Morphological and Behavioral Effects in Zebrafish Embryos after Exposure to Smoke Dyes
Abstract
1. Introduction
2. Materials and Methods
2.1. Analytical Chemistry
2.2. Blood-Brain Barrier Permeability
- [drug]equilibrium = ([drug]donor × VD + [drug]acceptor × VA)/(VD + VA);
- [drug]acceptor = (Aa/Ai × DF)acceptor;
- [drug]donor = (Aa/Ai × DF)donor;
- VD = 0.15 mL; VA = 0.30 mL; Area = 0.28 cm2; time = 14,400 s.
- Aa/Ai: Peak area ratio of NAC and the internal standard; DF: Dilution factor (13.5).
2.3. Statistical Analysis
3. Results and Discussion
3.1. Morphological Effects
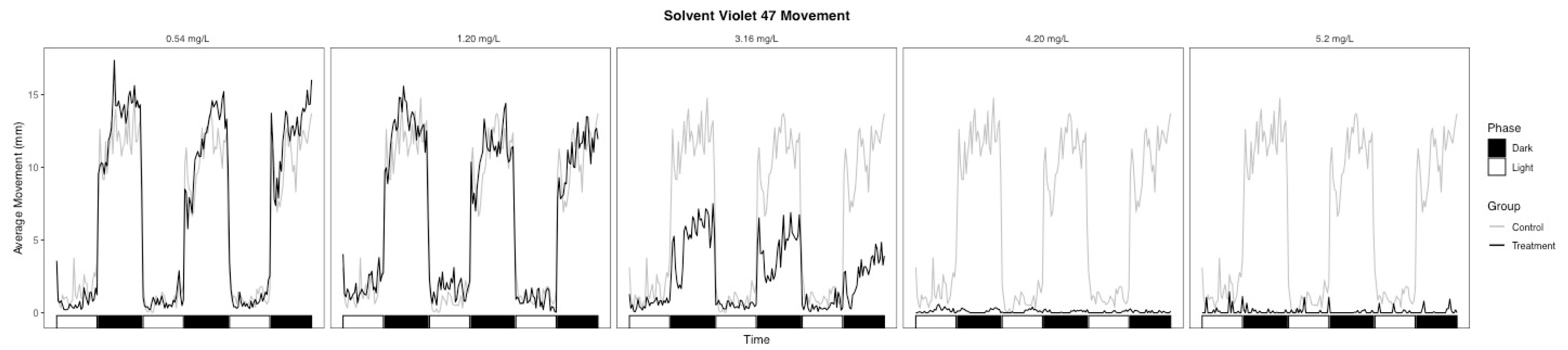
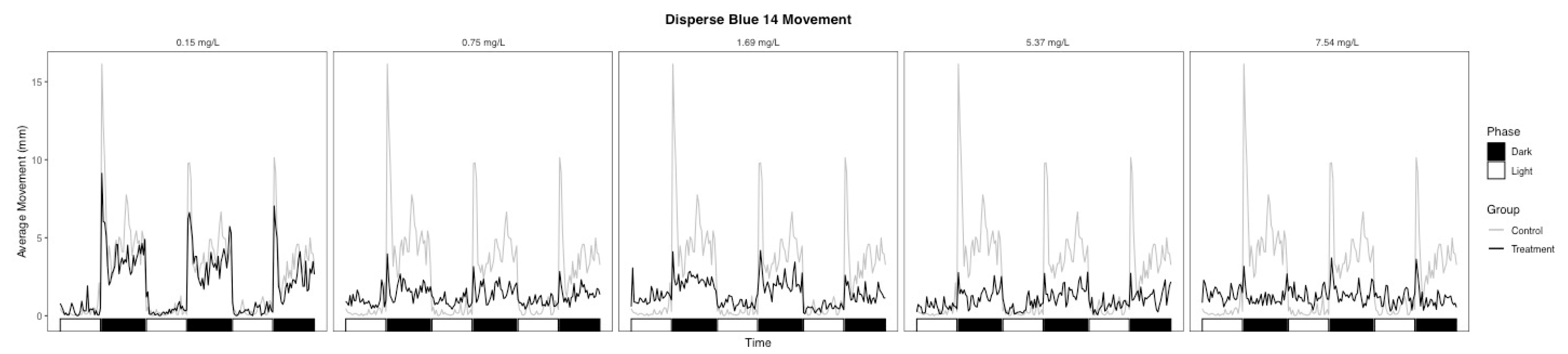
3.2. Behavioral Changes
3.3. Analytical Chemistry
3.4. Blood-Brain Barrier Permeability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabnis, R.W. Manufacture of dye intermediates, dyes, and their industrial applications. In Handbook of Industrial Chemistry and Biotechnology; Kent, J., Bommaraju, T., Barnicki, S., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Zaharia, C.; Suteu, D. Textile organic dyes—Characteristics, polluting effects and separation/elimination procedures from industrial effluents—A critical overview. In Organic Pollutants Ten Years after the Stockholm Convention—Environmental and Analytical Update; Puzyn, T., Ed.; InTechOpen: London, UK, 2012. [Google Scholar]
- Gonçalves*, I.M.C.; Gomes, A.; Brás, R.; Ferra, M.I.A.; Amorim, M.T.P.; Porter, R.S. Biological treatment of effluent containing textile dyes. Color. Technol. 2000, 116, 393–397. [Google Scholar] [CrossRef][Green Version]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Chaudhry, S.A. Arsenic: Toxic effects and remediation. In Advanced Materials for Wastewater Treatment; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–27. [Google Scholar]
- Piaskowski, K.; Świderska-Dąbrowska, R.; Zarzycki, P.K. Dye removal from water and wastewater using various physical, chemical, and biological processes. J. AOAC Int. 2018, 101, 1371–1384. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, P.K.; Gupta, R.; Singh, R.L. Treatment and recycling of wastewater from textile industry. In Advances in Biological Treatment of Industrial Waste Water and their Recycling for a Sustainable Future; Singh, R.L., Singh, R.P., Eds.; Springer: Singapore, 2019; pp. 225–266. [Google Scholar]
- Routoula, E.; Patwardhan, S.V. Degradation of anthraquinone dyes from effluents: A review focusing on enzymatic dye degradation with industrial potential. Environ. Sci. Technol. 2020, 54, 647–664. [Google Scholar] [CrossRef]
- Chaudhari, A.U.; Paul, D.; Dhotre, D.; Kodam, K.M. Effective biotransformation and detoxification of anthraquinone dye reactive blue 4 by using aerobic bacterial granules. Water Res. 2017, 122, 603–613. [Google Scholar] [CrossRef]
- Novotný, Č.; Dias, N.; Kapanen, A.; Malachová, K.; Vándrovcová, M.; Itävaara, M.; Lima, N. Comparative use of bacterial, algal and protozoan tests to study toxicity of azo- and anthraquinone dyes. Chemosphere 2006, 63, 1436–1442. [Google Scholar] [CrossRef]
- De Oliveira, G.A.R.; de Lapuente, J.; Teixidó, E.; Porredón, C.; Borràs, M.; de Oliveira, D.P. Textile dyes induce toxicity on zebrafish early life stages. Environ. Toxicol. Chem. 2016, 35, 429–434. [Google Scholar] [CrossRef]
- Parrott, J.L.; Bartlett, A.J.; Balakrishnan, V.K. Chronic toxicity of azo and anthracenedione dyes to embryo-larval fathead minnow. Environ. Pollut. 2016, 210, 40–47. [Google Scholar] [CrossRef]
- Hattori, M. Dyes, anthraquinone. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 1–45. [Google Scholar]
- ECHA. Skin Sensitising, Irritative and/or Corrosive…—Registry of Restriction Intentions until Outcome—ECHA. Available online: https://echa.europa.eu/registry-of-restriction-intentions/-/dislist/details/0b0236e182446136 (accessed on 19 October 2020).
- NICNAS. Anthraquinone-Based Dyes with Limited Data Availability: Human Health tier II Assessment. National Industrial Chemicals Notification and Assessment Scheme, IMAP tier II Assessment. Available online: https://www.industrialchemicals.gov.au/sites/default/files/Anthraquinone-based%20dyes%20with%20limited%20data%20availability_Human%20health%20tier%20II%20assessment.pdf (accessed on 19 October 2020).
- Albasher, G.; Maashi, N.; Alfarraj, S.; Almeer, R.; Albrahim, T.; Alotibi, F.; Bin-Jumah, M.; Mahmoud, A.M. Perinatal exposure to tartrazine triggers oxidative stress and neurobehavioral alterations in mice offspring. Antioxidants 2020, 9, 53. [Google Scholar] [CrossRef]
- Bakthavachalu, P.; Kannan, S.M.; Qoronfleh, M.W. Food color and autism: A meta-analysis. In Personalized Food Intervention and Therapy for Autism Spectrum Disorder Management; Essa, M.M., Qoronfleh, M.W., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 481–504. [Google Scholar]
- D’Amora, M.; Giordani, S. The utility of zebrafish as a model for screening developmental neurotoxicity. Front. Neurosci. 2018, 12, 976. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic effects and biomarkers of lead exposure: A review. Rev. Environ. Health 2009, 24, 15–45. [Google Scholar] [CrossRef] [PubMed]
- Eliceiri, B.P.; Gonzalez, A.M.; Baird, A. Zebrafish model of the blood-brain barrier: Morphological and permeability studies. Methods Mol. Biol. 2011, 686, 371–378. [Google Scholar] [PubMed]
- Truong, L.; Reif, D.M.; St Mary, L.; Geier, M.C.; Truong, H.D.; Tanguay, R.L. Multidimensional in vivo hazard assessment using zebrafish. Toxicol. Sci. 2014, 137, 212–233. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.R.; Noyes, P.D.; Tanguay, R.L. Advancements in zebrafish applications for 21st century toxicology. Pharmacol. Ther. 2016, 161, 11–21. [Google Scholar] [CrossRef]
- Wullimann, M.F. Secondary neurogenesis and telencephalic organization in zebrafish and mice: A brief review. Integr. Zool. 2009, 4, 123–133. [Google Scholar] [CrossRef]
- Gilbert, S.F. Developmental Biology, 6th ed.; Sinnauer Associates: Sunderland, MA, USA, 2010. [Google Scholar]
- Quiñonez-Silvero, C.; Hübner, K.; Herzog, W. Development of the brain vasculature and the blood-brain barrier in zebrafish. Dev. Biol. 2020, 457, 181–190. [Google Scholar] [CrossRef]
- Jeong, J.-Y.; Kwon, H.-B.; Ahn, J.-C.; Kang, D.; Kwon, S.-H.; Park, J.A.; Kim, K.-W. Functional and developmental analysis of the blood-brain barrier in zebrafish. Brain Res. Bull. 2008, 75, 619–628. [Google Scholar] [CrossRef]
- Xie, J.; Farage, E.; Sugimoto, M.; Anand-Apte, B. A novel transgenic zebrafish model for blood-brain and blood-retinal barrier development. BMC Dev. Biol. 2010, 10, 76. [Google Scholar] [CrossRef]
- Fleming, A.; Diekmann, H.; Goldsmith, P. Functional characterisation of the maturation of the blood-brain barrier in larval zebrafish. PLoS ONE 2013, 8, e77548. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Mandrell, D.; Truong, L.; Jephson, C.; Sarker, M.R.; Moore, A.; Lang, C.; Simonich, M.T.; Tanguay, R.L. Automated zebrafish chorion removal and single embryo placement: Optimizing throughput of zebrafish developmental toxicity screens. J. Lab. Autom. 2012, 17, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Reif, D.M.; Truong, L.; Mandrell, D.; Marvel, S.; Zhang, G.; Tanguay, R.L. High-throughput characterization of chemical-associated embryonic behavioral changes predicts teratogenic outcomes. Arch. Toxicol. 2016, 90, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Rabal, O.; Sánchez-Arias, J.A.; José-Enériz, E.S.; Agirre, X.; de Miguel, I.; Garate, L.; Miranda, E.; Sáez, E.; Roa, S.; Martínez-Climent, J.A.; et al. Detailed exploration around 4-aminoquinolines chemical space to navigate the lysine methyltransferase G9a and DNA methyltransferase biological spaces. J. Med. Chem. 2018, 61, 6546–6573. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Granato, M.; van Eeden, F.J.; Schach, U.; Trowe, T.; Brand, M.; Furutani-Seiki, M.; Haffter, P.; Hammerschmidt, M.; Heisenberg, C.P.; Jiang, Y.J.; et al. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Dev. Camb. Engl. 1996, 123, 399–413. [Google Scholar]
- Lindsey, B.W.; Smith, F.M.; Croll, R.P. From inflation to flotation: Contribution of the swimbladder to whole-body density and swimming depth during development of the zebrafish (Danio rerio). Zebrafish 2010, 7, 85–96. [Google Scholar] [CrossRef]
- Robertson, G.N.; Lindsey, B.W.; Dumbarton, T.C.; Croll, R.P.; Smith, F.M. The contribution of the swimbladder to buoyancy in the adult zebrafish (Danio rerio): A morphometric analysis. J. Morphol. 2008, 269, 666–673. [Google Scholar] [CrossRef]
- Shen, B.; Liu, H.-C.; Ou, W.-B.; Eilers, G.; Zhou, S.M.; Meng, F.G.; Li, C.Q.; Li, Y.Q. Toxicity induced by basic violet 14, direct red 28 and acid red 26 in zebrafish larvae. J. Appl. Toxicol. JAT 2015, 35, 1473–1480. [Google Scholar] [CrossRef]
- Abe, F.R.; Mendonça, J.N.; Moraes, L.A.B.; de Oliveira, G.A.R.; Gravato, C.; Soares, A.M.V.M.; de Oliveira, D.P. Toxicological and behavioral responses as a tool to assess the effects of natural and synthetic dyes on zebrafish early life. Chemosphere 2017, 178, 282–290. [Google Scholar] [CrossRef]


| Endpoint | Solvent Violet 47 | Disperse Blue 14 | ||
|---|---|---|---|---|
| LEL | p | LEL | p | |
| MO24 | - | - | - | - |
| DP24 | 38.5 | 3.84 × 10−5 | - | - |
| SM24 | - | - | - | - |
| NC24 | - | - | - | - |
| MORT | 38.5 | 0.002 | - | - |
| YSE | 29 | 0.001 | 35.6 | 6.3 × 10−6 |
| AXIS | 29 | 0.001 | 35.6 | 0.003 |
| EYE | 48 | 0.0005 | 35.6 | 0.003 |
| SNOU | 38.5 | 0.003 | 35.6 | 0.007 |
| JAW | 29 | 0.006 | 35.6 | 0.007 |
| OTIC | - | - | - | - |
| PE | 29 | 0.001 | 35.6 | 1.95 × 10−5 |
| BRAI | - | - | - | - |
| SOMI | - | - | - | - |
| PFIN | 48 | 0.0005 | 35.6 | 1.95 × 10−5 |
| CFIN | 38.5 | 0.01 | - | - |
| PIG | - | - | - | - |
| CIRC | - | - | - | - |
| TRUN | - | - | - | - |
| SWIM | - | - | - | - |
| NC | - | - | - | - |
| TR | 29 | 0.006 | - | - |
| Phase | Solvent Violet 47 | Disperse Blue 14 | ||||||
|---|---|---|---|---|---|---|---|---|
| LEL μM | p | Delta | Effect | LEL μM | p | Delta | Effect | |
| Light | 38.5 | 0.002 | −0.915 | Hypoactive | 35.6 | 0.003 | 0.963 | Hyperactive |
| Dark | 29 | 0.002 | −0.630 | Hypoactive | 1 | 0.007 | −0.270 | Hypoactive |
| Dye | MW | Nominal (mM) | Measured (mM) | Measured (mg/L) |
|---|---|---|---|---|
| SV47 | 240.26 | 40 | 18.147 | 4360 |
| DB14 | 266.29 | 40 | 22.64 | 6030 |
| Compound ID | Test Concentration | Incubation Time | Mean Pe (nm/s) | % Mean Recovery | Permeability |
|---|---|---|---|---|---|
| Solvent Violet 47 | 10 μM | 4 h | 1.586 | 113.5 | Moderate |
| Disperse Blue 14 | 10 μM | 4 h | 26.259 | 11.5 | High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
To, K.T.; St. Mary, L.; Wooley, A.H.; Wilbanks, M.S.; Bednar, A.J.; Perkins, E.J.; Truong, L.; Tanguay, R.L.; Garcia-Reyero, N. Morphological and Behavioral Effects in Zebrafish Embryos after Exposure to Smoke Dyes. Toxics 2021, 9, 9. https://doi.org/10.3390/toxics9010009
To KT, St. Mary L, Wooley AH, Wilbanks MS, Bednar AJ, Perkins EJ, Truong L, Tanguay RL, Garcia-Reyero N. Morphological and Behavioral Effects in Zebrafish Embryos after Exposure to Smoke Dyes. Toxics. 2021; 9(1):9. https://doi.org/10.3390/toxics9010009
Chicago/Turabian StyleTo, Kimberly T., Lindsey St. Mary, Allyson H. Wooley, Mitchell S. Wilbanks, Anthony J. Bednar, Edward J. Perkins, Lisa Truong, Robyn L. Tanguay, and Natàlia Garcia-Reyero. 2021. "Morphological and Behavioral Effects in Zebrafish Embryos after Exposure to Smoke Dyes" Toxics 9, no. 1: 9. https://doi.org/10.3390/toxics9010009
APA StyleTo, K. T., St. Mary, L., Wooley, A. H., Wilbanks, M. S., Bednar, A. J., Perkins, E. J., Truong, L., Tanguay, R. L., & Garcia-Reyero, N. (2021). Morphological and Behavioral Effects in Zebrafish Embryos after Exposure to Smoke Dyes. Toxics, 9(1), 9. https://doi.org/10.3390/toxics9010009








