Diacetyl Vapor Inhalation Induces Mixed, Granulocytic Lung Inflammation with Increased CD4+CD25+ T Cells in the Rat
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diacetyl Vapor Exposures
2.3. Bronchoalveolar Lavage Fluid (BALF), Tissue Harvest, and Histopathology
2.4. Flow Cytometry for T Cells in Whole Lung Homogenates
2.5. Bronchoalveolar Fluid (BALF) Total Protein and Interleukin-17A (IL-17a)
2.6. Statistical Analysis
3. Results
3.1. Mixed, Granulocytic Airways Inflammation with Adjacent Lymphoid Aggregates Following DA Vapor Exposures
3.2. Bronchoalveolar Lavage Fluid (BALF) Cell Counts and Differentials after DA Exposures
3.3. T Cell Populations within the Rat Lung after DA Exposure
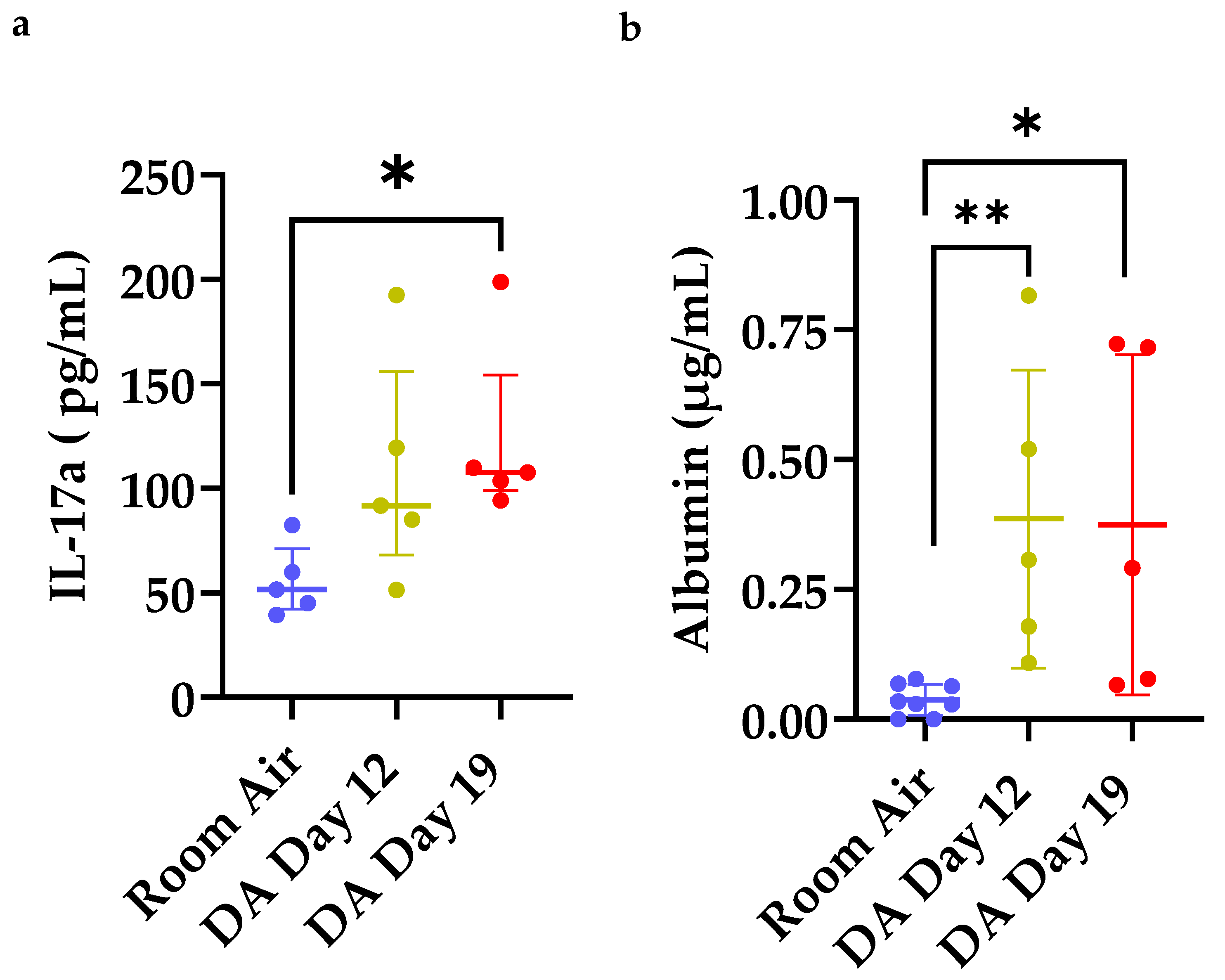
3.4. Increased IL-17a Expression and Total Albumin in Bronchoalveolar Lavage Fluid (BALF) after DA Exposure
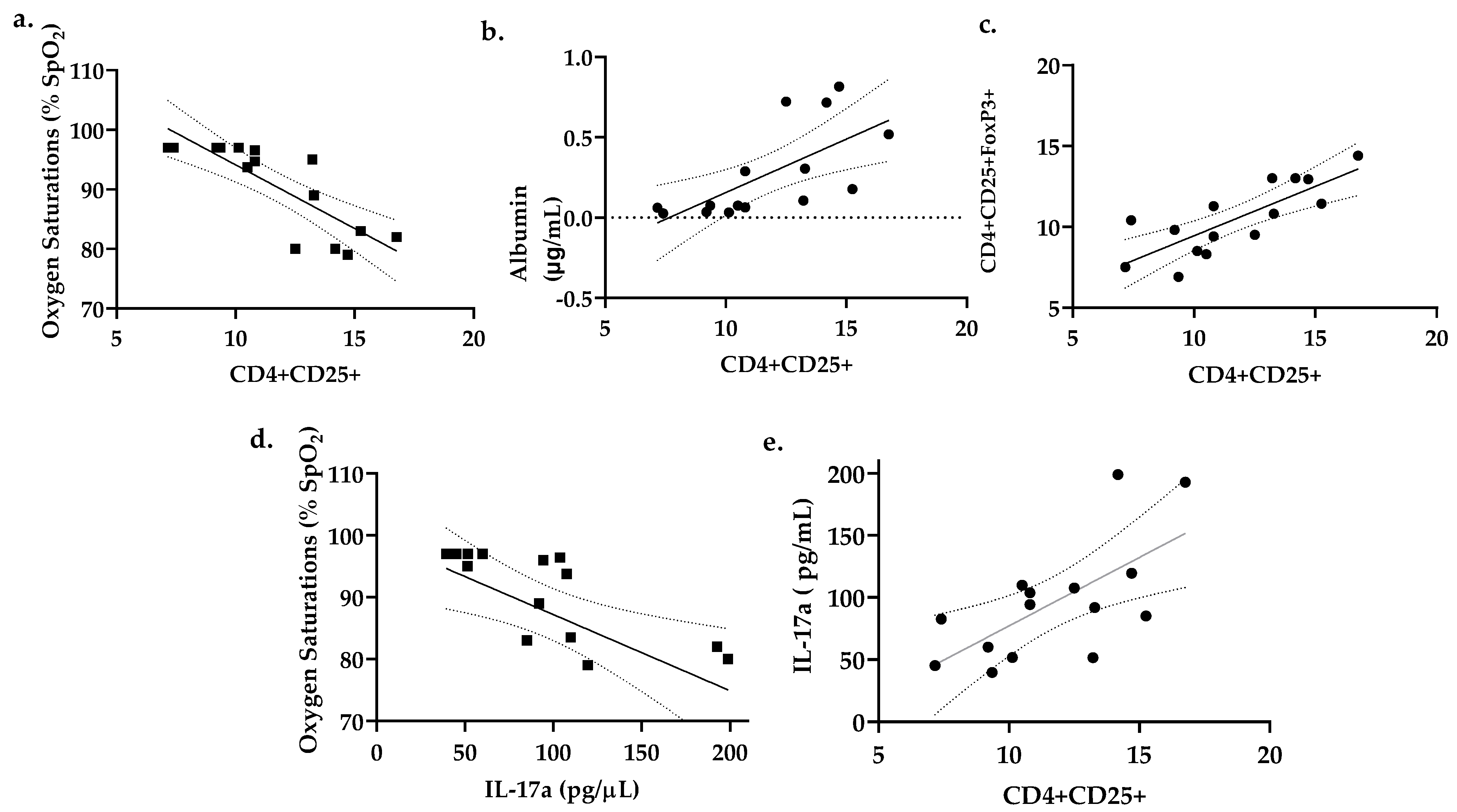
3.5. Percent CD4+CD25+ Lung T Cells Correlates with Reduced Oxygen Saturations and Increased Lung Permeability
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kreiss, K. Flavoring-related bronchiolitis obliterans. Curr. Opin. Allergy Clin. Immunol. 2007, 7, 162–167. [Google Scholar] [CrossRef]
- Hubbs, A.F.; Kreiss, K.; Cummings, K.J.; Fluharty, K.L.; O’Connell, R.; Cole, A.; Dodd, T.M.; Clingerman, S.M.; Flesher, J.R.; Lee, R.; et al. Flavorings-Related Lung Disease: A Brief Review and New Mechanistic Data. Toxicol. Pathol. 2019, 47, 1012–1026. [Google Scholar] [CrossRef]
- LeBouf, R.F.; Blackley, B.H.; Fortner, A.R.; Stanton, M.; Martin, S.B.; Groth, C.P.; McClelland, T.L.; Duling, M.G.; Burns, D.A.; Ranpara, A.; et al. Exposures and Emissions in Coffee Roasting Facilities and Cafes: Diacetyl, 2,3-Pentanedione, and Other Volatile Organic Compounds. Front. Public Health 2020, 8, 561740. [Google Scholar] [CrossRef]
- Mathews, J.M.; Watson, S.L.; Snyder, R.W.; Burgess, J.P.; Morgan, D.L. Reaction of the butter flavorant diacetyl (2,3-butanedione) with N-alpha-acetylarginine: A model for epitope formation with pulmonary proteins in the etiology of obliterative bronchiolitis. J. Agric. Food Chem. 2010, 58, 12761–12768. [Google Scholar] [CrossRef][Green Version]
- Kreiss, K.; Gomaa, A.; Kullman, G.; Fedan, K.; Simoes, E.J.; Enright, P.L. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N. Engl. J. Med. 2002, 347, 330–338. [Google Scholar] [CrossRef]
- Kanwal, R.; Kullman, G.; Piacitelli, C.; Boylstein, R.; Sahakian, N.; Martin, S.; Fedan, K.; Kreiss, K. Evaluation of flavorings-related lung disease risk at six microwave popcorn plants. J. Occup. Environ. Med. 2006, 48, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.; Winter, C.K. Diacetyl in Foods: A Review of Safety and Sensory Characteristics. Compr. Rev. Food Sci. Food Saf. 2015, 14, 634–643. [Google Scholar] [CrossRef]
- Allen, J.G.; Flanigan, S.S.; LeBlanc, M.; Vallarino, J.; MacNaughton, P.; Stewart, J.H.; Christiani, D.C. Flavoring Chemicals in E-Cigarettes: Diacetyl, 2,3-Pentanedione, and Acetoin in a Sample of 51 Products, Including Fruit-, Candy-, and Cocktail-Flavored E-Cigarettes. Environ. Health Perspect. 2016, 124, 733–739. [Google Scholar] [CrossRef]
- Palmer, S.M.; Flake, G.P.; Kelly, F.L.; Zhang, H.L.; Nugent, J.L.; Kirby, P.J.; Foley, J.F.; Gwinn, W.M.; Morgan, D.L. Severe airway epithelial injury, aberrant repair and bronchiolitis obliterans develops after diacetyl instillation in rats. PLoS ONE 2011, 6, e17644. [Google Scholar] [CrossRef]
- Morgan, D.L.; Jokinen, M.P.; Johnson, C.L.; Price, H.C.; Gwinn, W.M.; Bousquet, R.W.; Flake, G.P. Chemical Reactivity and Respiratory Toxicity of the alpha-Diketone Flavoring Agents: 2,3-Butanedione, 2,3-Pentanedione, and 2,3-Hexanedione. Toxicol. Pathol. 2016, 44, 763–783. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.L.; Flake, G.P.; Kirby, P.J.; Palmer, S.M. Respiratory toxicity of diacetyl in C57BL/6 mice. Toxicol. Sci. 2008, 103, 169–180. [Google Scholar] [CrossRef]
- Flake, G.P.; Morgan, D.L. Pathology of diacetyl and 2,3-pentanedione airway lesions in a rat model of obliterative bronchiolitis. Toxicology 2017, 388, 40–47. [Google Scholar] [CrossRef]
- Hubbs, A.F.; Goldsmith, W.T.; Kashon, M.L.; Frazer, D.; Mercer, R.R.; Battelli, L.A.; Kullman, G.J.; Schwegler-Berry, D.; Friend, S.; Castranova, V. Respiratory toxicologic pathology of inhaled diacetyl in sprague-dawley rats. Toxicol. Pathol. 2008, 36, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kim, S.Y.; House, E.; Olson, H.M.; Johnston, C.J.; Chalupa, D.; Hernady, E.; Mariani, T.J.; Clair, G.; Ansong, C.; et al. Repetitive diacetyl vapor exposure promotes ubiquitin proteasome stress and precedes bronchiolitis obliterans pathology. Arch. Toxicol. 2021, 95, 2469–2483. [Google Scholar] [CrossRef]
- Morgan, D.L.; Jokinen, M.P.; Price, H.C.; Gwinn, W.M.; Palmer, S.M.; Flake, G.P. Bronchial and bronchiolar fibrosis in rats exposed to 2,3-pentanedione vapors: Implications for bronchiolitis obliterans in humans. Toxicol. Pathol. 2012, 40, 448–465. [Google Scholar] [CrossRef]
- Martinu, T.; Kelly, F.L.; Sun, J.; Zhang, H.L.; Beasley, R.F.; Potts-Kant, E.N.; Flake, G.P.; Morgan, D.L.; Foster, W.M. T Cell-Deficiency Exacerbates Diacetyl-Induced Obliterative Bronchiolitis. J. Heart Lung Transpl. 2013, 32. [Google Scholar] [CrossRef]
- Banfi, A.; Tiszlavicz, L.; Szekely, E.; Petak, F.; Toth-Szuki, V.; Barati, L.; Bari, F.; Novak, Z. Development of bronchus-associated lymphoid tissue hyperplasia following lipopolysaccharide-induced lung inflammation in rats. Exp. Lung Res. 2009, 35, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.R.; Garibaldi, B.T.; Aggarwal, N.R.; Jenkins, J.; Limjunyawong, N.; Singer, B.D.; Chau, E.; Rabold, R.; Files, D.C.; Sidhaye, V.; et al. Foxp3+ regulatory T cells promote lung epithelial proliferation. Mucosal. Immunol. 2014, 7, 1440–1451. [Google Scholar] [CrossRef]
- Aaron, S.D.; Vandemheen, K.L.; Ramsay, T.; Zhang, C.; Avnur, Z.; Nikolcheva, T.; Quinn, A. Multi analyte profiling and variability of inflammatory markers in blood and induced sputum in patients with stable COPD. Respir. Res. 2010, 11, 41. [Google Scholar] [CrossRef]
- Hanidziar, D.; Koulmanda, M. Inflammation and the balance of Treg and Th17 cells in transplant rejection and tolerance. Curr. Opin. Organ. Transpl. 2010, 15, 411–415. [Google Scholar] [CrossRef]
- Mercer, R.R.; Russell, M.L.; Roggli, V.L.; Crapo, J.D. Cell number and distribution in human and rat airways. Am. J. Respir. Cell Mol. Biol. 1994, 10, 613–624. [Google Scholar] [CrossRef]
- Glanville, A.R.; Verleden, G.M.; Todd, J.L.; Benden, C.; Calabrese, F.; Gottlieb, J.; Hachem, R.R.; Levine, D.; Meloni, F.; Palmer, S.M.; et al. Chronic lung allograft dysfunction: Definition and update of restrictive allograft syndrome-A consensus report from the Pulmonary Council of the ISHLT. J. Heart Lung Transpl. 2019, 38, 483–492. [Google Scholar] [CrossRef]
- Shilling, R.A.; Wilkes, D.S. Role of Th17 cells and IL-17 in lung transplant rejection. Semin. Immunopathol. 2011, 33, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Wagner, S.R.; Wu, Q.; Shilling, R.A. IL-17A Blockade Attenuates Obliterative Bronchiolitis and IFN-γ Cellular Immune Response in Lung Allografts. Am. J. Respir. Cell Mol. Biol. 2017, 56, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Benson, H.L.; Vittal, R.; Mickler, E.A.; Presson, R.; Fisher, A.J.; Cummings, O.W.; Heidler, K.M.; Keller, M.R.; Burlingham, W.J.; et al. Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation. Am. J. Transpl. 2011, 11, 911–922. [Google Scholar] [CrossRef]
- Shi, Q.; Cao, H.; Liu, J.; Zhou, X.; Lan, Q.; Zheng, S.; Liu, Z.; Li, Q.; Fan, H. CD4+ Foxp3+ regulatory T cells induced by TGF-β, IL-2 and all-trans retinoic acid attenuate obliterative bronchiolitis in rat trachea transplantation. Int. Immunopharmacol. 2011, 11, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.; Salimian, J.; Bozorgmehr, M.; Vahedi, E.; Ghazvini, A.; Ghanei, M.; Panahi, Y. Assessment of Treg/Th17 axis role in immunopathogenesis of chronic injuries of mustard lung disease. J. Recept. Signal. Transduct. Res. 2016, 36, 531–541. [Google Scholar] [CrossRef]
- Iman, M.; Rezaei, R.; Azimzadeh Jamalkandi, S.; Shariati, P.; Kheradmand, F.; Salimian, J. Th17/Treg immunoregulation and implications in treatment of sulfur mustard gas-induced lung diseases. Expert Rev. Clin. Immunol. 2017, 13, 1173–1188. [Google Scholar] [CrossRef]
- Baraniuk, J.N.; Casado, B.; Pannell, L.K.; McGarvey, P.B.; Boschetto, P.; Luisetti, M.; Iadarola, P. Protein networks in induced sputum from smokers and COPD patients. Int. J. Chron. Obs. Pulmon. Dis. 2015, 10, 1957–1975. [Google Scholar] [CrossRef]
- Zhang, F.; Li, M.-y.; Lan, Y.-t.; Wang, C.-b. Imbalance of Th17/Tregs in rats with smoke inhalation-induced acute lung injury. Sci. Rep. 2016, 6, 21348. [Google Scholar] [CrossRef] [PubMed]
- Gurczynski, S.J.; Moore, B.B. IL-17 in the lung: The good, the bad, and the ugly. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L6–L16. [Google Scholar] [CrossRef]
- Diller, M.L.; Kudchadkar, R.R.; Delman, K.A.; Lawson, D.H.; Ford, M.L. Balancing Inflammation: The Link between Th17 and Regulatory T Cells. Mediat. Inflamm. 2016, 2016, 6309219. [Google Scholar] [CrossRef]
- Li, L.; Kim, J.; Boussiotis, V.A. IL-1beta-mediated signals preferentially drive conversion of regulatory T cells but not conventional T cells into IL-17-producing cells. J. Immunol. 2010, 185, 4148–4153. [Google Scholar] [CrossRef]
- Li, H.; Hua, F.; Zhao, C.; Liu, G.; Zhou, Q. Diagnotic value of the combined determination of telomerase activity in induced sputum, pleural effusion and fiberobronchoscopic biopsy samples in lung cancer. Zhongguo Fei Ai Za Zhi 2010, 13, 128–131. [Google Scholar] [CrossRef]
- Hubbs, A.F.; Fluharty, K.L.; Edwards, R.J.; Barnabei, J.L.; Grantham, J.T.; Palmer, S.M.; Kelly, F.; Sargent, L.M.; Reynolds, S.H.; Mercer, R.R.; et al. Accumulation of Ubiquitin and Sequestosome-1 Implicate Protein Damage in Diacetyl-Induced Cytotoxicity. Am. J. Pathol. 2016, 186, 2887–2908. [Google Scholar] [CrossRef][Green Version]
- Morris, J.B.; Hubbs, A.F. Inhalation dosimetry of diacetyl and butyric acid, two components of butter flavoring vapors. Toxicol. Sci. 2009, 108, 173–183. [Google Scholar] [CrossRef]
- Pengelly, I.; O’Shea, H.; Smith, G.; Coggins, M.A. Measurement of Diacetyl and 2,3-Pentanedione in the Coffee Industry Using Thermal Desorption Tubes and Gas Chromatography-Mass Spectrometry. Ann. Work Expo. Health 2019, 63, 415–425. [Google Scholar] [CrossRef]
- Beckett, E.M.; Cyrs, W.D.; Abelmann, A.; Monnot, A.D.; Gaffney, S.H.; Finley, B.L. Derivation of an occupational exposure limit for diacetyl using dose-response data from a chronic animal inhalation exposure study. J. Appl. Toxicol. 2019, 39, 688–701. [Google Scholar] [CrossRef]
- LeBouf, R.F.; Hawley, B.; Cummings, K.J. Potential Hazards Not Communicated in Safety Data Sheets of Flavoring Formulations, Including Diacetyl and 2,3-Pentanedione. Ann. Work Expo. Health 2019, 63, 124–130. [Google Scholar] [CrossRef]
- Hubbs, A.F.; Battelli, L.A.; Goldsmith, W.T.; Porter, D.W.; Frazer, D.; Friend, S.; Schwegler-Berry, D.; Mercer, R.R.; Reynolds, J.S.; Grote, A.; et al. Necrosis of nasal and airway epithelium in rats inhaling vapors of artificial butter flavoring. Toxicol. Appl. Pharm. 2002, 185, 128–135. [Google Scholar] [CrossRef]
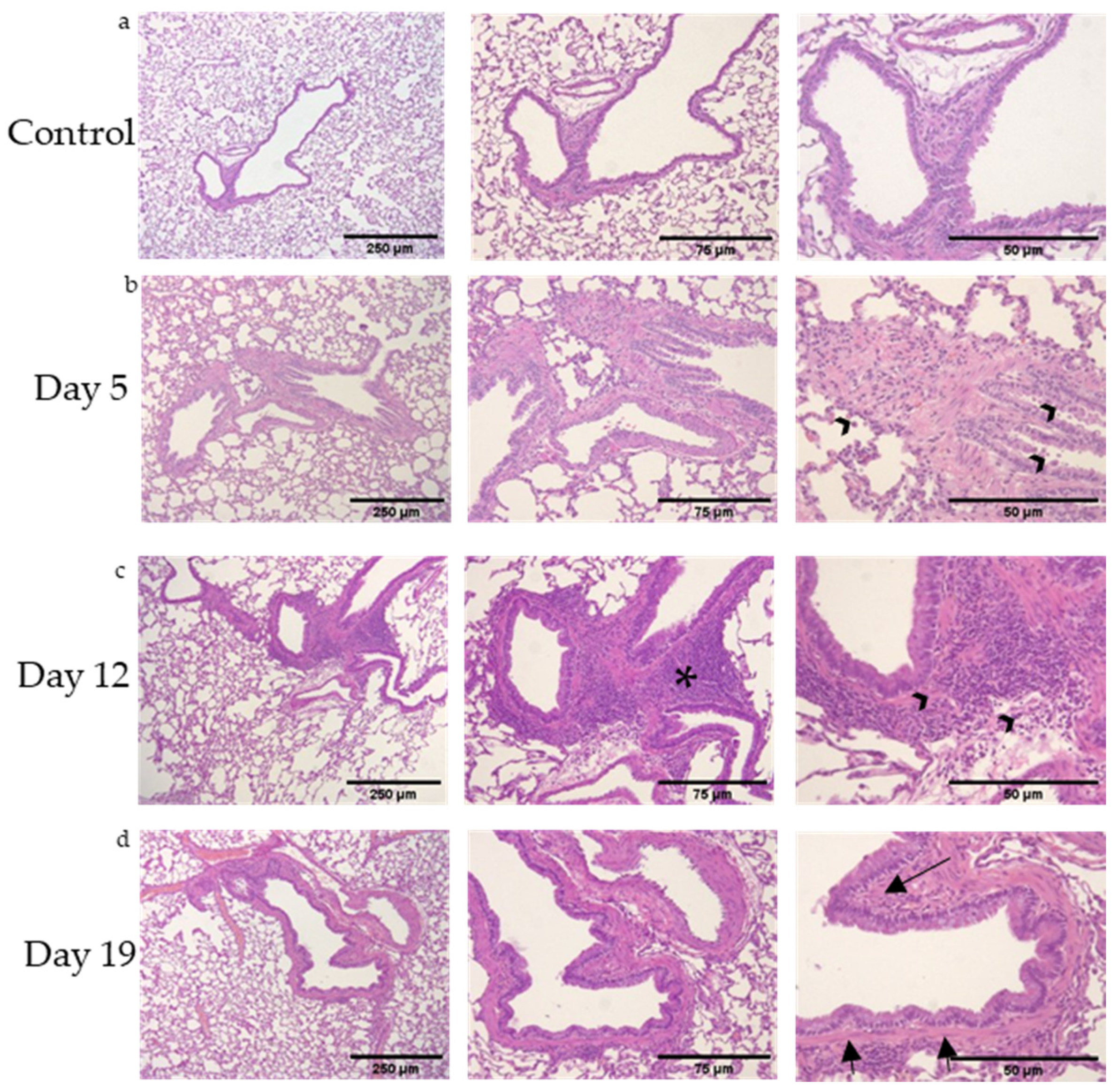
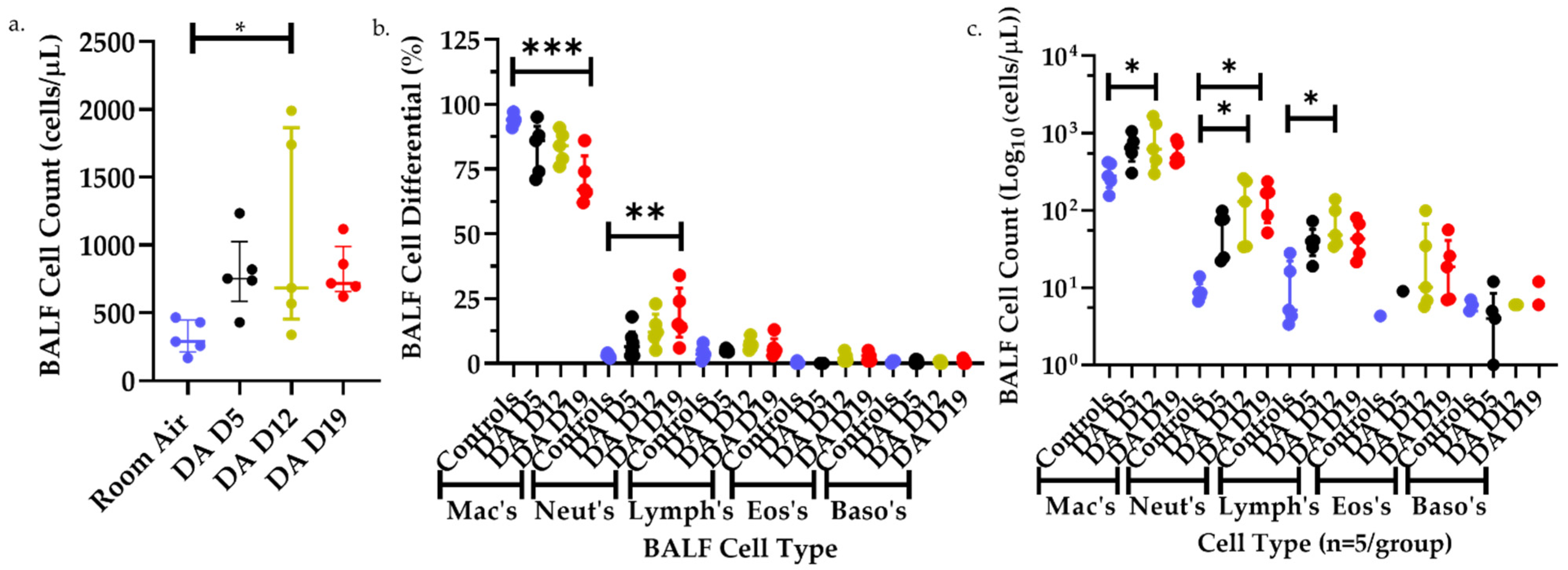
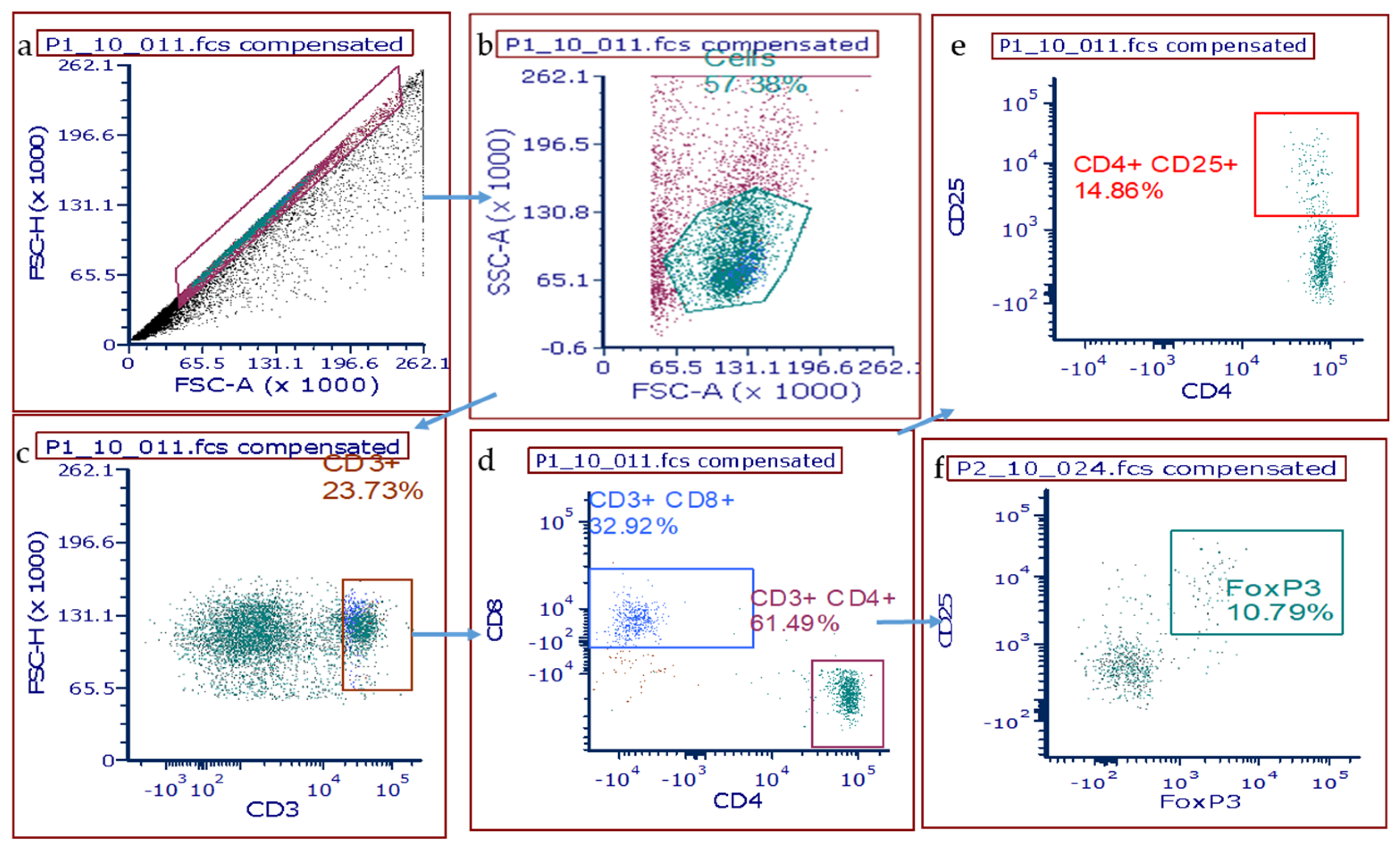
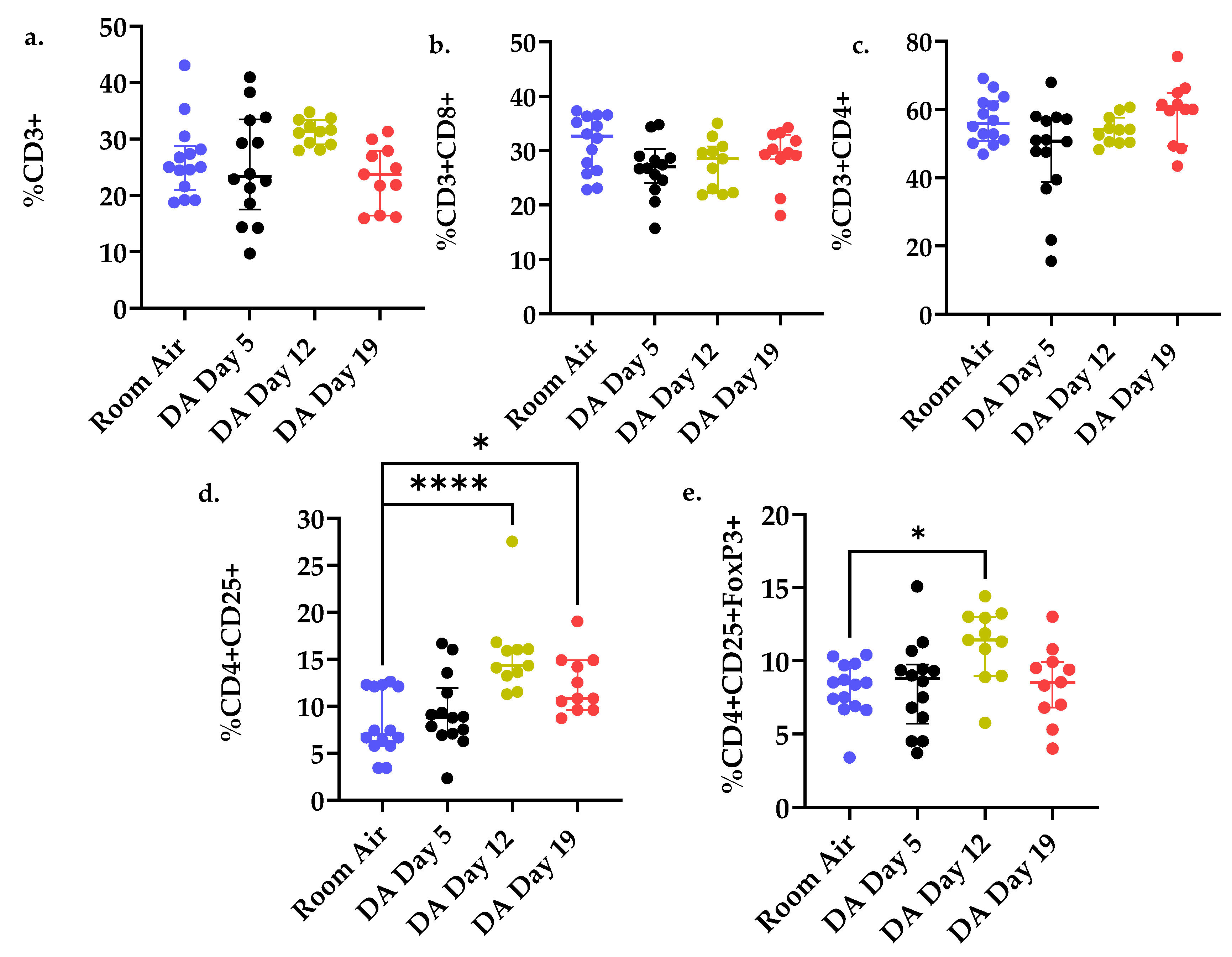
| Group (n = 5 Rats/ Time Point) | Extracellular Matrix Deposition /Rat Lung Section | Bronchial Lymphoid Aggregates /Rat Lung Section |
|---|---|---|
| Air Control | 0.0 +/− 0.0 | 0.0 +/− 0.0 |
| DA D5 | 0.8 +/− 0.5 | 1.6 +/− 0.5 |
| DA D12 | 2.0 +/− 1.0 | 2.6 +/− 0.5 |
| DA D19 | 2.6 +/− 1.5 | 3.0 +/− 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
House, E.L.; Kim, S.-Y.; Johnston, C.J.; Groves, A.M.; Hernady, E.; Misra, R.S.; McGraw, M.D. Diacetyl Vapor Inhalation Induces Mixed, Granulocytic Lung Inflammation with Increased CD4+CD25+ T Cells in the Rat. Toxics 2021, 9, 359. https://doi.org/10.3390/toxics9120359
House EL, Kim S-Y, Johnston CJ, Groves AM, Hernady E, Misra RS, McGraw MD. Diacetyl Vapor Inhalation Induces Mixed, Granulocytic Lung Inflammation with Increased CD4+CD25+ T Cells in the Rat. Toxics. 2021; 9(12):359. https://doi.org/10.3390/toxics9120359
Chicago/Turabian StyleHouse, Emma L., So-Young Kim, Carl J. Johnston, Angela M. Groves, Eric Hernady, Ravi S. Misra, and Matthew D. McGraw. 2021. "Diacetyl Vapor Inhalation Induces Mixed, Granulocytic Lung Inflammation with Increased CD4+CD25+ T Cells in the Rat" Toxics 9, no. 12: 359. https://doi.org/10.3390/toxics9120359
APA StyleHouse, E. L., Kim, S.-Y., Johnston, C. J., Groves, A. M., Hernady, E., Misra, R. S., & McGraw, M. D. (2021). Diacetyl Vapor Inhalation Induces Mixed, Granulocytic Lung Inflammation with Increased CD4+CD25+ T Cells in the Rat. Toxics, 9(12), 359. https://doi.org/10.3390/toxics9120359






