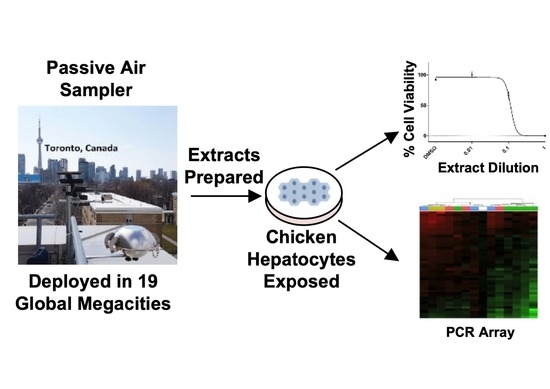
Cytotoxic and Transcriptomic Effects in Avian Hepatocytes Exposed to a Complex Mixture from Air Samples, and Their Relation to the Organic Flame Retardant Signature
Abstract
1. Introduction
2. Materials and Methods
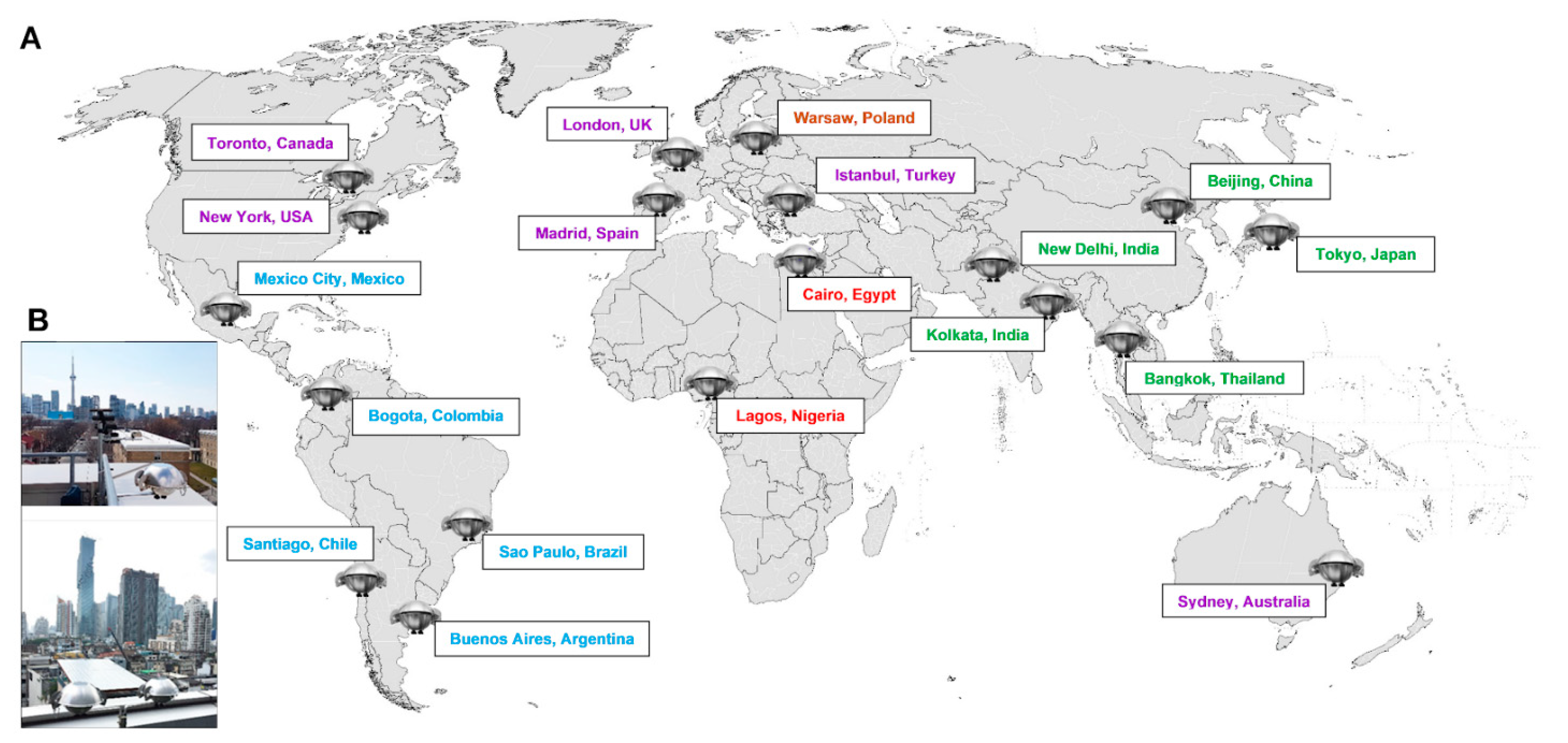
2.1. Passive Air Sampler Deployment and Extract Preparation
2.2. Preparation and Dosing of Chicken Embryonic Hepatocytes
2.3. Cell Viability Determination
2.4. Chicken ToxChip PCR Array
2.5. Data Analysis
3. Results and Discussion
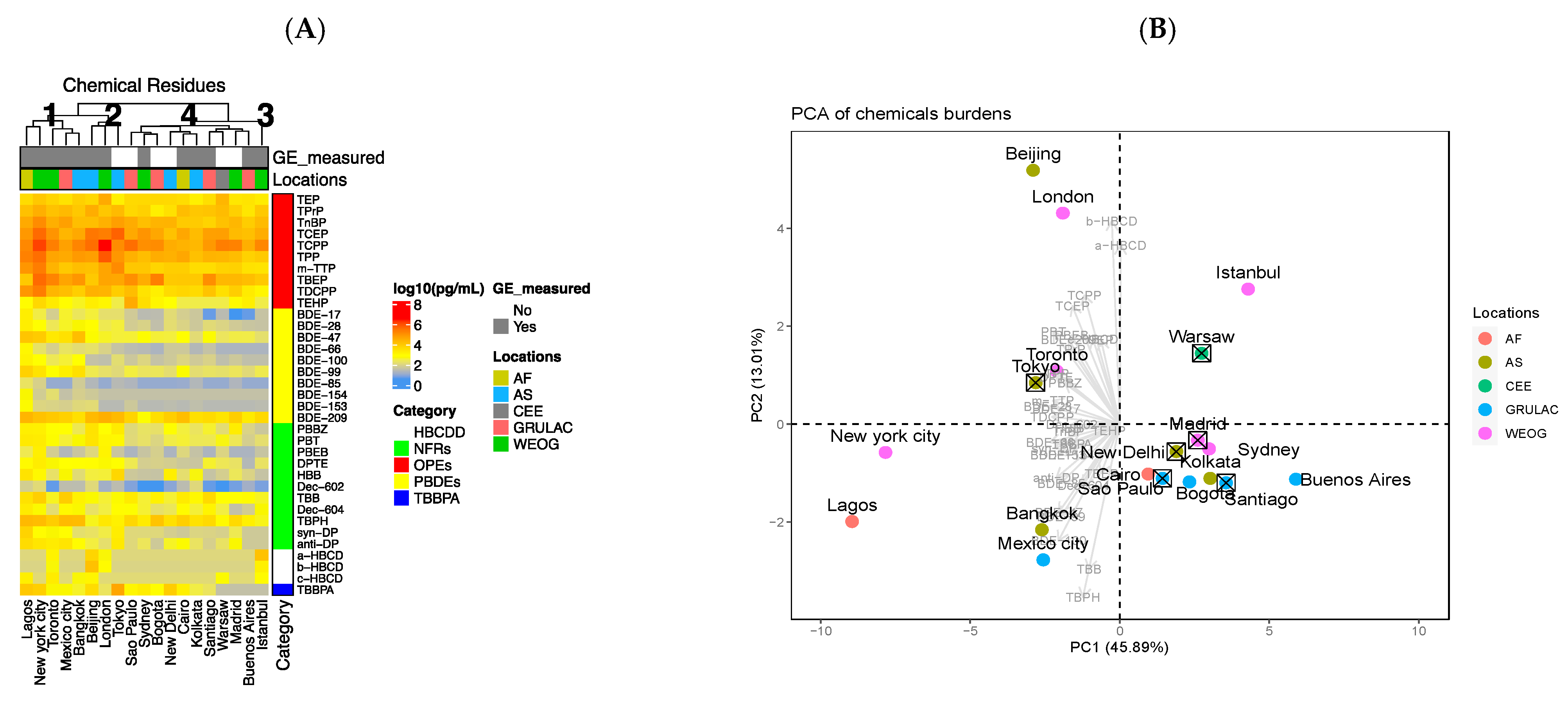
3.1. Organic Flame Retardant Concentrations Vary Greatly between Cities
3.2. PUF Extracts from Megacities Have Highly Variable Cytotoxicity
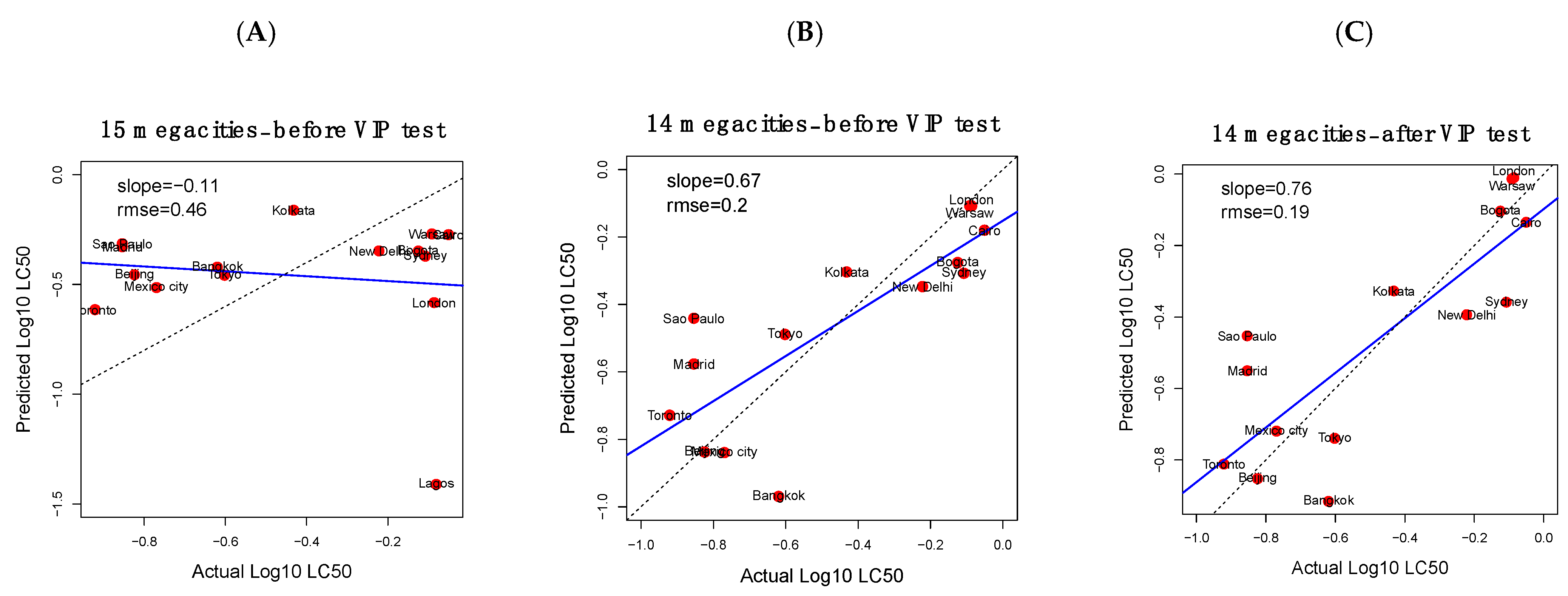
3.3. Cytotoxicity Could Be Estimated from OFR Concentrations Using PLS Regression
3.4. Gene Expression Results Are Variable among Megacities and Not Strongly Correlated to OFR Concentration
3.5. Limitations of the Study
- (i)
- Although the toxicological analysis conducted in this study is based on a unique data set representing average air concentrations from 19 megacities from around the world, information on the contaminant content of these samples is limited. The samples have so far been analyzed for one general class of contaminants—organic flame retardants—of which organophosphate flame retardants (OPFRs) were by far the dominant class. A future priority is to analyze the samples for a broader suite of contaminants, including for instance, polycyclic aromatic compounds, which could be especially relevant to urban population exposures and related biological responses. Transformation products of commercial chemicals such as the OPFRs that are abundant in air and formed through oxidation reactions have recently been shown to significantly contribute to exposure and risk assessment [33]. This highlights challenges in associating biological responses to individual classes of “known” chemicals when extremely large numbers of “unknown” chemicals are also present in air at toxicologically relevant concentrations. A more holistic approach to contaminant mixture assessment may be needed.
- (ii)
- Although best efforts were made to collect samples that were “representative” of a large area from each city, it is likely that megacities have a degree of heterogeneity in terms of contaminant mixtures in air, depending on the location and proximity to sources. This introduces some uncertainty in the characterization and comparison of results among cities. Additionally, in the current study, contaminant analysis and toxicological assessment were conducted on consecutive three-month samples from the same site, which may have introduced additional uncertainty associated with temporal variability of contaminants in air. Future work should evaluate the degree of heterogeneity in biological response and chemical profiles for air collected in different parts of a city with differing land-use characteristics (e.g., residential, traffic, industry, commercial etc.) and at different seasons. Some work on this topic has recently begun in Toronto [24,42], but not for the other megacities investigated here.
- (iii)
- In this study, in vitro effects were determined using avian hepatic cells to provide a theoretical basis for the approach. Future work should explore other in vitro models (e.g., human/mammalian lung cells) which could be more relevant for assessing human health risks. Although liver is a commonly used organ for toxicological studies, performing this assay with a broader range of cell types (e.g., lung epithelial, cardiomyocytes) would be warranted, especially given the negative health effects of air pollution associated with cardiovascular/respiratory endpoints [43,44].
- (iv)
- The dose range used for cell viability and gene expression evaluation was limited (i.e., selected to provide an initial qualitative comparison across megacities as opposed to permitting full transcriptomics dose-response analysis or comparison of relative potencies). For example, Toronto had the lowest LC50 value (Table 1) and the most pronounced gene dysregulation (Cluster 4; Figure 3A); however, the concentration used for gene expression analysis (0.1) was similar to the median lethal concentration (0.12; Table 1).
- (v)
- Finally, gene expression data are based on a reduced transcriptome (43 genes), meaning that a large percentage of biological pathways/processes were not considered in the analysis.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; von Gunten, U.; Wehrli, B. The Challenge of Micropollutants in Aquatic Systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef]
- Neale, P.; Altenburger, R.; Ait-Aissa, S.; Brion, F.; Busch, W.; Umbuzeiro, G.; Denison, M.S.; Du Pasquier, D.; Hilscherová, K.; Hollert, H.; et al. Development of a bioanalytical test battery for water quality monitoring: Fingerprinting identified micropollutants and their contribution to effects in surface water. Water Res. 2017, 123, 734–750. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, X.; Cai, Z. Analytical chemistry of the persistent organic pollutants identified in the Stockholm Convention: A review. Anal. Chim. Acta 2013, 790, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shoeib, M.; Harner, T. Characterization and Comparison of Three Passive Air Samplers for Persistent Organic Pollutants. Environ. Sci. Technol. 2002, 36, 4142–4151. [Google Scholar] [CrossRef]
- Escher, B. Bioanalytical Tools in Water Quality Assessment; Iwa Publishing: London, UK, 2011; p. 267. [Google Scholar]
- Liu, J.; Zhang, X. Comparative toxicity of new halophenolic DBPs in chlorinated saline wastewater effluents against a marine alga: Halophenolic DBPs are generally more toxic than haloaliphatic ones. Water Res. 2014, 65, 64–72. [Google Scholar] [CrossRef]
- Yang, Y.; Komaki, Y.; Kimura, S.Y.; Hu, H.-Y.; Wagner, E.D.; Mariñas, B.J.; Plewa, M.J. Toxic Impact of Bromide and Iodide on Drinking Water Disinfected with Chlorine or Chloramines. Environ. Sci. Technol. 2014, 48, 12362–12369. [Google Scholar] [CrossRef] [PubMed]
- Crump, U.; Williams, K.L.; Chiu, S.; Letcher, R.J.; Periard, L.; Kennedy, S.W. Biochemical and Transcriptomic Effects of Herring Gull Egg Extracts from Variably Contaminated Colonies of the Laurentian Great Lakes in Chicken Hepatocytes. Environ. Sci. Technol. 2015, 49, 10190–10198. [Google Scholar] [CrossRef]
- Crump, D.; Williams, K.L.; Chiu, S.; Periard, L.; Letcher, R.J. A rapid method of preparing complex organohalogen extracts from avian eggs: Applications to in vitro toxicogenomics screening. Environ. Toxicol. Chem. 2019, 38, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Mundy, L.J.; Williams, K.L.; Chiu, S.; Pauli, B.D.; Crump, D. Extracts of Passive Samplers Deployed in Variably Contaminated Wetlands in the Athabasca Oil Sands Region Elicit Biochemical and Transcriptomic Effects in Avian Hepatocytes. Environ. Sci. Technol. 2019, 53, 9192–9202. [Google Scholar] [CrossRef]
- Xia, P.; Crump, D.; Chiu, S.; Chan, H.-M.; O’Brien, J.M. Toxicogenomic Assessment of Complex Chemical Signatures in Double-Crested Cormorant Embryos from Variably Contaminated Great Lakes Sites. Environ. Sci. Technol. 2020, 54, 7504–7512. [Google Scholar] [CrossRef]
- Li, S.; Villeneuve, D.L.; Berninger, J.P.; Blackwell, B.; Cavallin, J.E.; Hughes, M.N.; Jensen, K.M.; Jorgenson, Z.; Kahl, M.D.; Schroeder, A.L.; et al. An integrated approach for identifying priority contaminant in the Great Lakes Basin—Investigations in the Lower Green Bay/Fox River and Milwaukee Estuary areas of concern. Sci. Total Environ. 2017, 579, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Pujolar, J.M.; Jacobsen, M.W.; Als, T.D.; Frydenberg, J.; Munch, K.; Jónsson, B.; Jian, J.B.; Cheng, L.; Maes, G.E.; Bernatchez, L.; et al. Genome-wide single-generation signatures of local selection in the panmictic e uropean eel. Mol. Ecol. 2014, 23, 2514–2528. [Google Scholar] [CrossRef] [PubMed]
- Zahaby, Y.; Xia, P.; Crump, D.; Provencher, J.F.; Thomas, P.J.; Pauli, B.; Braune, B.M.; Franckowiak, R.P.; Gendron, M.; Savard, G.; et al. ToxChip PCR Arrays for Two Arctic-Breeding Seabirds: Applications for Regional Environmental Assessments. Environ. Sci. Technol. 2021, 55, 7521–7530. [Google Scholar] [CrossRef] [PubMed]
- Stanek, L.W.; Brown, J.S.; Stanek, J.; Gift, J.; Costa, D.L. Air Pollution Toxicology--A Brief Review of the Role of the Science in Shaping the Current Understanding of Air Pollution Health Risks. Toxicol. Sci. 2010, 120, S8–S27. [Google Scholar] [CrossRef] [PubMed]
- Escher, B.I.; Stapleton, H.M.; Schymanski, E.L. Tracking complex mixtures of chemicals in our changing environment. Science 2020, 367, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Érseková, A.; Hilscherová, K.; Klánová, J.; Giesy, J.P.; Novák, J. Effect-based assessment of passive air samples from four countries in eastern europe. Environ. Monit. Assess. 2014, 186, 3905–3916. [Google Scholar] [CrossRef]
- Kennedy, K.; Macova, M.; Bartkow, M.E.; Hawker, D.W.; Zhao, B.; Denison, M.S.; Mueller, J. Effect based monitoring of seasonal ambient air exposures in Australia sampled by PUF passive air samplers. Atmos. Pollut. Res. 2010, 1, 50–58. [Google Scholar] [CrossRef][Green Version]
- Pozo, K.; Harner, T.; Wania, F.; Muir, D.C.G.; Jones, K.C.; Barrie, L.A. Toward a Global Network for Persistent Organic Pollutants in Air: Results from the GAPS Study. Environ. Sci. Technol. 2006, 40, 4867–4873. [Google Scholar] [CrossRef]
- Saini, A.; Harner, T.; Chinnadhurai, S.; Schuster, J.K.; Yates, A.; Sweetman, A.; Aristizabal-Zuluaga, B.H.; Jiménez, B.; Manzano, C.A.; Gaga, E.O.; et al. GAPS-megacities: A new global platform for investigating persistent organic pollutants and chemicals of emerging concern in urban air. Environ. Pollut. 2020, 267, 115416. [Google Scholar] [CrossRef]
- Bogdal, C.; Wang, Z.; Buser, A.M.; Scheringer, M.; Gerecke, A.C.; Schmid, P.; Müller, C.E.; MacLeod, M.; Hungerbühler, K. Emissions of polybrominated diphenyl ethers (PBDEs) in Zurich, Switzerland, determined by a combination of measurements and modeling. Chemosphere 2014, 116, 15–23. [Google Scholar] [CrossRef]
- Chakraborty, P.; Zhang, G.; Cheng, H.; Balasubramanian, P.; Li, J.; Jones, K.C. Passive air sampling of polybrominated diphenyl ethers in New Delhi, Kolkata, Mumbai and Chennai: Levels, homologous profiling and source apportionment. Environ. Pollut. 2017, 231, 1181–1187. [Google Scholar] [CrossRef]
- Rodgers, T.F.M.; Truong, J.; Jantunen, L.M.; Helm, P.A.; Diamond, M.L. Organophosphate Ester Transport, Fate, and Emissions in Toronto, Canada, Estimated Using an Updated Multimedia Urban Model. Environ. Sci. Technol. 2018, 52, 12465–12474. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Clarke, J.; Jariyasopit, N.; Rauert, C.; Schuster, J.K.; Halappanavar, S.; Evans, G.J.; Su, Y.; Harner, T. Flame retardants in urban air: A case study in Toronto targeting distinct source sectors. Environ. Pollut. 2019, 247, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Rauert, C.; Schuster, J.K.; Eng, A.; Harner, T. Global Atmospheric Concentrations of Brominated and Chlorinated Flame Retardants and Organophosphate Esters. Environ. Sci. Technol. 2018, 52, 2777–2789. [Google Scholar] [CrossRef]
- Crump, D.; Chiu, S.; Kennedy, S.W. Effects of Tris(1,3-dichloro-2-propyl) phosphate and Tris(1-chloropropyl) phosphate on Cytotoxicity and mRNA Expression in Primary Cultures of Avian Hepatocytes and Neuronal Cells. Toxicol. Sci. 2012, 126, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Head, J.A.; O’Brien, J.; Kennedy, S.W. EXPOSURE TO 3,3′,4,4′,5-pentachlorobiphenyl during embryonic development has a minimal effect on the cytochrome p4501a response to 2,3,7,8-tetrachlorodibenzo-p-dioxin in cultured chicken embryo hepatocytes. Environ. Toxicol. Chem. 2006, 25, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.; Crump, D.; Egloff, C.; Chiu, S.; Kennedy, S.W. Use of an avian hepatocyte assay and the avian toxchip polymerse chain reaction array for testing prioritization of 16 organic flame retardants. Environ. Toxicol. Chem. 2014, 33, 573–582. [Google Scholar] [CrossRef]
- Pagé-Larivière, F.; Chiu, S.; Jones, S.P.; Farhat, A.; Crump, D.; O’Brien, J.M. Prioritization of 10 organic flame retardants using an avian hepatocyte toxicogenomic assay. Environ. Toxicol. Chem. 2018, 37, 3134–3144. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time pcr data by the comparative c t method. Nat. Protoc. 2008, 3, 1101. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
- Helland, I.S. Partial least squares regression and statistical models. Scand. J. Stat. 1990, 17, 97–114. [Google Scholar]
- Liu, Q.; Li, L.; Zhang, X.; Saini, A.; Li, W.; Hung, H.; Hao, C.; Li, K.; Wentzell, J.; Huo, C.; et al. Uncovering New Global Risks from Commercial Chemicals in Air. Nature 2021. Accepted. [Google Scholar]
- Su, G.; Letcher, R.J.; Crump, U.; Farmahin, R.; Giesy, J.P.; Kennedy, S.W. Photolytic Degradation Products of Two Highly Brominated Flame Retardants Cause Cytotoxicity and mRNA Expression Alterations in Chicken Embryonic Hepatocytes. Environ. Sci. Technol. 2014, 48, 12039–12046. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Letcher, R.J.; Farmahin, R.; Crump, D. Photolysis of highly brominated flame retardants leads to time-dependent dioxin-responsive mRNA expression in chicken embryonic hepatocytes. Chemosphere 2018, 194, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Manning, G.E.; Farmahin, R.; Crump, D.; Zhang, X.; Kennedy, S.W. Relative Potencies of Aroclor Mixtures Derived from Avian in Vitro Bioassays: Comparisons with Calculated Toxic Equivalents. Environ. Sci. Technol. 2013, 47, 8852–8861. [Google Scholar] [CrossRef]
- Blackwell, B.R.; Ankley, G.T.; Bradley, P.M.; Houck, K.; Makarov, S.S.; Medvedev, A.V.; Swintek, J.; Villeneuve, D.L. Potential Toxicity of Complex Mixtures in Surface Waters from a Nationwide Survey of United States Streams: Identifying in Vitro Bioactivities and Causative Chemicals. Environ. Sci. Technol. 2019, 53, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ji, C.; Yin, X.; Yan, L.; Lu, M.; Zhao, M. Thyroid hormone-disrupting activity and ecological risk assessment of phosphorus-containing flame retardants by in vitro, in vivo and in silico approaches. Environ. Pollut. 2016, 210, 27–33. [Google Scholar] [CrossRef]
- Guigueno, M.F.; Fernie, K.J. Birds and flame retardants: A review of the toxic effects on birds of historical and novel flame retardants. Environ. Res. 2017, 154, 398–424. [Google Scholar] [CrossRef]
- Crump, D.; Williams, K.L.; Chiu, S.; Zhang, Y.; Martin, J.W. Athabasca Oil Sands Petcoke Extract Elicits Biochemical and Transcriptomic Effects in Avian Hepatocytes. Environ. Sci. Technol. 2017, 51, 5783–5792. [Google Scholar] [CrossRef]
- Farhat, A.; Crump, D.; Porter, E.; Chiu, S.; Letcher, R.J.; Su, G.; Kennedy, S.W. Time-dependent effects of the flame retardant tris(1,3-dichloro-2-propyl) phosphate (TDCPP) on mRNA expression, in vitro and in ovo, reveal optimal sampling times for rapidly metabolized compounds. Environ. Toxicol. Chem. 2014, 33, 2842–2849. [Google Scholar] [CrossRef]
- Halappanavar, S.; Wu, D.; Boyadzhiev, A.; Solorio-Rodriguez, S.; Williams, A.; Jaroyasopit, N.; Saini, A.; Harner, T. Toxicity screening of air extracts representing different source sectors in the city of Toronto: In vitro oxidative stress, pro-inflammatory response, and toxicogenomic analysis. Mutat. Res.—Genet. Toxicol. Environ. Mutagenesis 2021, 872, 503415. [Google Scholar] [CrossRef] [PubMed]
- González-Díaz, S.; Arias-Cruz, A.; Macouzet-Sánchez, C.; Partida-Ortega, A. Impact of air pollution in respiratory allergic diseases. Med. Univ. 2016, 18, 212–215. [Google Scholar] [CrossRef]
- Teoldi, F.; Lodi, M.; Benfenati, E.; Colombo, A.; Baderna, D. Air quality in the Olona Valley and in vitro human health effects. Sci. Total Environ. 2017, 579, 1929–1939. [Google Scholar] [CrossRef] [PubMed]


| Chemical Profile | Gene Expression Profile | |||||
|---|---|---|---|---|---|---|
| Location Name | Total OFR | LC50 | Cluster a | PC1 b | Cluster c | PC1 d |
| Toronto, Canada | 652,000 | 0.12 | 1 | −2.1 | 4 | 10.83 |
| Madrid, Spain | 298,000 | 0.14 | 4 | 2.61 | ND | ND |
| Sao Paulo, Brazil | 682,000 | 0.14 | 4 | 1.43 | ND | ND |
| Beijing, China | 878,000 | 0.15 | 2 | −2.9 | 3 | −0.42 |
| Mexico City, Mexico | 304,000 | 0.17 | 1 | −2.56 | 3 | 3.55 |
| Bangkok, Thailand | 458,000 | 0.24 | 1 | −2.61 | 2 | −3.03 |
| Tokyo, Japan | 1,106,000 | 0.25 | 2 | −2.81 | ND | ND |
| Kolkata, India | 85,000 | 0.37 | 4 | 3.02 | 1 | −6.93 |
| New Delhi, India | 150,000 | 0.60 | 4 | 1.89 | ND | ND |
| Bogota, Colombia | 473,000 | 0.76 | 4 | 3.55 | ND | ND |
| Sydney, Australia | 303,000 | 0.78 | 4 | 2.98 | 3 | 4.22 |
| Warsaw, Poland | 388,000 | 0.81 | 4 | 2.74 | ND | ND |
| London, UK | 4,606,000 | 0.81 | 2 | −1.9 | 3 | 7.81 |
| Lagos, Nigeria | 747,000 | 0.83 | 1 | −8.95 | 1 | −4.80 |
| Cairo, Egypt | 188,000 | 0.89 | 4 | 0.95 | 1 | −6.79 |
| Buenos Aires, Argentina | 89,000 | - | 4 | 5.89 | 2 | −0.60 |
| Istanbul, Turkey | 250,000 | - | 3 | 4.29 | 3 | 3.38 |
| New York, USA | 3,292,000 | - | 1 | −7.83 | 2 | −2.85 |
| Santiago, Chile | 323,000 | ND | 4 | 2.33 | 1 | −4.37 |
| Regression with log (OFR) | - | p = 0.8628 | p = 0.0071 * | p = 0.0012 * | p = 0.2785 | p = 0.3131 |
| - | r2 = 0.0024 | r2 = 0.3546 | r2 = 0.4698 | r2 = 0.1057 | r2 = 0.0922 | |
| Regression with log (LC50) | p = 0.8628 | - | p = 0.21893 | p = 0.6370 | p = 0.1235 | p = 0.5471 |
| r2 = 0.0024 | - | r2 = 0.1138 | r2 = 0.0176 | r2 = 0.3045 | r2 = 0.0541 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, K.; Xia, P.; Crump, D.; Saini, A.; Harner, T.; O’Brien, J. Cytotoxic and Transcriptomic Effects in Avian Hepatocytes Exposed to a Complex Mixture from Air Samples, and Their Relation to the Organic Flame Retardant Signature. Toxics 2021, 9, 324. https://doi.org/10.3390/toxics9120324
Ha K, Xia P, Crump D, Saini A, Harner T, O’Brien J. Cytotoxic and Transcriptomic Effects in Avian Hepatocytes Exposed to a Complex Mixture from Air Samples, and Their Relation to the Organic Flame Retardant Signature. Toxics. 2021; 9(12):324. https://doi.org/10.3390/toxics9120324
Chicago/Turabian StyleHa, Kelsey, Pu Xia, Doug Crump, Amandeep Saini, Tom Harner, and Jason O’Brien. 2021. "Cytotoxic and Transcriptomic Effects in Avian Hepatocytes Exposed to a Complex Mixture from Air Samples, and Their Relation to the Organic Flame Retardant Signature" Toxics 9, no. 12: 324. https://doi.org/10.3390/toxics9120324
APA StyleHa, K., Xia, P., Crump, D., Saini, A., Harner, T., & O’Brien, J. (2021). Cytotoxic and Transcriptomic Effects in Avian Hepatocytes Exposed to a Complex Mixture from Air Samples, and Their Relation to the Organic Flame Retardant Signature. Toxics, 9(12), 324. https://doi.org/10.3390/toxics9120324