Assessment of the Suitability of Melilotus officinalis for Phytoremediation of Soil Contaminated with Petroleum Hydrocarbons (TPH and PAH), Zn, Pb and Cd Based on Toxicological Tests
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Description
2.2. Biopreparation Characteristics
2.3. Biodegradation of Petroleum Pollutants in Ex-Situ Conditions
2.4. Plant Selection, Experiment Description
2.4.1. Physical and Chemical Tests of Soil and Biomass
2.4.2. Extraction of Analytes from Soil and Biomass, and Methods Used to Determine the Analytes
2.4.3. Calculation of Phytoremediation Process Parameters
2.5. Toxicological Tests of Soil
2.5.1. PhytotoxkitTM
2.5.2. Ostracodtoxkit FTM
2.5.3. Microtox®/DeltaTox
2.5.4. MARA
2.6. Statistical Analysis
3. Results
3.1. Soil Tests
3.2. Evaluation of the Biodegradation Efficiency of TPH and PAH
3.2.1. Evaluation of the Biodegradation of TPH and PAH Based on Chromatographic Analyses
3.2.2. Ecotoxicological Assessment
3.3. Phytoremediation Soil
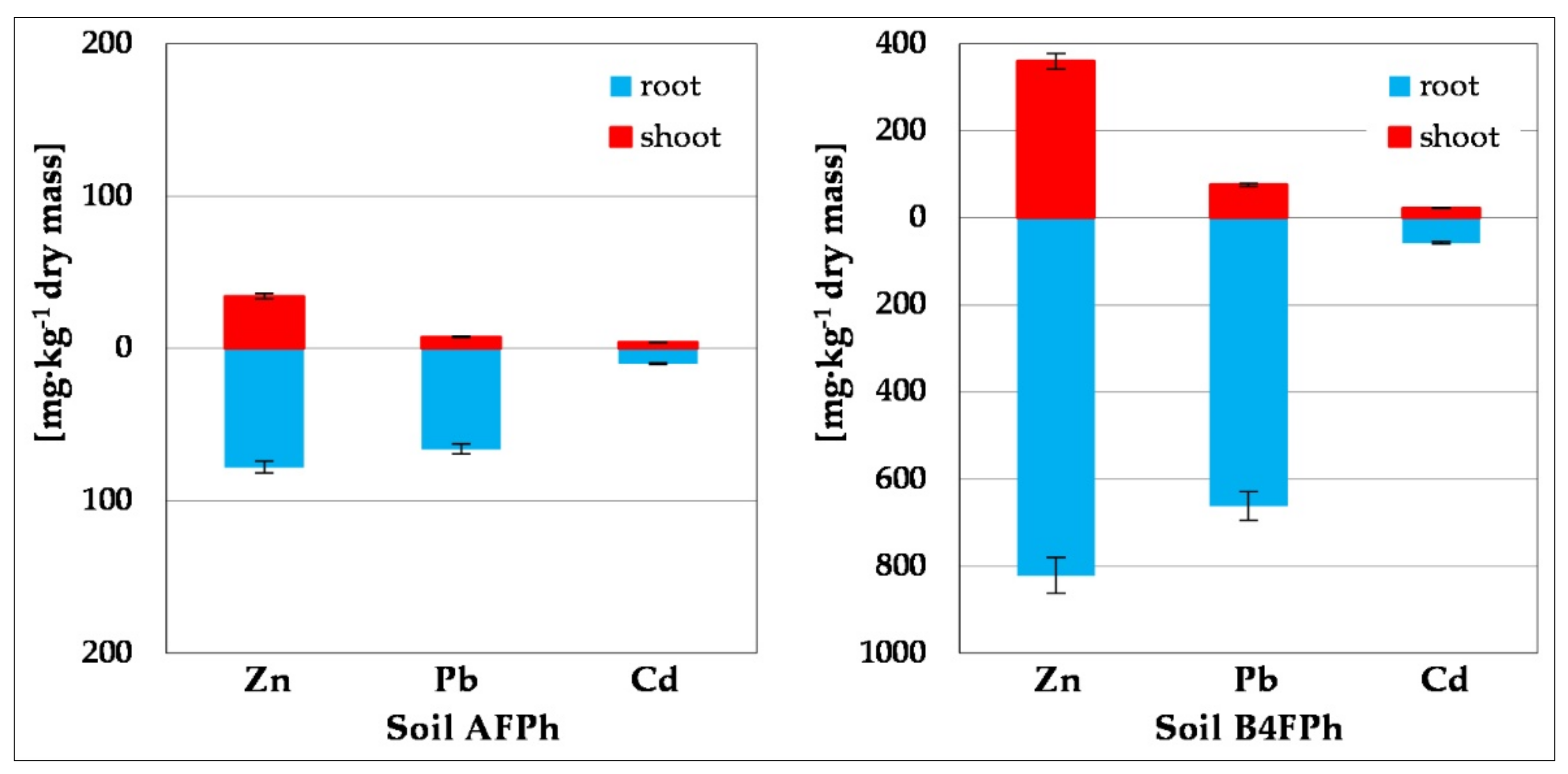
Plant Material Analysis
3.4. Toxicological Tests of Soil
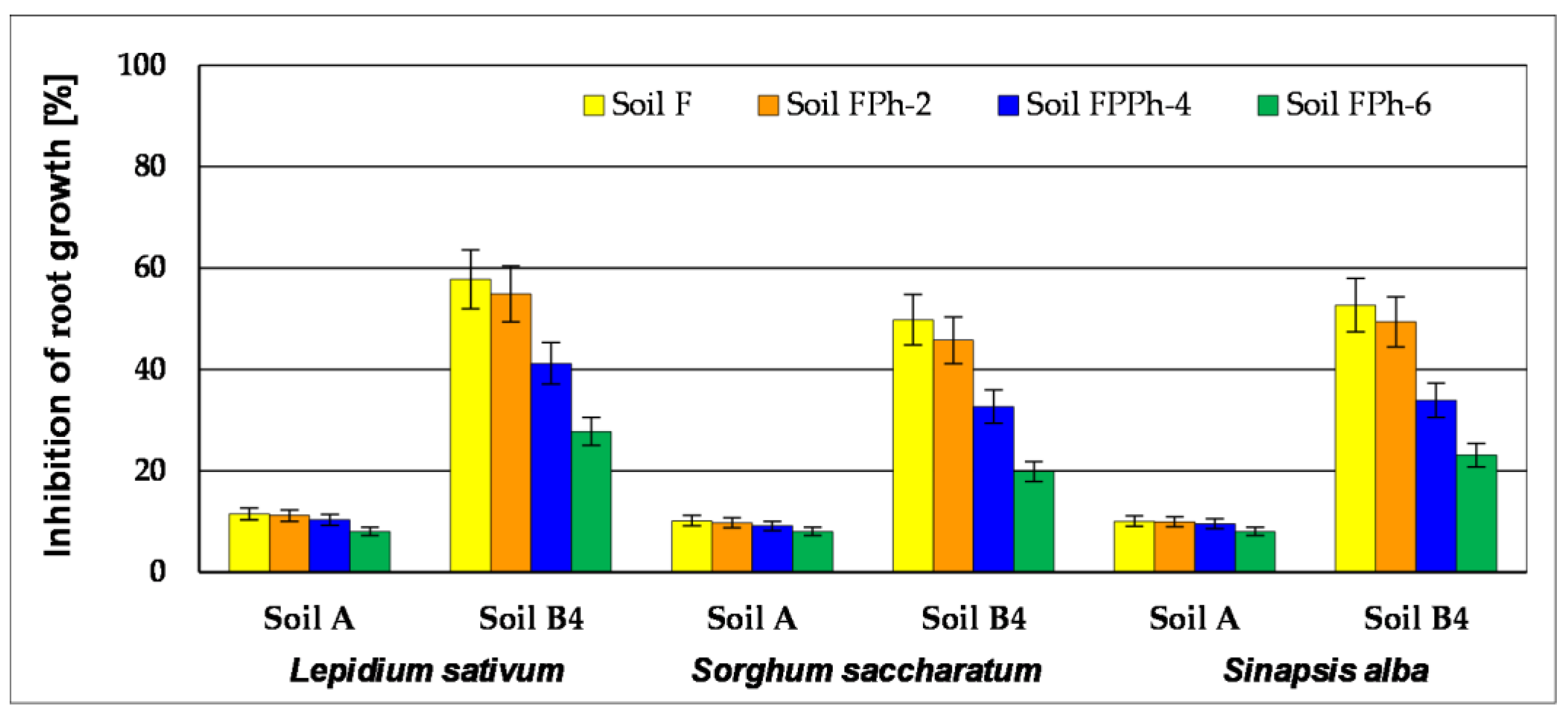
3.4.1. PhytotoxkitTM
3.4.2. Ostracodtoxikit FTM
3.4.3. Microtox®SPT
3.4.4. The MARA Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steliga, T.; Jakubowicz, P.; Kapusta, P. Optimisation research of petroleum hydrocarbon biodegradation in weathered drilling wastes from waste pits. Waste Manag. Res. 2010, 28, 1065–1075. [Google Scholar] [CrossRef]
- Steliga, T.; Jakubowicz, P.; Kapusta, P.; Kluk, D. Study on biodegradation of drilling wastes contaminated with petroleum hydrocarbons. Przemysł Chem. 2018, 97, 1666–1675. [Google Scholar] [CrossRef]
- de Souza, R.B.; Maziviero, T.G.; Christofoletti, C.A.; Pinheiro, T.G.; Fontanetti, C.S. Soil contamination with heavy metals and petroleum derivates: Impact on edaphic fauna and remediation strategies. Soil Process. Curr. Trends Qual. Assess. 2013, 6, 175–203. [Google Scholar] [CrossRef]
- Xi, Y.; Song, Y.; Johnson, D.M.; Li, M.; Liu, H.; Huang, Y. Se enhanced phytoremediation of diesel in soil by Trifolium repens. Ecotoxicol. Environ. Saf. 2018, 154, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Moubasher, H.A.; Hegazy, A.K.; Mohamed, N.H.; Moustafa, Y.M.; Kabiel, H.F.; Hamad, A.A. Phytoremediation of soils polluted with crude petroleum oil using Bassia scoparia and its associated rhizosphere microorganisms. Int. Biodeterior. Biodegrad. 2015, 98, 113–120. [Google Scholar] [CrossRef]
- Steliga, T.; Kapusta, P.; Jakubowicz, P. Changes in toxicity during in situ bioremediation of weathered drill wastes contaminated with petroleum hydrocarbons. Bioresour. Technol. 2012, 125, 1–10. [Google Scholar] [CrossRef]
- Steliga, T.; Kluk, D. Assessment of the composition of pollution of soil contaminated with TPH and PAHs for the development of the bioremediation technology. Prace INiG PIB 2017, 215, 1–211. [Google Scholar] [CrossRef]
- Wu, M.; Dick, W.A.; Li, W.; Wang, X.; Yang, Q.; Wang, T.; Xu, L.; Zhang, M.; Chen, L. Bioaugmentation and biostimulation of hydrocarbon degradation and the microbial community in a petroleum-contaminated soil. Int. Biodeterior. Biodegrad. 2016, 107, 158–164. [Google Scholar] [CrossRef]
- Nwankwegu, A.S.; Orji, M.U.; Onwosi, C.O. Studies on organic and in-organic biostimulants in bioremediation of diesel-contaminated arable soil. Chemosphere 2016, 162, 148–156. [Google Scholar] [CrossRef]
- Wang, S.Y.; Kuo, Y.; Hong, C.A.; Chang, Y.M.; Kao, C.M. Bioremediation of diesel and lubricant oil-contaminated soils using enhanced landfarming system. Chemosphere 2016, 164, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Abbasian, F.; Lockington, R.; Mallavarapu, M.; Naidu, R. A comprehensive review of aliphatic hydrocarbon biodegradation by bacteria. Appl. Biochem. Biotechnol. 2015, 176, 670–699. [Google Scholar] [CrossRef]
- Wilkes, H.; Buckel, W.; Golding, B.T.; Rabus, R. Metabolism of hydrocarbons in n-Alkane—utilizing anaerobic bacteria. J. Mol. Microbiol. Biotechnol. 2016, 26, 138–151. [Google Scholar] [CrossRef]
- Karamalidis, A.K.; Evangelou, A.C.; Karabika, E.; Koukkou, A.I.; Drainas, C.; Voudrias, E.A. Laboratory scale bioremediation of petroleum-contaminated soil by indigenous microorganisms and added Pseudomonas aeruginosa strain Spet. Bioresour. Technol. 2010, 101, 6545–6552. [Google Scholar] [CrossRef]
- Sarkar, J.; Kazy, S.K.; Gupta, A.; Dutta, A.; Mohapatra, B.; Roy, A.; Bera, P.; Mitra, A.; Sar, P. Biostimulation of indigenous microbial community for bioremediation of petroleum refinery sludge. Front. Microbiol. 2016, 7, 1407. [Google Scholar] [CrossRef]
- Guarino, C.; Spada, V.; Sciarrillo, R. Assessment of three approaches of bioremediation (Natural Attenuation, Landfarming and Bioagumentation—Assistited Landfarming) for a petroleum hydrocarbons contaminated soil. Chemosphere 2017, 170, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Meckenstock, R.U.; Boll, M.; Mouttaki, H.; Koelschbach, J.S.; Tarouco, P.; Weyrauch, P.; Dong, X.; Himmelberg, A.M. Anaerobic degradation of benzene and polycyclic aromatic hydrocarbons. J. Mol. Microbiol. Biotechnol. 2016, 26, 92–118. [Google Scholar] [CrossRef]
- Roy, A.; Dutta, A.; Pal, S.; Gupta, A.; Sarkar, J.; Chatterjeea, A.; Saha, A.; Sarkar, P.; Sar, P.; Kazy, S.K. Biostimulation and bioaugmentation of native microbial community accelerated bioremediation of oil refinery sludge. Bioresour. Technol. 2018, 253, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Liu, X.; Chen, X.; Hu, X.; Cao, L.; Yuan, X. Biodegradation of crude oil by a defined co-culture of indigenous bacterial consortium and exogenous Bacillus subtilis. Bioresour. Technol. 2017, 224, 327–332. [Google Scholar] [CrossRef]
- Pugazhendi, A.; Qari, H.; Al-Badry Basahi, J.M.; Godon, J.J.; Dhavamani, J. Role of a halothermophilic bacterial consortium for the biodegradation of PAHs and the treatment of petroleum wastewater at extreme conditions. Int. Biodeterior. Biodegrad. 2017, 121, 44–54. [Google Scholar] [CrossRef]
- Guarino, C.; Zuzolo, D.; Marziano, M.; Conte, B.; Baiamonte, G.; Morra, L.; Benotti, D.; Gresia, D.; Stacul, E.R.; Cicchella, D.; et al. Investigation and Assessment for an effective approach to the reclamation of Polycyclic Aromatic Hydrocarbon (PAHs) contaminated site: SIN Bagnoli, Italy. Sci. Rep. 2019, 9, 11522. [Google Scholar] [CrossRef]
- Azadi, D.; Shojaei, H. Biodegradation of polycyclic aromatic hydrocarbons, phenol and sodium sulfate by Nocardia species isolated and characterized from Iranian ecosystems. Sci. Rep. 2020, 10, 21860. [Google Scholar] [CrossRef] [PubMed]
- Gojgic-Cvijovic, G.D.; Milic, J.S.; Solevic, T.M.; Beskoski, V.P.; Ilic, M.V.; Djokic, L.S.; Narancic, T.M.; Vrvic, M.M. Biodegradation of petroleum sludge and petroleum polluted soil by a bacterial consortium: A laboratory study. Biodegradation 2012, 23, 1–14. [Google Scholar] [CrossRef]
- Steliga, T. Role of fungi in bioremediation of petroleum hydrocarbons in drill waste. Pol. J. Environ. Stud. 2012, 21, 471–479. [Google Scholar]
- Alagić, S.Č.; Maluckov, B.S.; Radojičić, V.B. How can plants manage polycyclic aromatic hydrocarbons? May these effects represent a useful tool for an effective soil remediation? A review. Clean Technol. Environ. Policy 2015, 17, 597–614. [Google Scholar] [CrossRef]
- Kathi, S.; Khan, A.B. Phytoremediation approaches to PAH contaminated soil. Indian J. Sci. Technol. 2011, 4, 56–63. [Google Scholar] [CrossRef]
- Ghazaryan, K.A.; Movsesyan, H.S.; Minkina, T.M.; Sushkova, S.N.; Rajput, V.D. The identification of phytoextraction potential of Melilotus officinalis and Amaranthus retroflexus growing on copper-and molybdenum-polluted soils. Environ. Geochem. Health 2021, 43, 1327–1335. [Google Scholar] [CrossRef]
- Parrish, Z.D.; Banks, M.K.; Schwab, A.P. Effect of root death and decay on dissipation of polycyclic aromatic hydrocarbons in the rhizosphere of yellow sweet clover and tall fescue. J. Environ. Qual. 2005, 34, 207–216. [Google Scholar] [CrossRef]
- Li, N.; Liu, R.; Chen, J.; Zhou, J. Enhanced phytoremediation of PAHs and cadmium contaminated soils by a Mycobacterium. Sci. Total Environ. 2021, 754, 141198. [Google Scholar] [CrossRef]
- Blinova, I.; Bityukova, L.; Kasemets, K.; Ivask, A.; Käkinen, A.; Kurvet, I.; Bondarenko, O.; Kanarbik, L.; Sihtmäe, M.; Aruoja, V.; et al. Environmental hazard of oil shale combustion fly ash. J. Hazard. Mater. 2012, 229–230, 192–200. [Google Scholar] [CrossRef]
- Paskuliakova, A.; McGowan, T.; Tonry, S.; Touzet, N. Phycoremediation of landfill leachate with the chlorophyte Chlamydomonas sp. SW15aRL and evaluation of toxicity pre and post treatment. Ecotoxicol. Environ. Saf. 2018, 147, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Afyuni, M.; Hajabbasi, M.A.; Nourbakhsh, F.; Sabzalian, M.R.; Christensen, J.H. Phytoremediation of an aged petroleum contaminated soil using endophyte infected and non-infected grasses. Chemosphere 2010, 81, 1084–1090. [Google Scholar] [CrossRef]
- Verma, S.; Dubey, R.S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Petriccione, M.; Di Patre, D.; Ferrante, P.; Papa, S.; Bartoli, G.; Fioretto, A.; Scortichini, M. Effects of pseudomonas fluorescens seed bioinoculation on heavy metal accumulation for mirabilis jalapa phytoextraction in smelter-contaminated soil. Water Air Soil Pollut. 2013, 224, 1645. [Google Scholar] [CrossRef]
- Wolf, J.J.; Beatty, S.W.; Seastedt, T.R. Soil characteristics of Rocky Mountain National Park grasslands invaded by Melilotus officinalis and M. alba. J. Biogeogr. 2004, 31, 415–424. [Google Scholar] [CrossRef]
- Nedjimi, B. Phytoremediation: A sustainable environmental technology for heavy metals decontamination. Review Paper. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]
- Fernández, R.; Bertrand, A.; García, J.I.; Tamés, R.S.; Gonzáleza, A. Lead accumulation and synthesis of non-protein thiolic peptides in selected clones of Melilotus alba and Melilotus officinalis. Environ. Exp. Bot. 2012, 78, 18–24. [Google Scholar] [CrossRef]
- Kluk, D.; Steliga, T. Evaluation of toxicity changes in soil contaminated with nickel and petroleum-derived substances in phytoremediation processes. Nafta-Gaz 2016, 4, 230–241. [Google Scholar] [CrossRef]
- Han, Y.; Wu, X.; Gu, J.; Zhao, J.; Huang, S.; Yuan, H.; Fu, J. Effects of organic acids on the photosynthetic and antioxidant properties and accumulations of heavy metals of Melilotus officinalis grown in Cu tailing. Environ. Sci. Pollut. Res. 2016, 23, 17901–17909. [Google Scholar] [CrossRef]
- Morariu, F.; Mâsu, S.; Lixandru, B.; Popescu, D. Restoration of ecosystems destroyed by the fly ash dump using different plant species. Anim. Sci. Biotechnol. 2013, 46, 180–184. [Google Scholar]
- Andresen, E.; Peiter, E.; Küpper, H. Trace metal metabolism in plants. J. Exp. Bot. 2018, 69, 909–954. [Google Scholar] [CrossRef]
- Robson, D.B.; Germida, J.J.; Farrell, R.E.; Knight, J.D. Phytoremediation of hydrocarbon-contaminated soil using native plants. In Soils and Crops Workshop; Harvest, Saskatoon, SK, Canada, 2001; 551–556.
- Robson, D.B.; Germida, J.J.; Farrell, R.E.; Knight, J.D. Ability of cold-tolerant plants to grow in hydrocarbon-contaminated soil. Int. J. Phytore. 2003, 5, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Panchenko, L.; Muratova, A.; Dubrovskaya, E.; Golubev, S.; Turkovskaya, O. Dynamics of natural revegetation of hydrocarbon-contaminated soil and remediation potential of indigenous plant species in the steppe zone of the southern Volga Uplands. Environ. Sci. Pollut. Res. 2018, 25, 3260–3274. [Google Scholar] [CrossRef]
- Godheja, J.; Shekhar, S.K.; Modi, D.R. Bacterial rhizoremediation of petroleum hydrocarbons (PHC). In Plant-Microbe Interactions in Agro-Ecological Perspectives; Springer: Singapore, 2017; pp. 495–519. [Google Scholar] [CrossRef]
- Muratova, A.Y.; Dmitrieva, T.V.; Panchenko, L.V.; Turkovskaya, O.V. Phytoremediation of oil-sludge—contaminated soil. Int. J. Phytoremediat. 2008, 10, 486–502. [Google Scholar] [CrossRef] [PubMed]
- Parrish, D.Z.; Banks, M.K.; Schwab, A.P. Effectiveness of phytoremediation as a secondary treatment for polycyclic aromatic hydrocarbons (PAHs) in composted soil. Int. J. Phytoremediat. 2004, 6, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Olson, P.E.; Castro, A.; Joern, M.; Du Teau, N.M.; Pilon-Smits, E.A.H.; Reardon, K.F. Comparison of plant families in a greenhouse phytoremediation study on an aged polycyclic aromatic hydrocarbon-contaminated soil. J. Environ. Qual. 2007, 6, 1461–1469. [Google Scholar] [CrossRef]
- Foucault, Y.; Durand, M.J.; Tack, K.; Schreck, E.; Geret, F.; Leveque, T.; Pradère, P.; Goix, S.; Dumat, C. Use of ecotoxicity test and ecoscores to improve the management of polluted soils: Case of a secondary lead smelter plant. J. Hazard. Mater. 2013, 246–247, 291–299. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Jośko, I.; Kuśmierz, M.; Futa, B.; Wielgosz, E.; Ligęza, S.; Pranagal, J. Microbiological, biochemical and ecotoxicological evaluation of soils in the area of biochar production in relation to polycyclic aromatic hydrocarbon content. Geoderma 2014, 213, 502–511. [Google Scholar] [CrossRef]
- Niyommaneerat, W.; Nakajima, F.; Tobino, T.; Yamamoto, K. Development of a chronic sediment toxicity test using the benthic ostracod Heterocypris incongruens and their application to toxicity assessments of urban road dust. Ecotoxicol. Environ. Saf. 2017, 143, 266–274. [Google Scholar] [CrossRef]
- Baran, A.; Tarnawski, M. Phytotoxkit/Phytotestkit and Microtox® as tools for toxicity assessment of sediments. Ecotoxicol. Environ. Saf. 2013, 98, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Sekutowski, T.; Bartniak, M. Usage of microbiotest Phytotoxkit in detecting of allelopathic potential of phalaris arundinacea. J. Res. Appl. Agric. Eng. 2009, 54, 88–93. [Google Scholar]
- Kucaj, W.F.; Rygielski, K.; Cybulska, K. Optimizing the use of the Phytotoxkit test to assess the toxicity of soil contaminated with creosote. Pol. J. Soil Sci. 2019, 52, 153–164. [Google Scholar] [CrossRef][Green Version]
- Fai, P.B.; Grant, A. An assessment of the potential of the microbial assay for risk assessment (MARA) for ecotoxicological testing. Ecotoxicology 2010, 19, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Gabrielson, J.; Kühn, I.; Colque-Navarro, P.; Hart, M.; Iversen, A.; McKenzie, D.; Möllby, R. Microplate-based microbial assay for risk assessment and (eco)toxic fingerprinting of chemicals. Anal. Chim. Acta 2003, 485, 121–130. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Bartminski, P.; Rózyło, K.; Debicki, R.; Oleszczuk, P. Ecotoxicological assessment of residues from different biogas production plants used as fertilizer for soil. J. Hazard. Mater. 2015, 298, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, W.; Dick, W.A.; Ye, X.; Chen, K.; Kost, D.; Liming, C. Bioremediation of hydrocarbon degradation in a petroleum contaminated soil and microbial population and activity determination. Chemosphere 2017, 169, 124–130. [Google Scholar] [CrossRef]
- Xu, R.; Obbard, J.P. Biodegradation of polycyclic aromatic hydrocarbons in oil-contaminated beach sediments treated with nutrient amendments. J. Environ. Qual. 2004, 33, 861–867. [Google Scholar] [CrossRef]
- Duke, J.A. Handbook of Legumes of World Economic Importance; Plenum Press: New York, NY, USA, 1981. [Google Scholar]
- van Riper, L.C.; Larson, D.L. Role of invasive Melilotus officinalis in two native plant communities. Plant Ecol. 2009, 200, 129–139. [Google Scholar] [CrossRef]
- Krzciuk, K.; Gałuszka, A. Prospecting for hyperaccumulators of trace elements: A review. J. Crit. Rev. Biotechnol. 2015, 35, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Kluk, D. Analyzing of environmental samples using ion chromatography. Nafta-Gaz 2014, 1, 46–56. [Google Scholar]
- Steliga, T.; Kluk, D. Application of Festuca arundinacea in phytoremediation of soils contaminated with Pb, Ni, Cd and petroleum hydrocarbons. Ecotoxicol. Environ. Saf. 2020, 194, 110409. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Brooks, R.R. Terrestrial higher plants which hyperaccumulate metallic elements-a review of their distribution, ecology and phytochemistry. Biorecovery 1989, 1, 81–126. [Google Scholar]
- Wadhia, K.; Dando, T.; Thompson, K.C. Intralaboratory evaluation of Microbial Assay for Risk Assessment (MARA) for potential application in the implementation of the Water Framework Directive (WFD). J. Environ. Monit. 2007, 9, 953–958. [Google Scholar] [CrossRef]
- Suja, F.; Rahim, F.; Taha, M.R.; Hambali, N.; Razali, M.R.; Khalid, A.; Hamzah, A. Effects of local microbial bioaugmentation and biostimulation on the bioremediation of total petroleum hydrocarbons (TPH) in crude oil contaminated soil based on laboratory and field observations. Int. Biodeterior. Biodegrad. 2014, 90, 115–122. [Google Scholar] [CrossRef]
- Xia, M.; Liu, Y.; Taylor, A.A.; Fu, D.; Khan, A.R.; Terrya, N. Crude oil depletion by bacterial strains isolated from a petroleum hydrocarbon impacted solid waste management site in California. Int. Biodeterior. Biodegr. 2017, 123, 70–77. [Google Scholar] [CrossRef]
- Liang, L.; Song, X.; Kong, J.; Shen, C.; Huang, T.; Hu, Z. Anaerobic biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by a facultative anaerobe Pseudomonas sp. JP1. Biodegradation 2014, 25, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Safdari, M.S.; Kariminia, H.R.; Nejad, Z.G.; Fletcher, T.H. Study potential of indigenous pseudomonas aeruginosa and bacillus subtilis in bioremediation of diesel-contaminated water. Water Air Soil Pollut. 2017, 228, 37. [Google Scholar] [CrossRef]
- Liu, P.-W.G.; Chang, T.C.; Whang, L.-M.; Kao, C.-H.; Pan, P.-T.; Cheng, S.-S. Bioremediation of petroleum hydrocarbon contaminated soil: Effects of strategies and microbial community shift. Int. Biodeterior. Biodegrad 2011, 65, 1119–1127. [Google Scholar] [CrossRef]
- Koolivand, A.; Rajaei, M.S.; Ghanadzadeh, M.J.; Saeedi, R.; Abtahi, H.; Godini, K. Bioremediation of storage tank bottom sludge by using a two-stage composting system: Effect of mixing ratio and nutrients addition. Bioresour. Technol. 2017, 235, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Komilis, D.P.; Vrohidou, A.E.K.; Voudrias, E.A. Kinetics of aerobic bioremediation of a diesel-contaminated sandy soil: Effect of nitrogen addition. Water Air Soil Pollut. 2010, 208, 193–208. [Google Scholar] [CrossRef]
- Peng, S.; Zhou, Q.; Cai, Z.; Zhang, Z. Phytoremediation of petroleum contaminated soils by Mirabilis Jalapa L. in a greenhouse plot experiment. J. Hazard. Mater. 2009, 168, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Lone, M.I.; He, Z.; Stoffella, P.J.; Yang, X. Phytoremediation of heavy metal polluted soils and water: Progresses and perspectives. J. Zhejiang Univ. Sci. B 2008, 9, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Chiaberge, S.; Pedron, F.; Barbafieri, M.; Petruzzelli, G.; Vocciante, M.; Franchi, E.; Pietrini, I. Dealing with complex contamination: A novel approach with a combined bio-phytoremediation strategy and effective analytical techniques. J. Environ. Manag. 2021, 288, 112381. [Google Scholar] [CrossRef]
- Namkoong, W.; Hwang, E.Y.; Choi, J.Y. Bioremediation of diesel-contaminated soil with composting. Environ. Pollut. 2002, 119, 23–31. [Google Scholar] [CrossRef]
- Xu, R.; Lau, N.L.A.; Ng, K.L.; Obbard, J.P. Application of a slow-release fertilizer for oil bioremediation in beach sediment. J. Environ. Qual. 2004, 33, 1210–1216. [Google Scholar] [CrossRef]
- Hamamura, N.; Olson, S.H.; Ward, D.M.; Inskeep, W.P. Microbial population dynamics associated with crude-oil biodegradation in diverse soils. Appl. Environ. Microbiol. 2006, 72, 6316–6324. [Google Scholar] [CrossRef]
- Husaini, A.; Roslan, H.A.; Hii, K.S.Y.; Ang, C.H. Biodegradation of aliphatic hydrocarbon by indigenous fungi isolated from used motor oil contaminated sites. World J. Microbiol. Biotechno. 2008, 24, 2789–2797. [Google Scholar] [CrossRef]
- Erdogan, R.; Zaimoglu, Z. The characteristics of phytoremediation of soil and leachate polluted by landfills. In Advances in Bioremediation of Wastewater and Polluted Soil; Intech Open: London, UK, 2015; Chapter 10; pp. 227–246. [Google Scholar] [CrossRef]
- Nworie, O.E.; Qin, J.; Lin, C. Trace element uptake by herbaceous plants from the soils at a multiple trace element-contaminated site. Toxics 2019, 7, 3. [Google Scholar] [CrossRef]
- Guo, X.; Luo, L.; Ma, Y.; Zhang, S. Sorption of polycyclic aromatic hydrocarbons on particulate organic matters. J. Hazard. Mater. 2010, 173, 130–136. [Google Scholar] [CrossRef]
- Ji, Y.; Mao, G.; Wang, Y.; Bartlam, M. Structural insights into diversity and n-alkane biodegradation mechanisms of alkane hydroxylases. Front. Microbiol. 2013, 4, 58. [Google Scholar] [CrossRef]
- Agnello, A.C.; Bagard, M.; van Hullebusch, E.D.; Esposito, G.; Huguenot, D. Comparative bioremediation of heavy metals and petroleum hydrocarbons co-contaminated soil by natural attenuation, phytoremediation, bioaugmentation and bioaugmentation-assisted phytoremediation. Sci. Total Environ. 2016, 563–564, 693–703. [Google Scholar] [CrossRef]
- Haichar, F.Z.; Santaella, C.; Heulin, T.; Achouak, W. Root exudates mediated interactions belowground. Soil Biol. Biochem. 2014, 77, 69–80. [Google Scholar] [CrossRef]
- Gupta, P.; Rani, R.; Usmani, Z.; Chandra, A.; Kumar, V. The role of plant-associated bacteria in phytoremediation of trace metals in contaminated soils. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, J.S., Singh, D.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 5; pp. 69–76. [Google Scholar] [CrossRef]
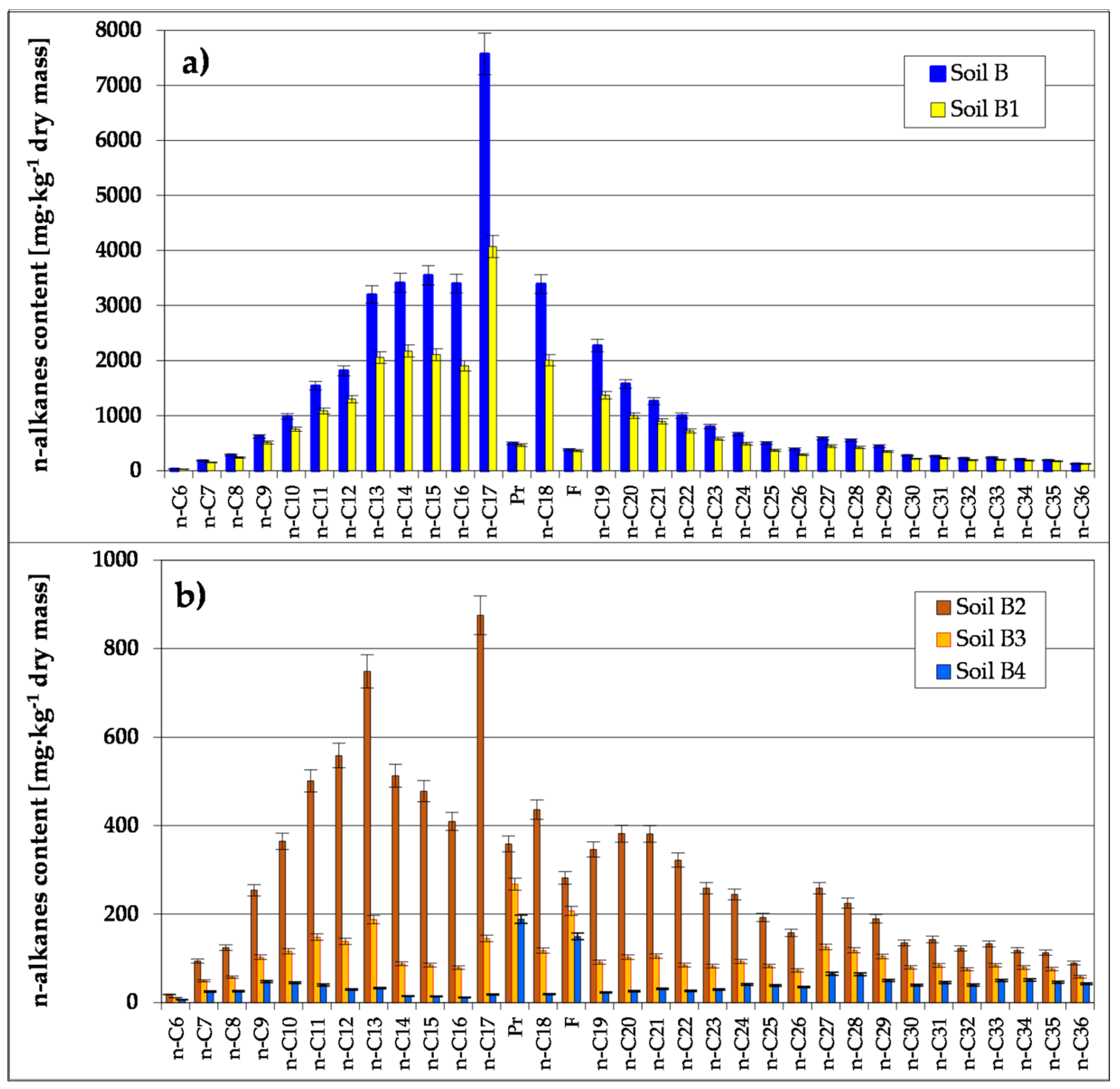
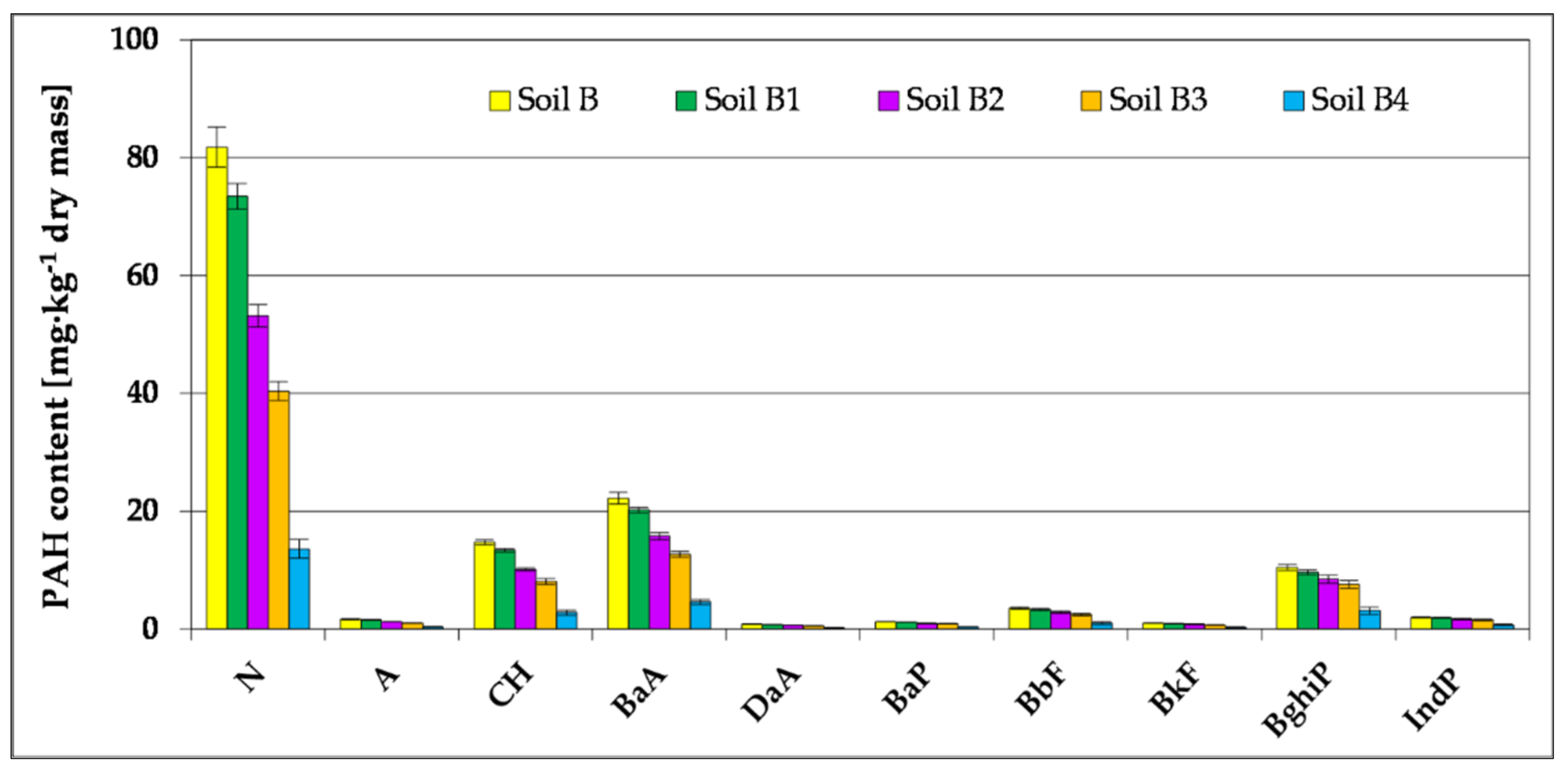
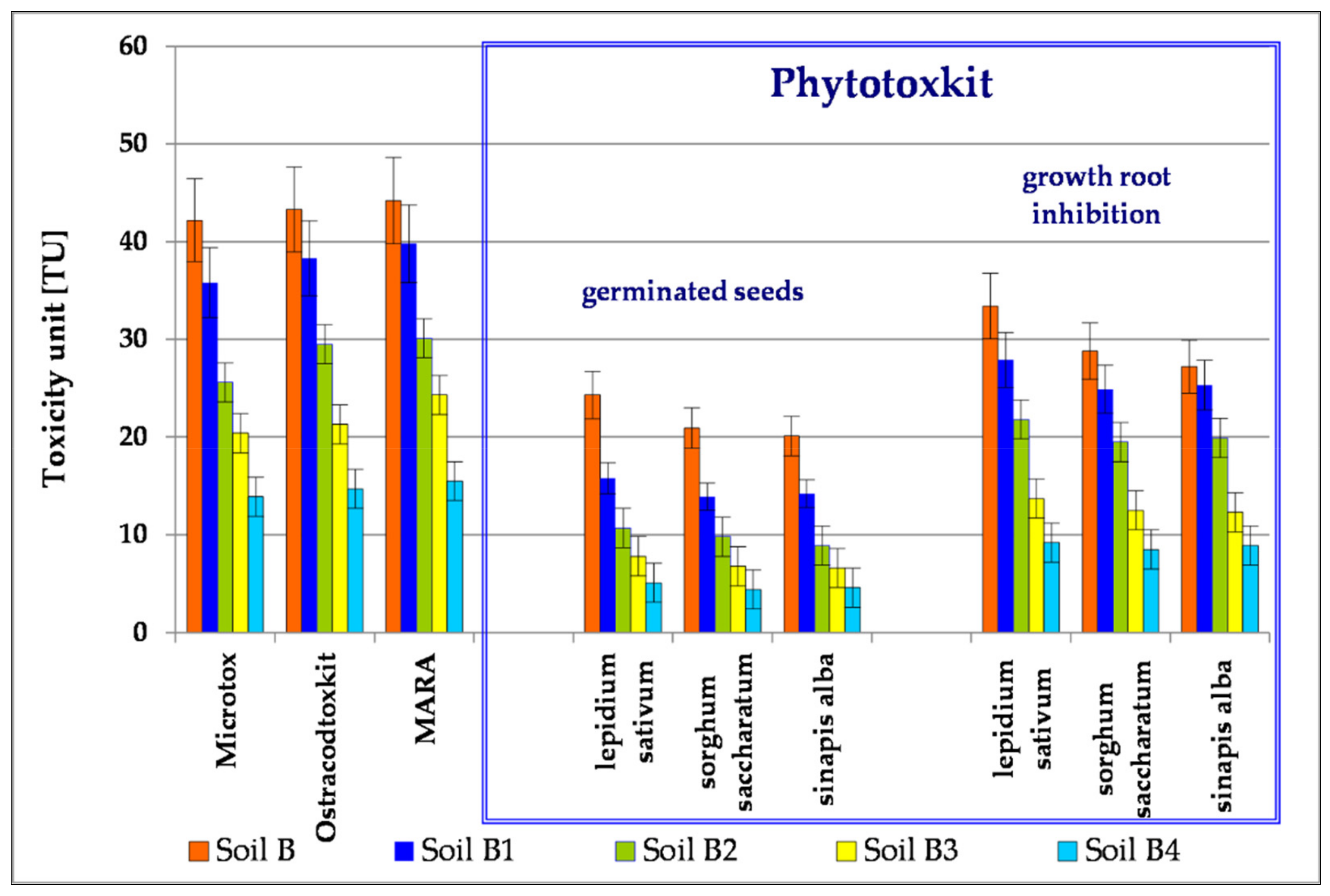
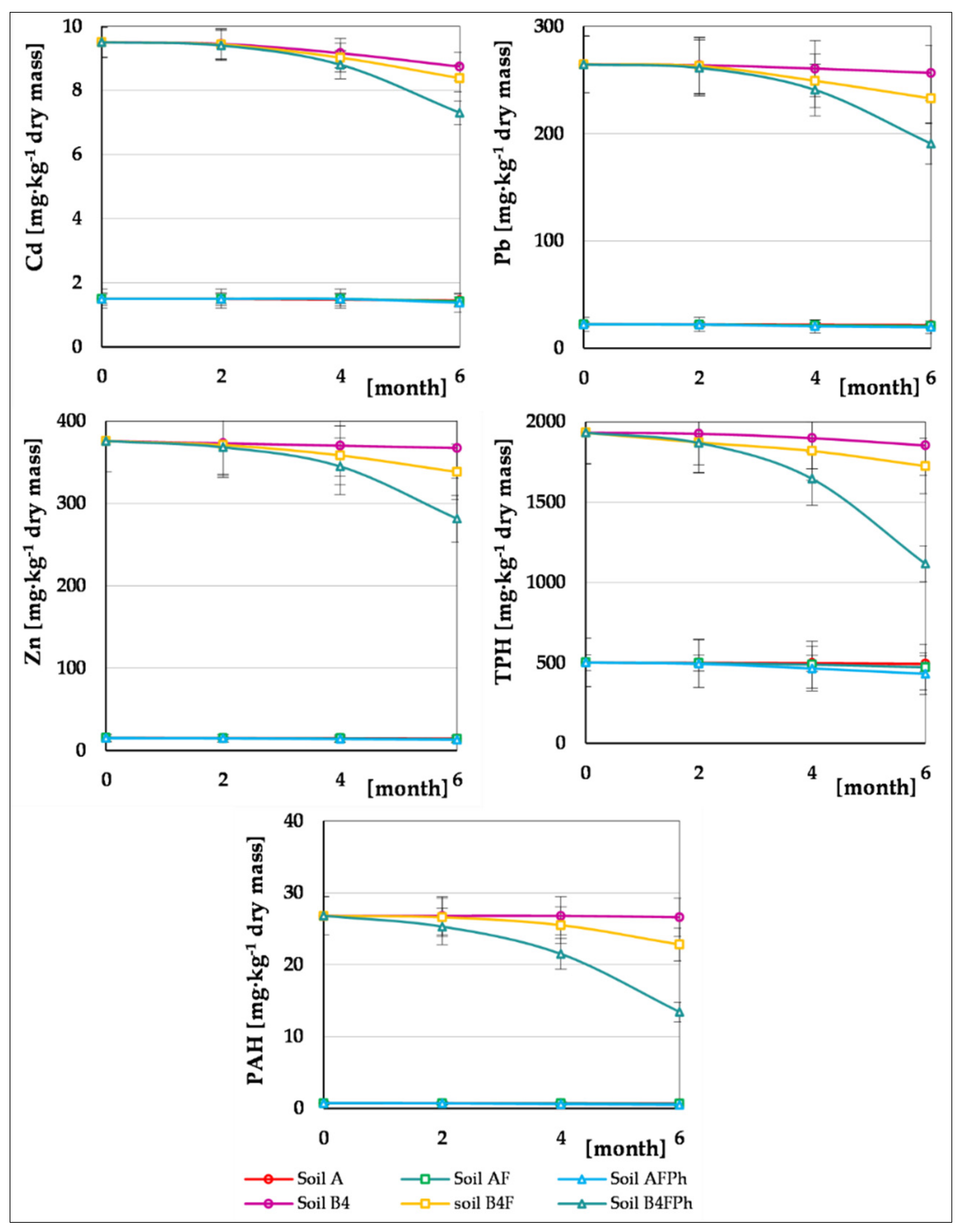




| Parameter of the Chromatograph | Calibration Standards | ||||
|---|---|---|---|---|---|
| Temperature (°C) | Capillary Coumn | Carrier Gas | |||
| Injector | Detector | Temperature Program | |||
| Chromatographic analysis of aliphatic hydrocarbons—TPH, nC6—nC44, pristane, phytane (Clarus 580 GC) | |||||
| 290 °C | FID, 300 °C | 28 °C—isothermal run in 2 min 28–105 °C—temp. increase rate 10 °C min−1 105–285 °C—temp. increase rate 5 °C min−1 285 °C—isothermal run in 10 min−1 | Quadrex 007-1 30 m × 0.53 mm | He, 20 mL min−1 | reference soil: BAM—K010 Supelco No. D2807 (nC6–nC40) Restek No. A029668 (Fuel Oil Degradation Mix nC17, pristane, nC18, phytane) |
| Chromatographic analysis of polycyclic aromatic hydrocarbons—PAH (Clarus 500 GC) | |||||
| 320 °C | FID, 320 °C | 40 °C—isothermal run in 2 min 40–240 °C—temp. increase rate 30 °C min−1 240–320 °C—temp. increase rate 8 °C min−1 320 °C—isothermal run in 10 min−1 | RTX-440 30 m × 0.25 mm | H2, 8 mL min−1 | Reference soil containing 16 PAHs: BAM—ERM-CC013 Mixture of 16 PAHs in chloroform: nr 31011 (2000 μg ml−1 each), nr 31264 (500–1000 μg ml−1), nr 31451 (100–200 μg ml−1) made by Restek |
| Parameter | Soil AFPh | Soil B4FPh | ||||
|---|---|---|---|---|---|---|
| Zn | Pb | Cd | Zn | Pb | Cd | |
| TF | 0.44 ± 0.02 | 0.12 ± 0.01 | 0.40 ± 0.02 | 0.44 ± 0.02 | 0.11 ± 0.01 | 0.40 ± 0.02 |
| BCFshoot | 2.30 ± 0.12 | 0.34 ± 0.02 | 2.62 ± 0.12 | 0.96 ± 0.08 | 0.29 ± 0.03 | 2.42 ± 0.18 |
| BCFroot | 5.23 ± 0.32 | 2.96 ± 0.16 | 6.53 ± 0.45 | 2.19 ± 0.16 | 2.50 ± 0.18 | 6.02 ± 0.60 |
| Germination (%) | Soil A | Soil B4 | ||||||
|---|---|---|---|---|---|---|---|---|
| AF | AFPh-2 | AFPh-4 | AFPh-6 | B4F | B4FPh-2 | B4FPh-4 | B4FPh-6 | |
| Lepidium sativum | 93 ± 1 | 97 ± 1 | 97 ± 1 | >99 ± 1 | 55 ± 3 | 68 ± 3 | 84 ± 2 | 90 ± 1 |
| Sorghum saccharatum | 97 ± 1 | >99 ± 1 | ≥99 ± 1 | >99 ± 1 | 67 ± 3 | 79 ± 2 | 90 ± 1 | 97 ± 1 |
| Sinapis alba | 97 ± 1 | 97 ± 1 | >99 ± 1 | >99 ± 1 | 60 ± 3 | 75 ± 2 | 90 ± 1 | 93 ± 1 |
| Parameter | Soil A | Soil B4 | ||||||
|---|---|---|---|---|---|---|---|---|
| AF | AFPh-2 | AFPh-4 | Ph-6 | B4F | B4FPh-2 | B4FPh-4 | B4FPh-6 | |
| EC 50 [mg soil dm−3] | 35,712 ± 3687 | 50,369 ± 5032 | N | N | 6451 ± 728 | 6971 ± 815 | 10,556 ± 1136 | 14,759 ± 1680 |
| TU | 2.5 | 1.9 | – | – | 13.5 | 12.2 | 8.5 | 5.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steliga, T.; Kluk, D. Assessment of the Suitability of Melilotus officinalis for Phytoremediation of Soil Contaminated with Petroleum Hydrocarbons (TPH and PAH), Zn, Pb and Cd Based on Toxicological Tests. Toxics 2021, 9, 148. https://doi.org/10.3390/toxics9070148
Steliga T, Kluk D. Assessment of the Suitability of Melilotus officinalis for Phytoremediation of Soil Contaminated with Petroleum Hydrocarbons (TPH and PAH), Zn, Pb and Cd Based on Toxicological Tests. Toxics. 2021; 9(7):148. https://doi.org/10.3390/toxics9070148
Chicago/Turabian StyleSteliga, Teresa, and Dorota Kluk. 2021. "Assessment of the Suitability of Melilotus officinalis for Phytoremediation of Soil Contaminated with Petroleum Hydrocarbons (TPH and PAH), Zn, Pb and Cd Based on Toxicological Tests" Toxics 9, no. 7: 148. https://doi.org/10.3390/toxics9070148
APA StyleSteliga, T., & Kluk, D. (2021). Assessment of the Suitability of Melilotus officinalis for Phytoremediation of Soil Contaminated with Petroleum Hydrocarbons (TPH and PAH), Zn, Pb and Cd Based on Toxicological Tests. Toxics, 9(7), 148. https://doi.org/10.3390/toxics9070148






