Acrylamide Content in Breast Milk: The Evaluation of the Impact of Breastfeeding Women’s Diet and the Estimation of the Exposure of Breastfed Infants to Acrylamide in Breast Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Study Group
2.1.2. Breast Milk Sample Collection
2.1.3. Dietary Intake Assessment
2.2. METHODS
2.2.1. Determining the Acrylamide Level in Breast Milk
Chemicals
Preparation of Breast Milk Samples and LC–MS/MS Analysis
2.2.2. Estimation of the Exposure of Breastfed Infants to Acrylamide Present in Breast Milk
2.2.3. Estimation of the Exposure of Breastfeeding Women to Dietary Acrylamide
2.2.4. Statistical Evaluation
3. Results
3.1. The Characteristics of the Breastfeeding Women and Their Children
3.2. LC–MS/MS Method Validation
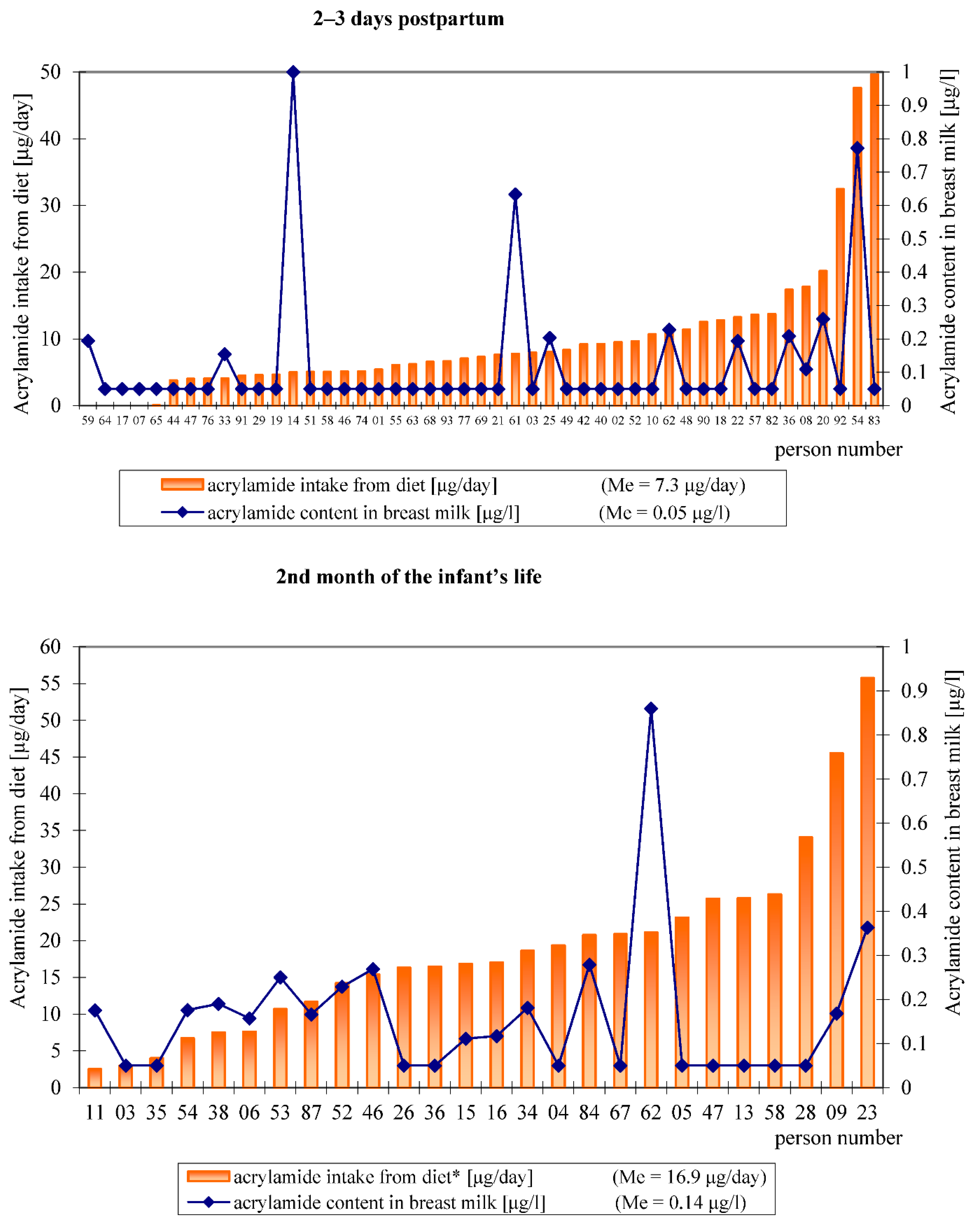
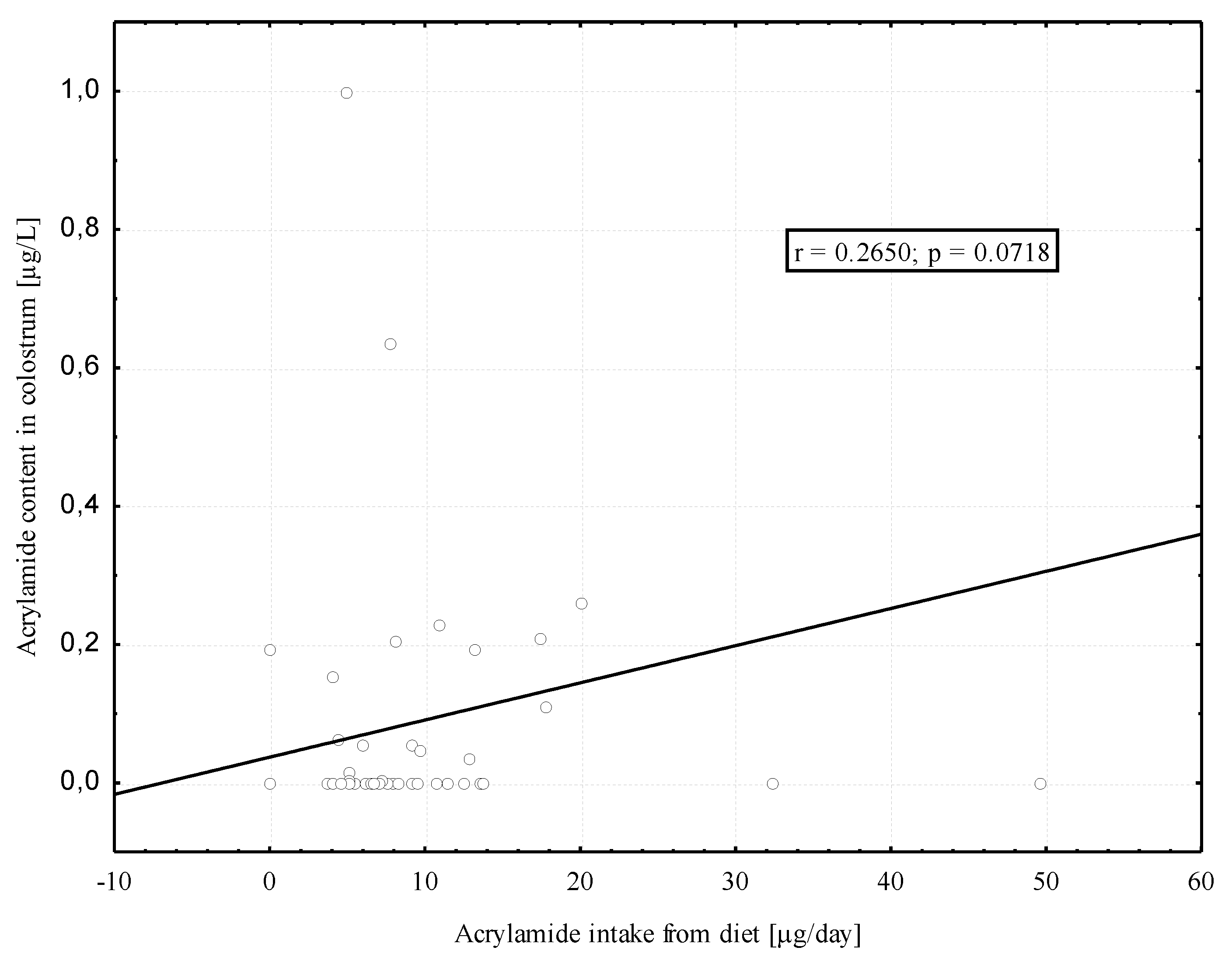
3.3. The Acrylamide Level in Breast Milk
3.4. Dietary Acrylamide Intake of Breastfeeding Women
3.5. The Estimation of the Infants’ Exposure to Acrylamide Present in Breast Milk and the Breastfeeding Women’s Exposure to Acrylamide from Their Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Health Topics/Breastfeeding. Available online: https://www.who.int/health-topics/breastfeeding (accessed on 12 June 2021).
- Hall, B. Uniformity of human milk. Am. J. Clin. Nutr. 1979, 32, 304–312. [Google Scholar] [CrossRef] [Green Version]
- Koletzko, B.; Godfrey, K.M.; Poston, L.; Szajewska, H.; Van Goudoever, J.B.; De Waard, M.; Brands, B.; Grivell, R.M.; Deussen, A.R.; Dodd, J.M.; et al. Nutrition during pregnancy, lactation, and early childhood and its implications for maternal and long-term child health: The Early Nutrition Project recommendations. Ann. Nutr. Metab. 2019, 74, 93–106. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer: Acrylamide. Summary of Data Reported and Evaluation. W: IARC Monographs on the Evaluations of Carcinogenic Risks to Humans; Some industrial Chemicals; IARC: Lyon, France, 1994; Volume 60, pp. 389–433. Available online: https://monograpfs.iarc.fr/iarc-monographs-on-the-evaluation-of-carcinogenic-risks-to-human-61/ (accessed on 12 March 2020).
- Bergmark, E. Hemoglobin adducts of acrylamide and acrylonitrile in laboratory workers, smokers and nonsmokers. Chem. Res. Toxicol. 1997, 10, 78–84. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhang, S.; Wang, H.; Li, G.; Zhang, Z.; Li, F.; Dong, X.; Hu, F. Neurological and electroneuromyographic assessment of the adverse effects of acrylamide on occupationally exposed workers. Scand. J. Work Environ. Heath 1989, 15, 125–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Tornqvist, M. Acrylamide: A cooking carcinogen? Chem. Res. Toxicol. 2000, 13, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Tőrnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef] [PubMed]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef]
- Stadler, R.H.; Scholz, G. Acrylamide: An update on current knowledge in analysis, levels in food, mechanisms of formation, and potential strategies of control. Nutr. Rev. 2004, 62, 449–467. [Google Scholar] [CrossRef]
- European Food Safety Authority. Panel on Contaminants in the Food Chain: Scientific Opinion on acrylamide in food. EFSA J. 2015, 13, 4104. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/4104 (accessed on 13 March 2020).
- Smith, C.J.; Perfetti, T.A.; Rumple, M.A.; Rodgman, A.; Doolittle, D.J. “IARC Group 2A Carcinogens” Reported in Cigarette Mainstream Smoke. Food Chem. Toxicol. 2000, 38, 371–383. [Google Scholar] [CrossRef]
- Moldoveanu, S.C.; Gerardi, A.R. Acrylamide analysis in tobacco, alternative tobacco products, and cigarette smoke. J. Chromatogr. Sci. 2011, 49, 234–242. [Google Scholar] [CrossRef]
- Mojska, H.; Gielecińska, I.; Cendrowski, A. Acrylamide content in cigarette mainstream smoke and estimation of exposure to acrylamide from tobacco smoke in Poland. Ann. Agric. Environ. Med. 2016, 23, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Sansano, M.; Heredia, A.; Peinado, I.; Andres, A. Dietary acrylamide: What happens during digestion. Food Chem. 2017, 237, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Hamzalioglu, A.; Gokmen, V. Investigation of the reactions of acrylamide during in vitro multistep enzymatic digestion of thermally processed foods. Food Funct. 2015, 6, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Hamzalioglu, A.; Gokmen, V. Investigation and kinetic evaluation of the reactions of hydroxymethylfurfural with amino and thiol groups of amino acids. Food Chem. 2018, 240, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Bolger, P.M.; Leblanc, J.-C.; Setzer, R.W. Application of the Margin of Exposure (MoE) approach to substances in food that are genotoxic and carcinogenic. Example: Acrylamide (CAS No. 79-06-1). Food Chem. Toxicol. 2010, 48, S25–S33. [Google Scholar] [CrossRef]
- JECFA. Evaluation of Certain Food Additives and Contaminants; 72nd Report of the Joint FAO/WHO Expert Committee on Food Additive; WHO Technical Report Series; World Health Organization: Rome, Italy, 2011; p. 959. [Google Scholar]
- Von Tungeln, L.S.; Doerge, D.R.; Gamboa da Costa, G.; Marques, M.M.; Witt, W.M.; Koturbash, I.; Pogribny, I.P.; Beland, F.A. Tumorigenicity of acrylamide and its metabolite glycidamide in the neonatal mouse bioassay. Int. J. Cancer 2012, 131, 2008–2015. [Google Scholar] [CrossRef] [Green Version]
- Bjellaas, T.; Stølen, L.H.; Haugen, M.; Paulsen, J.E.; Alexander, J.; Lundanes, E.; Becher, G. Urinary acrylamide metabolites as biomarker for short-term dietary exposure to acrylamide. Food Chem. Toxicol. 2007, 45, 1020–1026. [Google Scholar] [CrossRef]
- Boettcher, M.I.; Schettgen, T.; Kütting, B.; Pischetsrieder, M.; Angerer, J. Mercapturic acids of acrylamide and glycidamide as biomarkers of the internal exposure to acrylamide in the general population. Mutat. Res. 2005, 580, 167–176. [Google Scholar] [CrossRef]
- Goempel, K.; Tedsen, L.; Ruenz, M.; Bakuradze, T.; Schipp, D.; Galan, J.; Eisenbrand, G.; Richling, E. Biomarker monitoring of controlled dietary acrylamide exposure indicates consistent human endogenous background. Arch. Toxicol. 2017, 91, 3551–3560. [Google Scholar] [CrossRef]
- Vikström, A.C.; Warholm, M.; Paulsson, B.; Axmon, A.; Wirfält, E.; Törnqvist, M. Hemoglobin adducts as a measure of variations in exposure to acrylamide in food and comparison to questionnaire data. Food Chem. Toxicol. 2012, 50, 2531–2539. [Google Scholar] [CrossRef]
- Annola, K.; Karttunen, V.; Keski-Rahkonen, P.; Myllynen, P.; Segerbäck, D.; Heinonen, S.; Vähäkangas, K. Transplacental transfer of acrylamide and glycidamide are comparable to that of antipyrine in perfused human placenta. Toxicol. Lett 2008, 182, 50–56. [Google Scholar] [CrossRef]
- Tyl, R.W.; Friedman, M.A. Effects of acrylamide on rodent reproductive performance. Reprod. Toxicol. 2003, 17, 1–13. [Google Scholar] [CrossRef]
- Manson, J.; Brabec, M.J.; Buelke-Sam, J.; Carlson, G.P.; Chapin, R.E.; Favor, J.B.; Fischer, L.J.; Hattis, D.; Lees, P.S.J.; Perreault-Darney, S.; et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of acrylamide. Birth Defects Res. B Dev. Reprod. Toxicol. 2005, 74, 17–113. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Salles, T.; von Stedingk, H.; Granum, B.; Gützkow, K.B.; Rydberg, P.; Törnqvist, M.; Mendez, M.A.; Brunborg, G.; Brantsæter, A.L.; Meltzer, H.M.; et al. Dietary acrylamide intake during pregnancy and fetal growth results from the Norwegian Mother and Child Cohort study (MoBa). Environ. Health Perspect. 2013, 121, 374–379. [Google Scholar] [CrossRef]
- Pedersen, M.; von Stedingk, H.; Botsivali, M.; Agramunt, S.; Alexander, J.; Brunborg, G.; Chatzi, L.; Fleming, S.; Fthenou, E.; Granum, B.; et al. NewGeneris Consortium. Birth weight, head circumference, and prenatal exposure to acrylamide from maternal diet: The European prospective mother-child study (NewGeneris). Environ. Health Perspect. 2012, 120, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Kadawathagedara, M.; Chan Hon Tong, A.; Heude, B.; Forhan, A.; Charles, M.-A.; Sirot, V.; Botton, J. The EDEN mother-child cohort study group: Dietary acrylamide intake during pregnancy and anthropometry at birth in the French EDEN mother-child cohort study. Environ. Res. J. 2016, 149, 189–196. [Google Scholar] [CrossRef]
- Hogervorst, J.; Vesper, H.W.; Madhloum, N.; Gyselaers, W.; Nawrot, T. Cord blood acrylamide levels and birth size, and interactions with genetic variants in acrylamide-metabolising genes. Environ. Health 2021, 20, 35. [Google Scholar] [CrossRef]
- Nagata, C.; Konishi, K.; Wada, K.; Tamura, T.; Goto, Y.; Koda, S.; Mizuta, F.; Iwasa, S. Material acrylamide intake during pregnancy and sex hormone levels in maternal and umbilical cord blood and birth size of offspring. Nutr. Cancer 2019, 71, 77–82. [Google Scholar] [CrossRef]
- Friedman, M.A.; Tyl, R.W.; Marr, M.C.; Myers, C.B.; Gerling, F.S.; Ross, W.P. Effects of lactational administration of acrylamide on rat dams and offspring. Reprod. Toxicol. 1999, 13, 511–520. [Google Scholar] [CrossRef]
- Takahashi, M.; Shibutani, M.; Nakahigashi, J.; Sakaguchi, N.; Inoue, K.; Morikawa, T.; Yoshida, M.; Nishikawa, A. Limited lactational transfer of acrylamide to rat offspring on maternal oral administration during the gestation and lactation periods. Arch. Toxicol. 2009, 83, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Pabst, K.; Mathar, W.; Palavinskas, R.; Meisel, H.; Bluthgen, A.; Klaffke, H. Acrylamide-occurence in mixed concentrate feed dairy cows and carry-over into milk. Food Addit. Contam. 2005, 22, 210–213. [Google Scholar] [CrossRef]
- Sörgel, F.; Weissenbacher, R.; Kinzig-Schippers, M.; Hofmann, A.; Illauer, M.; Skott, A.; Landersdorfer, C. Acrylamide: Increased concentrations in homemade food and first evidence of its variable absorption from food, variable metabolism and placental and breast milk transfer in humans. Chemotherapy 2002, 48, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Fohgelberg, P.; Rosén, J.; Hellenäs, K.-E.; Abramsson-Zetterberg, L. The acrylamide intake via some common baby food for children in Sweden during their first year of life—An improved method for analysis of acrylamide. Food Chem. Toxicol. 2005, 43, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Mojska, H.; Gielecińska, I.; Zielińska, A.; Winiarek, J.; Sawicki, W. Estimation of exposure to dietary acrylamide based on mercapturic acids level in urine of Polish women post partum and an assessment of health risk. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 288–295. [Google Scholar] [CrossRef]
- Palczewska, I.; Niedźwiecka, Z. Wskaźniki rozwoju somatycznego dzieci i młodzieży warszawskiej. Med. Wieku Rozw. 2001, 5 (Suppl. I), 18–118. [Google Scholar]
- Szponar, L.; Wolnicka, K.; Rychlik, E. Album Fotografii Produktów i Potraw; Prace IŻŻ 96; Instytut Żywności i Żywienia: Warszawa, Poland, 2000. [Google Scholar]
- Szajewska, H.; Socha, P.; Horvath, A.; Rybak, A.; Dobrzańska, A.; Borszewska-Kornacka, M.K.; Chybicka, A.; Czerwionka-Szaflarska, M.; Gajewska, D.; Helwich, E.; et al. Zasady żywienia zdrowych niemowląt. Zalecenia Polskiego Towarzystwa Gastroenterologii, Hepatologii i Żywienia Dzieci. Standardy. Med. Pediatr. 2014, 11, 321–338. [Google Scholar]
- Mojska, H.; Gielecińska, I.; Szponar, L.; Ołtarzewski, M. Estimation of the dietary acrylamide exposure of the Polish population. Food Chem. Toxicol. 2010, 48, 2090–2096. [Google Scholar] [CrossRef]
- Mojska, H.; Gielecińska, I.; Świderska, K. Zawartość akryloamidu w różnych rodzajach pieczywa w Polsce. Bromat. Chem. Toksykol. 2011, 64, 768–772. [Google Scholar]
- Mojska, H.; Gielecińska, I.; Stoś, K.; Jarosz, M. Zawartość akryloamidu w żywności w Polsce w świetle aktualnych zaleceń Unii Europejskiej. Probl. Hig. I Epidemiol. 2011, 92, 625–628. [Google Scholar]
- Mojska, H.; Gielecińska, I. Studies of acrylamide level in coffee and coffee substitutes: Influence of raw material and manufacturing conditions. Rocz. Panst. Zakl. Hig. 2013, 64, 173–181. [Google Scholar]
- Gielecińska, I.; Mojska, H. Zawartość akryloamidu w produktach ziemniaczanych i zbożowych przygotowanych w warunkach domowych. Bromat. Chem. Toksykol. 2019, 52, 240–244. [Google Scholar]
- Li, D.; Wang, P.; Liu, Y.; Hu, X.; Chen, F. Metabolism of Acrylamide: Interindividual and interspecies differences as well as the application as biomarkers. Curr. Drug Metab. 2016, 17, 317–326. [Google Scholar] [CrossRef]
- Timmermann, C.A.G.; Mølck, S.S.; Kadawathagedara, M.; Bjerregaard, A.A.; Törnqvist, M.; Brantsæter, A.L.; Petersen, M. A review of dietary intake of acrylamide in humans. Toxics 2021, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Freisling, H.; Moskal, A.; Ferrari, P.; Nicolas, G.; Knaze, V.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; Nailler, L.; Teucher, B.; Grote, V.A.; et al. Dietary acrylamide intake of adults in the European Prospective Investigation into Cancer and Nutrition differs greatly according to geographical region. Eur. J. Nutr. 2013, 52, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Khaneghah, A.M.; Fakhri, Y.; Nemtollahi, A.; Seilani, F.; Vasseghian, Y. The concentration of acrylamide in different food products: A global systematic review, meta-analysis, and meta-regression. Food Rev. Int. 2020, 1–19. [Google Scholar] [CrossRef]
- Mojska, H.; Gielecińska, I.; Stoś, K. Determination of acrylamide level in commercial baby foods and an assessment of infant dietary exposure. Food Chem. Toxicol. 2012, 50, 2722–2728. [Google Scholar] [CrossRef] [PubMed]
- Basaran, B.; Aydin, F. Determination of acrylamide levels in infant formulas and baby biscuits sold in Turkey. Lett. Appl. Nanobiosci. 2021, 11, 3155–3165. [Google Scholar]
- Neal-Kluever, A.; Aungst, J.; Gu, Y.; Hatwell, K.; Muldoon-Jacobs, K.; Liem, A.; Ogungbesan, A.; Shackelford, M. Infant toxicology: State of the science and considerations in evaluation of safety. Food Chem. Toxicol. 2014, 70, 68–83. [Google Scholar] [CrossRef]
- Treluyer, J.M.; Cheron, G.; Sonnier, M.; Cresteil, T. Cytochrome P450 expression in sudden infant death syndrome. Biochem. Pharmacol. 1996, 52, 497–504. [Google Scholar] [CrossRef]

| Parameters | Median (Range) or Number of People (%) | |
|---|---|---|
| Breastfeeding Women | ||
| 2–3 days postpartum (n = 47) | 2nd month of breastfeeding (n = 26) | |
| Age (years) | 31 (21–38) | 30 (23–38) |
| Body weight (kg) | 61 (57–96) | 60 (50–98) |
| Education: | ||
| – Primary and vocational | 2 (4.3) | – |
| – Secondary and high school | 16 (34.0) | 5 (19.2) |
| – University | 29 (61.7) | 21 (80.8) |
| Place of residence: | ||
| – City | 43 (91.5) | 26 (100) |
| – Countryside | 4 (8.5) | – |
| Pregnancy duration (weeks): | 39 (34–41) | 39 (37–41) |
| Natural birth | 27 (57.4) | 18 (69.2) |
| Caesarean section | 20 (42.6) | 8 (30.8) |
| Which birth: | ||
| – First | 30 (63.8) | 12 (46.2) |
| – Second + | 17 (36.2) | 14 (53.8) |
| Energy of all-day diet (kcal/day) | 1395 a*1 (0 *2–2116) | 1966 b (913–2871) |
| Infants | ||
| Birth weight (n = 47) | Body weight in the 2nd month of life *3 (n = 26) | |
| Body weight (kg): | ||
| – Girls (n = 18/8) | 3.13 (2.03–3.76) | 5.0 |
| – Boys (n = 29/18) | 3.56 (2.10–4.69) | 5.6 |
| Colostrum (2–3 Days Postpartum) | Mature Milk (2nd Month after Birth) | ||
|---|---|---|---|
| No. of Participants | Acrylamide Level in Breast Milk (μg/L) | No. of Participant | Acrylamide Level in Breast Milk (μg/L) |
| 01, 02, 03 *1, 07, 10, 17, 18, 19, 21, 29, 40, 42, 44, 46, 47, 48, 49, 51, 52, 55, 57, 58, 63, 64, 65, 68, 69, 74, 76, 77, 82, 83, 90, 91, 92, 93 *2 | <0.1 | 03, 04, 05, 13, 26, 28, 35, 36, 47, 58, 67 *2 | <0.1 |
| 08 | 0.11 | 06 | 0.16 |
| 14 | 1.00 | 09 | 0.17 |
| 20 | 0.26 | 11 | 0.18 |
| 22 | 0.19 | 15 | 0.11 |
| 25 | 0.20 | 16 | 0.12 |
| 33 | 0.15 | 23 | 0.36 |
| 36 | 0.21 | 34 | 0.18 |
| 54 | 0.77 | 38 | 0.19 |
| 59 | 0.19 | 46 | 0.27 |
| 61 | 0.63 | 52 | 0.23 |
| 62 | 0.23 | 53 | 0.25 |
| 54 | 0.18 | ||
| 62 | 0.86 | ||
| 84 | 0.28 | ||
| 87 | 0.17 | ||
| Me (n = 47) | 0.05 *3 | Me (n = 26) | 0.14 *3 |
| Product Group | Acrylamide Level (µg/kg) A | |
|---|---|---|
| Mean | Min ÷ Max | |
| French fries | 576 | 157 ÷ 2175 |
| Wheat–rye bread | 31 | 10 ÷ 99 |
| Rye bread | 98 | 87 ÷ 110 |
| Wholemeal rye bread | 43 | 10 ÷ 108 |
| Graham bread | 67 | 58 ÷ 90 |
| Pumpernickel | 190 | 33 ÷ 430 |
| Wheat rolls | 50 | 23 ÷ 85 |
| Graham rolls | 51 | 27 ÷ 83 |
| Yeast cake and cookies | 13 | 10 ÷ 21 |
| Milk rolls | 14 | 10 ÷ 24 |
| Crispbread | 430 | 65 ÷ 1271 |
| Rusks | 20 B | 6 ÷ 47 B |
| Cookies | 231 | 37 ÷ 1178 |
| Crackers | 859 | 566 ÷ 2017 |
| Salty sticks | 227 | 62 ÷ 879 |
| Corn flakes | 128 | 15 ÷ 414 |
| Oat flakes | 23 | 11 ÷ 41 |
| Semolina | 1.5 B | – |
| Crepes | 47 | 43 ÷ 52 |
| Crumpets/pancakes | 65 | 44 ÷ 75 |
| Potato pancakes | 1069 | 650 ÷ 2110 |
| Coffee substitutes (powder) | 818 | 528 ÷ 1145 |
| Roast coffee (powder) | 250 | 61 ÷ 699 |
| Instant coffee (powder) | 358 | 152 ÷ 830 |
| Parameter Tested | 2–3 Days Postpartum (n = 47) | 2nd Month of Infant’s Life (n = 26) | ||||
|---|---|---|---|---|---|---|
| Me | P 95 | Min–Max | Me | P 95 | Min–Max | |
| Acrylamide intake from diet (μg/person/day) | 7.3 a*1 | 32.4 | 0.00–49.6 | 16.9 b | 45.5 | 2.5–55.8 |
| Acrylamide exposure (μg/kg bw/day) | 0.11 a*1 | 0.41 | 0.00–0.66 | 0.50 b | 4.84 | 0.02–5.06 |
| MOE (BMDL10 = 0.18 mg/kg bw/day) *2 | 1636 | 439 | 273 *3 | 360 | 37 | 9000–36 |
| MOE (BMDL10 = 0.31 mg/kg bw/day) *2 | 2818 | 756 | 470 *3 | 620 | 64 | 15,500–61 |
| Parameter Tested | 2–3 Days Postpartum (n = 47) | 2nd Month of Infant’s Life (n = 26) | ||||
|---|---|---|---|---|---|---|
| Me | P 95 | Min–Max | Me | P 95 | Min–Max | |
| Acrylamide intake from breast milk *1 *2 (μg/person/day) | 0.008–0.012 a*3 | 0.101–0.152 | 0.008–0.240 | 0.099 b | 0.261 | 0.036–0.619 |
| Acrylamide exposure (μg/kg bw/day) | 0.003–0.004 a*3 | 0.026–0.040 | 0.002–0.044 | 0.018 b | 0.047 | 0.006–0.111 |
| MOE (BMDL10 = 0.18 mg/kg bw/day) *4 | 60,000–45,000 | 6923–4500 | 90,000–4090 | 10,000 | 3830 | 30,000–16,225 |
| MOE (BMDL10 = 0.31 mg/kg bw/day) *4 | 103,333–77,500 | 11,923–7750 | 155,000–7045 | 17,222 | 6596 | 51,667–2793 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mojska, H.; Gielecińska, I.; Winiarek, J.; Sawicki, W. Acrylamide Content in Breast Milk: The Evaluation of the Impact of Breastfeeding Women’s Diet and the Estimation of the Exposure of Breastfed Infants to Acrylamide in Breast Milk. Toxics 2021, 9, 298. https://doi.org/10.3390/toxics9110298
Mojska H, Gielecińska I, Winiarek J, Sawicki W. Acrylamide Content in Breast Milk: The Evaluation of the Impact of Breastfeeding Women’s Diet and the Estimation of the Exposure of Breastfed Infants to Acrylamide in Breast Milk. Toxics. 2021; 9(11):298. https://doi.org/10.3390/toxics9110298
Chicago/Turabian StyleMojska, Hanna, Iwona Gielecińska, Joanna Winiarek, and Włodzimierz Sawicki. 2021. "Acrylamide Content in Breast Milk: The Evaluation of the Impact of Breastfeeding Women’s Diet and the Estimation of the Exposure of Breastfed Infants to Acrylamide in Breast Milk" Toxics 9, no. 11: 298. https://doi.org/10.3390/toxics9110298
APA StyleMojska, H., Gielecińska, I., Winiarek, J., & Sawicki, W. (2021). Acrylamide Content in Breast Milk: The Evaluation of the Impact of Breastfeeding Women’s Diet and the Estimation of the Exposure of Breastfed Infants to Acrylamide in Breast Milk. Toxics, 9(11), 298. https://doi.org/10.3390/toxics9110298





