) | LOD (ng/mL) | 0.10–0.62 | [116] |
| LOQ | 0.33–2.06 | |||||
| RSD (%) | - | |||||
| Rec. (%) | 82–118 | |||||
| DEHP, DBP, DiNP, DiDP, and DEP | Water (10 mL) | DLLME (250 µL Heptane, 1 g NaCl) | GC-FID/TRB-Meta X5 (30 m × 0.25 mm i.d. × 0.25 μm) | LOD (ng/mL) | 2.0–19 | [117] |
| LOQ (ng/mL) | 4.0–48 | |||||
| RSD (%) | <10 | |||||
| Rec. (%) | 82–102 | |||||
| DBP, BBP, BDE, and DOP | Wines (2 mL) | SPME (0.2 g NaCl, PDMS/DVB) | GC-MS/HP-5 (60 m × 0.25 μm i.d. × 0.25 μm) | LOD (µg/L) | 0.03–0.11 | [69] |
| LOQ (µg/L) | 0.09–0.36 | |||||
| RSD (%) | <13 | |||||
| Rec. (%) | 80–108 | |||||
| DMP, DBP, DAP, and DEHP | Whisky (10 mL) | IT-SPME (15 % w/v NaCl and TPA/LDH) | HPLC-UV/ODS-3 (250 m × 4.6 μm i.d. × 5 μm) | LOD (µg/L) | 0.01–0.1 | [92] |
| LOQ (µg/L) | 0.03–0.2 | |||||
| RSD (%) | <7 | |||||
| Rec. (%) | 92–112 | |||||
| DMP, DEP, DIBP, and DBP | Yogurt (1 g), Water (1 g), and Edible oil (1 g) | HFLMP-SPME (monolithic fiber, 6 µL n-hexane) | GC-FID/BP-5 (25 m × 0.32 mm i.d. × 0.5 µm) | LOD (µg/L) | 0.01–0.03 | [93] |
| LOQ (µg/L) | 0.03–0.12 | |||||
| RSD (%) | <5 | |||||
| Rec. (%) | 96–100 | |||||
| Extraction Technique | Advantages | Disadvantages | |
|---|---|---|---|
| LLE |
|
| |
| DLLME assisted (UVA-DLLME, VSLLME, VALLME, VS-EDLLME) |
|
| |
| Soxhlet |
|
| |
| ASE |
|
| |
| UAE |
|
| |
| Microwave assisted |
|
| |
| SPE |
|
| |
| MSPE |
|
| |
| MSDP |
|
| |
| SPME |
|
| |
| QuEChERS-dSPE |
|
| |
| Analytical platforms | |||
| GC | FID |
|
|
| EI-MS |
|
| |
| LC | UV |
|
|
| ESI-MS |
|
| |
| HRMS |
|
| |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luís, C.; Algarra, M.; Câmara, J.S.; Perestrelo, R. Comprehensive Insight from Phthalates Occurrence: From Health Outcomes to Emerging Analytical Approaches. Toxics 2021, 9, 157. https://doi.org/10.3390/toxics9070157
Luís C, Algarra M, Câmara JS, Perestrelo R. Comprehensive Insight from Phthalates Occurrence: From Health Outcomes to Emerging Analytical Approaches. Toxics. 2021; 9(7):157. https://doi.org/10.3390/toxics9070157
Chicago/Turabian StyleLuís, Catarina, Manuel Algarra, José S. Câmara, and Rosa Perestrelo. 2021. "Comprehensive Insight from Phthalates Occurrence: From Health Outcomes to Emerging Analytical Approaches" Toxics 9, no. 7: 157. https://doi.org/10.3390/toxics9070157
APA StyleLuís, C., Algarra, M., Câmara, J. S., & Perestrelo, R. (2021). Comprehensive Insight from Phthalates Occurrence: From Health Outcomes to Emerging Analytical Approaches. Toxics, 9(7), 157. https://doi.org/10.3390/toxics9070157