Degradation Products of Polychlorinated Biphenyls and Their In Vitro Transformation by Ligninolytic Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extracellular Liquid Harvest
2.3. Enzyme Activity
2.4. In Vitro Biotransformation Experiment with Pleurotus ostreatus
2.5. In Vitro Biotransformation Experiment with Irpex lacteus and Chlorobenzaldehydes
2.6. Sample Extraction and Derivatization
2.7. GC–MS Analysis
2.8. Data Analysis
3. Results
3.1. Enzyme Activity
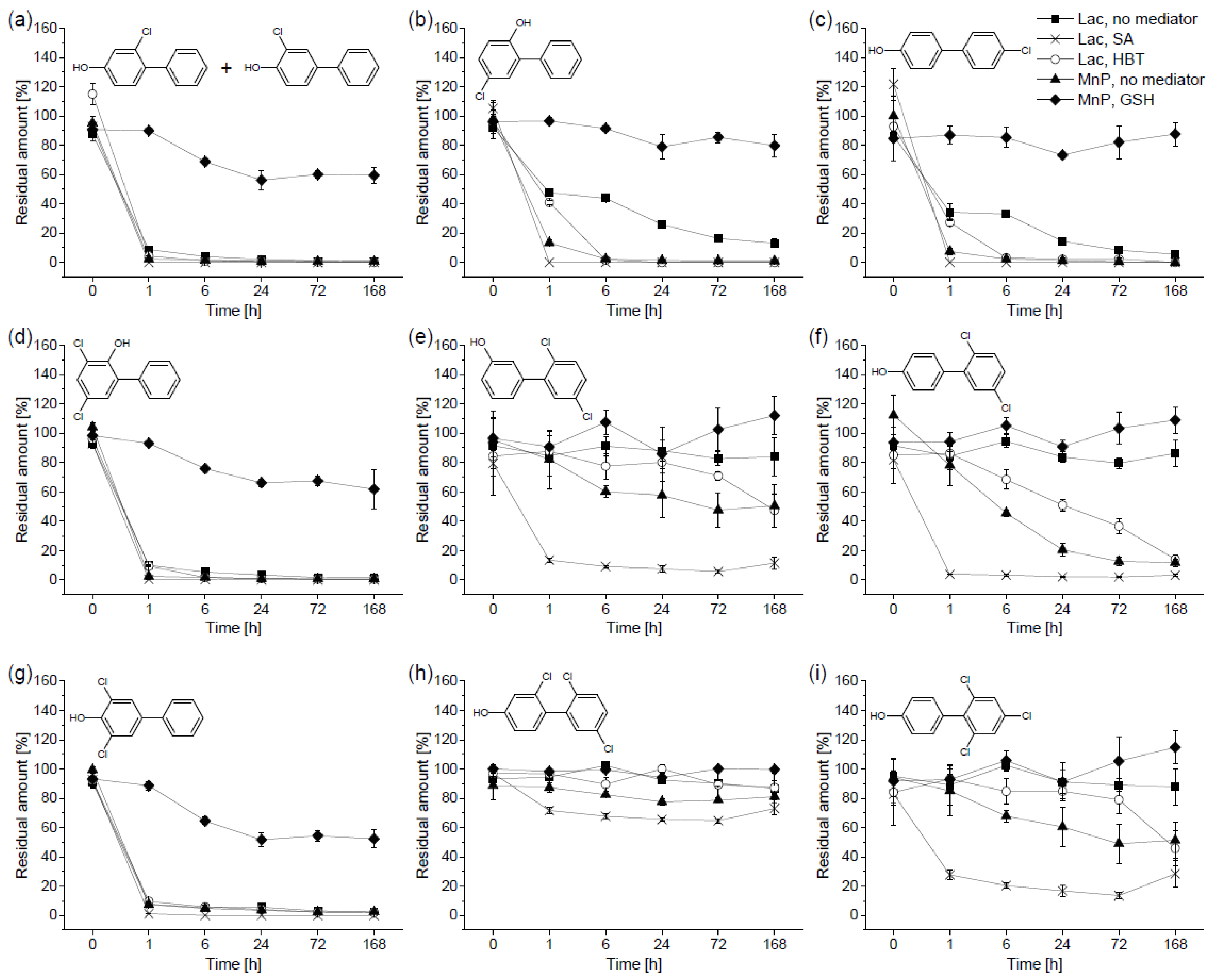
3.2. Biotransformation of Hydroxylated Polychlorinated Biphenyls
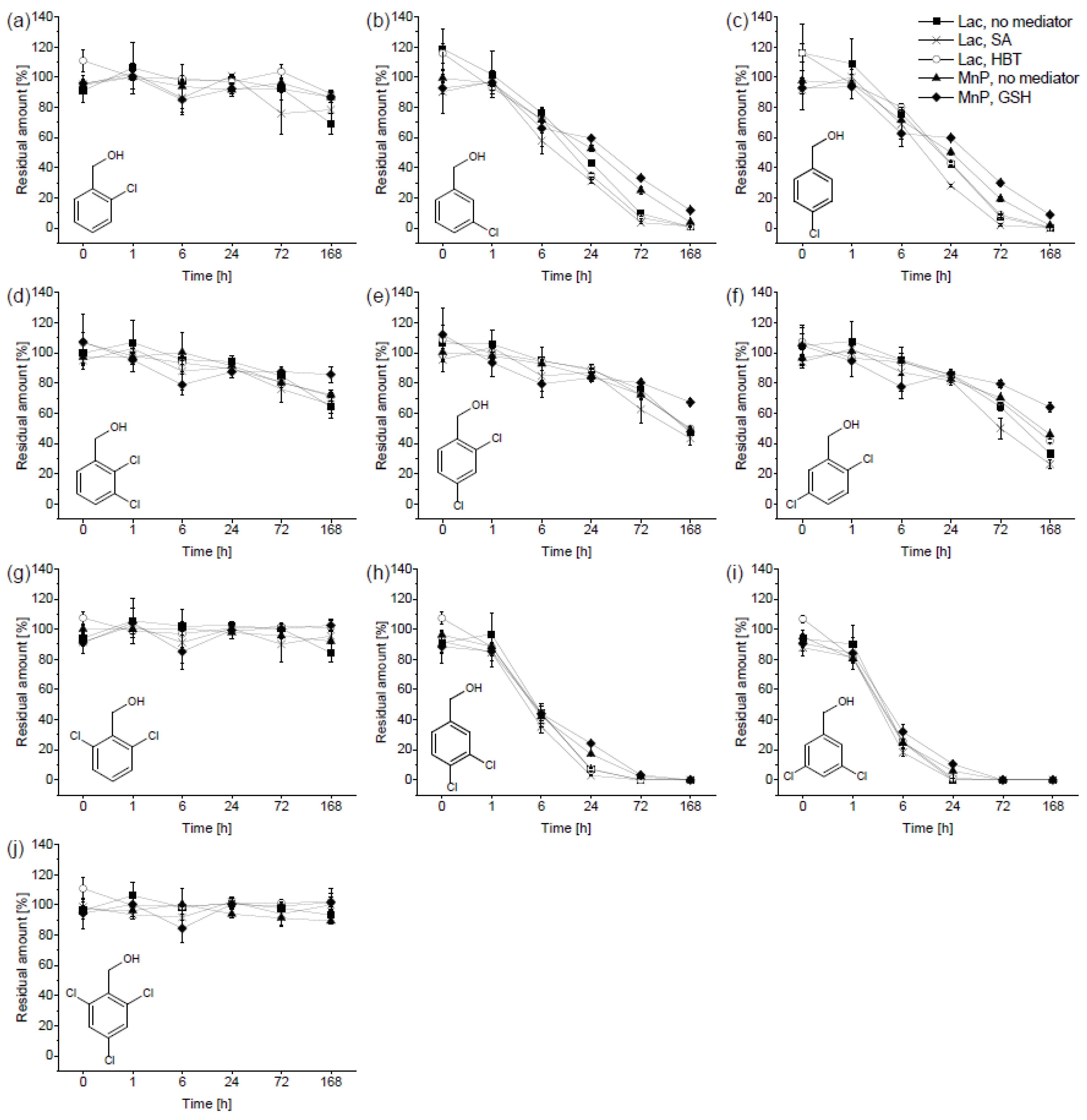
3.3. Biotransformation of Chlorobenzyl Alcohols
3.4. Biotransformation of Chlorobenzaldehydes
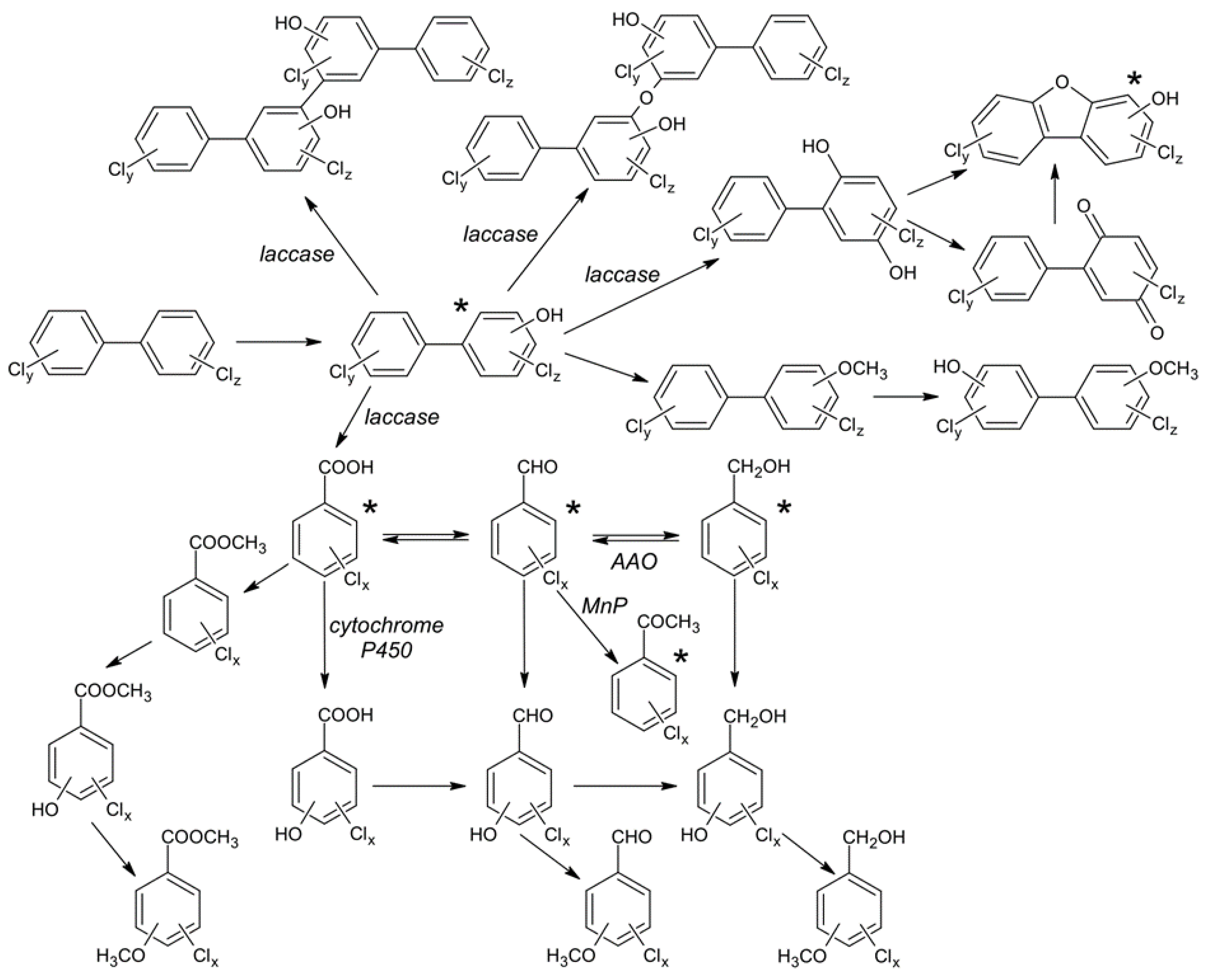
4. Discussion
4.1. Biotransformation of Hydroxylated Polychlorinated Biphenyls
4.2. Biotransformation of Chlorobenzyl Alcohols
4.3. Biotransformation of Chlorobenzaldehydes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Čvančarová, M.; Křesinová, Z.; Filipová, A.; Covino, S.; Cajthaml, T. Biodegradation of PCBs by ligninolytic fungi and characterization of the degradation products. Chemosphere 2012, 88, 1317–1323. [Google Scholar] [CrossRef]
- Kamei, I.; Kogura, R.; Kondo, R. Metabolism of 4,4′-dichlorobiphenyl by white-rot fungi Phanerochaete chrysosporium and Phanerochaete sp. MZ142. Appl. Microbiol. Biotechnol. 2006, 72, 566–575. [Google Scholar] [CrossRef]
- Keum, Y.S.; Li, Q.X. Fungal laccase-catalyzed degradation of hydroxy polychlorinated biphenyls. Chemosphere 2004, 56, 23–30. [Google Scholar] [CrossRef]
- Muzikář, M.; Křesinová, Z.; Svobodová, K.; Filipová, A.; Čvančarová, M.; Cajthamlová, K.; Cajthaml, T. Biodegradation of chlorobenzoic acids by ligninolytic fungi. J. Hazard. Mater. 2011, 196, 386–394. [Google Scholar] [CrossRef]
- Marek, R.F.; Martinez, A.; Hornbuckle, K.C. Discovery of hydroxylated polychlorinated biphenyls (OH-PCBs) in sediment from a Lake Michigan waterway and original commercial Aroclors. Environ. Sci. Technol. 2013, 47, 8204–8210. [Google Scholar] [CrossRef] [PubMed]
- Ueno, D.; Darling, C.; Alaee, M.; Campbell, L.; Pacepavicius, G.; Teixeira, C.; Muir, D. Detection of hydroxylated polychlorinated biphenyls (OH-PCBs) in the abiotic environment: Surface water and precipitation from Ontario, Canada. Environ. Sci. Technol. 2007, 41, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Bytingsvik, J.; Lie, E.; Aars, J.; Derocher, A.E.; Wiig, Ø.; Jenssen, B.M. PCBs and OH-PCBs in polar bear mother–cub pairs: A comparative study based on plasma levels in 1998 and 2008. Sci. Total Environ. 2012, 417–418, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Linderholm, L.; Charles, M.J.; Athanasiadou, M.; Petrik, J.; Kocan, A.; Drobna, B.; Trnovec, T.; Bergman, Å.; Hertz-Picciotto, I. Polychlorinated biphenyls and their hydroxylated metabolites (OH-PCBs) in pregnant women from eastern Slovakia. Environ. Health Perspect. 2007, 115, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Nomiyama, K.; Tsujisawa, Y.; Ashida, E.; Yachimori, S.; Eguchi, A.; Iwata, H.; Tanabe, S. Mother to Fetus Transfer of Hydroxylated Polychlorinated Biphenyl Congeners (OH-PCBs) in the Japanese Macaque (Macaca fuscata): Extrapolation of Exposure Scenarios to Humans. Environ. Sci. Technol. 2020, 54, 11386–11395. [Google Scholar] [CrossRef]
- Kunisue, T.; Tanabe, S. Hydroxylated polychlorinated biphenyls (OH-PCBs) in the blood of mammals and birds from Japan: Lower chlorinated OH-PCBs and profiles. Chemosphere 2009, 74, 950–961. [Google Scholar] [CrossRef]
- Liu, J.; Hu, D.; Jiang, G.; Schnoor, J.L. In vivo biotransformation of 3,3′,4,4′-tetrachlorobiphenyl by whole plants–poplars and switchgrass. Environ. Sci. Technol. 2009, 43, 7503–7509. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zeng, M.; Wang, L. Atmospheric oxidation mechansim of polychlorinated biphenyls (PCBs) initiated by OH radicals. Chemosphere 2020, 240, 124756. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhu, L.; Pan, L.; Wei, Z.; Song, Y.; Zhang, Y.; Qu, L.; Zhan, Y. Detection of methoxylated and hydroxylated polychlorinated biphenyls in sewage sludge in China with evidence for their microbial transformation. Sci. Rep. 2016, 6, 29782. [Google Scholar] [CrossRef]
- Tehrani, R.; Van Aken, B. Hydroxylated polychlorinated biphenyls in the environment: Sources, fate, and toxicities. Environ. Sci. Pollut. Res. 2014, 21, 6334–6345. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, K.; Gadupudi, G.S.; Lehmler, H.J.; Ludewig, G.; Duffel, M.W.; Robertson, L.W. Sources and toxicities of phenolic polychlorinated biphenyls (OH-PCBs). Environ. Sci. Pollut. Res. 2018, 25, 16277–16290. [Google Scholar] [CrossRef]
- Lans, M.C.; Klasson-Wehler, E.; Willemsen, M.; Meussen, E.; Safe, S.; Brouwer, A. Structure-dependent, competitive interaction of hydroxy-polychlorobiphenyls, -dibenzo-p-dioxins and -dibenzofurans with human transthyretin. Chem. Biol. Interact. 1993, 88, 7–21. [Google Scholar] [CrossRef]
- Kamata, R.; Shiraishi, F.; Nakajima, D.; Takigami, H.; Shiraishi, H. Mono-hydroxylated polychlorinated biphenyls are potent aryl hydrocarbon receptor ligands in recombinant yeast cells. Toxicol. In Vitro 2009, 23, 736–743. [Google Scholar] [CrossRef]
- Schultz, A.; Jonas, U.; Hammer, E.; Schauer, F. Dehalogenation of Chlorinated Hydroxybiphenyls by Fungal Laccase. Appl. Environ. Microbiol. 2001, 67, 4377–4381. [Google Scholar] [CrossRef]
- Fujihiro, S.; Higuchi, R.; Hisamatsu, S.; Sonoki, S. Metabolism of hydroxylated PCB congeners by cloned laccase isoforms. Appl. Microbiol. Biotechnol. 2009, 82, 853–860. [Google Scholar] [CrossRef]
- Kordon, K.; Mikolasch, A.; Schauer, F. Oxidative dehalogenation of chlorinated hydroxybiphenyls by laccases of white-rot fungi. Int. Biodeterior. Biodegrad. 2010, 64, 203–209. [Google Scholar] [CrossRef]
- Stella, T.; Covino, S.; Křesinová, Z.; D’Annibale, A.; Petruccioli, M.; Čvančarová, M.; Cajthaml, T. Chlorobenzoic acid degradation by Lentinus (Panus) tigrinus: In vivo and in vitro mechanistic study-evidence for P-450 involvement in the transformation. J. Hazard. Mater. 2013, 260, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Stella, T.; Covino, S.; Čvančarová, M.; Filipová, A.; Petruccioli, M.; D’Annibale, A.; Cajthaml, T. Bioremediation of long-term PCB-contaminated soil by white-rot fungi. J. Hazard. Mater. 2017, 324, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, E.; Yamamoto, E.; Numata, A.; Kawano, T.; Shin, T.; Murao, S. Structures of the Laccase-Catalyzed Oxidation Products of Hydroxy-Benzoic Acids in the Presence of ABTS [2,2′-Azino-di-(3-ethylbenzothiazoline-6-sulfonic Acid)]. Agric. Biol. Chem. 1986, 50, 1355–1357. [Google Scholar] [CrossRef]
- Ngo, T.T.; Lenhoff, H.M. A sensitive and versatile chromogenic assay for peroxidase and peroxidase-coupled reactions. Anal. Biochem. 1980, 105, 389–397. [Google Scholar] [CrossRef]
- Šnajdr, J.; Valášková, V.; Merhautová, V.; Cajthaml, T.; Baldrian, P. Activity and spatial distribution of lignocellulose-degrading enzymes during forest soil colonization by saprotrophic basidiomycetes. Enzyme Microb. Technol. 2008, 43, 186–192. [Google Scholar] [CrossRef]
- Tien, M.; Kirk, T.K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proc. Natl. Acad. Sci. USA 1984, 81, 2280–2284. [Google Scholar] [CrossRef]
- Covino, S.; Svobodová, K.; Křesinová, Z.; Petruccioli, M.; Federici, F.; D’Annibale, A.; Čvančarová, M.; Cajthaml, T. In vivo and in vitro polycyclic aromatic hydrocarbons degradation by Lentinus (Panus) tigrinus CBS 577.79. Bioresour. Technol. 2010, 101, 3004–3012. [Google Scholar] [CrossRef]
- Linhartová, L.; Michalíková, K.; Šrédlová, K.; Cajthaml, T. Biodegradability of Dental Care Antimicrobial Agents Chlorhexidine and Octenidine by Ligninolytic Fungi. Molecules 2020, 25, 400. [Google Scholar] [CrossRef]
- Hong, J.E.; Pyo, H.; Park, S.J.; Lee, W. Determination of hydroxy-PCBs in urine by gas chromatography/mass spectrometry with solid-phase extraction and derivatization. Anal. Chim. Acta 2005, 531, 249–256. [Google Scholar] [CrossRef]
- Kuroki, H.; Hattori, R.; Haraguchi, K.; Masuda, Y. Synthesis and mass spectral properties of polychlorinated dibenzofuran (PCDF) metabolites. Chemosphere 1987, 16, 1641–1647. [Google Scholar] [CrossRef]
- Ferreira, P.; Medina, M.; Guillén, F.; Martínez, M.J.; Van Berkel, W.J.H.; Martínez, Á.T. Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated alcohols. Biochem. J. 2005, 389, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Nikolaivits, E.; Siaperas, R.; Agrafiotis, A.; Ouazzani, J.; Magoulas, A.; Gioti, A.; Topakas, E. Functional and transcriptomic investigation of laccase activity in the presence of PCB29 identifies two novel enzymes and the multicopper oxidase repertoire of a marine-derived fungus. Sci. Total Environ. 2021, 775, 145818. [Google Scholar] [CrossRef]
- Takagi, S.; Shirota, C.; Sakaguchi, K.; Suzuki, J.; Sue, T.; Nagasaka, H.; Hisamatsu, S.; Sonoki, S. Exoenzymes of Trametes versicolor can metabolize coplanar PCB congeners and hydroxy PCB. Chemosphere 2007, 67, S54–S57. [Google Scholar] [CrossRef]
- Mota, M.I.F.; Rodrigues Pinto, P.C.; Loureiro, J.M.; Rodrigues, A.E. Recovery of Vanillin and Syringaldehyde from Lignin Oxidation: A Review of Separation and Purification Processes. Sep. Purif. Rev. 2016, 45, 227–259. [Google Scholar] [CrossRef]
- Nguyen, L.N.; van de Merwe, J.P.; Hai, F.I.; Leusch, F.D.L.; Kang, J.; Price, W.E.; Roddick, F.; Magram, S.F.; Nghiem, L.D. Laccase–syringaldehyde-mediated degradation of trace organic contaminants in an enzymatic membrane reactor: Removal efficiency and effluent toxicity. Bioresour. Technol. 2016, 200, 477–484. [Google Scholar] [CrossRef]
- Jiang, G.X.; Niu, J.F.; Zhang, S.P.; Zhang, Z.Y.; Xie, B. Prediction of biodegradation rate constants of hydroxylated polychlorinated biphenyls by fungal laccases from Trametes versicolor and Pleurotus ostreatus. Bull. Environ. Contam. Toxicol. 2008, 81, 1–6. [Google Scholar] [CrossRef]
- Baldrian, P. Increase of laccase activity during interspecific interactions of white-rot fungi. FEMS Microbiol. Ecol. 2004, 50, 245–253. [Google Scholar] [CrossRef]
- Pawlik, A.; Wójcik, M.; Rułka, K.; Motyl-Gorzel, K.; Osińska-Jaroszuk, M.; Wielbo, J.; Marek-Kozaczuk, M.; Skorupska, A.; Rogalski, J.; Janusz, G. Purification and characterization of laccase from Sinorhizobium meliloti and analysis of the lacc gene. Int. J. Biol. Macromol. 2016, 92, 138–147. [Google Scholar] [CrossRef]
- Qin, X.; Sun, X.; Huang, H.; Bai, Y.; Wang, Y.; Luo, H.; Yao, B.; Zhang, X.; Su, X. Oxidation of a non-phenolic lignin model compound by two Irpex lacteus manganese peroxidases: Evidence for implication of carboxylate and radicals. Biotechnol. Biofuels 2017, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qin, X.; Hao, Z.; Luo, H.; Yao, B.; Su, X. Degradation of Four Major Mycotoxins by Eight Manganese Peroxidases in Presence of a Dicarboxylic Acid. Toxins 2019, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Hofrichter, M.; Ziegenhagen, D.; Vares, T.; Friedrich, M.; Jäger, M.G.; Fritsche, W.; Hatakka, A. Oxidative decomposition of malonic acid as basis for the action of manganese peroxidase in the absence of hydrogen peroxide. FEBS Lett. 1998, 434, 362–366. [Google Scholar] [CrossRef]
- Rodríguez, E.; Nuero, O.; Guillén, F.; Martínez, A.T.; Martínez, M.J. Degradation of phenolic and non-phenolic aromatic pollutants by four Pleurotus species: The role of laccase and versatile peroxidase. Soil Biol. Biochem. 2004, 36, 909–916. [Google Scholar] [CrossRef]
- Sack, U.; Hofrichter, M.; Fritsche, W. Degradation of polycyclic aromatic hydrocarbons by manganese peroxidase of Nematoloma frowardii. FEMS Microbiol. Lett. 1997, 152, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Van Aken, B.; Cameron, M.D.; Stahl, J.D.; Plumat, A.; Naveau, H.; Aust, S.D.; Agathos, S.N. Glutathione-mediated mineralization of 14C-labeled 2-amino-4,6-dinitrotoluene by manganese-dependent peroxidase H5 from the white-rot fungus Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 2000, 54, 659–664. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šrédlová, K.; Šírová, K.; Stella, T.; Cajthaml, T. Degradation Products of Polychlorinated Biphenyls and Their In Vitro Transformation by Ligninolytic Fungi. Toxics 2021, 9, 81. https://doi.org/10.3390/toxics9040081
Šrédlová K, Šírová K, Stella T, Cajthaml T. Degradation Products of Polychlorinated Biphenyls and Their In Vitro Transformation by Ligninolytic Fungi. Toxics. 2021; 9(4):81. https://doi.org/10.3390/toxics9040081
Chicago/Turabian StyleŠrédlová, Kamila, Kateřina Šírová, Tatiana Stella, and Tomáš Cajthaml. 2021. "Degradation Products of Polychlorinated Biphenyls and Their In Vitro Transformation by Ligninolytic Fungi" Toxics 9, no. 4: 81. https://doi.org/10.3390/toxics9040081
APA StyleŠrédlová, K., Šírová, K., Stella, T., & Cajthaml, T. (2021). Degradation Products of Polychlorinated Biphenyls and Their In Vitro Transformation by Ligninolytic Fungi. Toxics, 9(4), 81. https://doi.org/10.3390/toxics9040081





