Acute Poisoning with Rhabdomyolysis in the Intensive Care Unit: Risk Factors for Acute Kidney Injury and Renal Replacement Therapy Requirement
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Parameter Definitions
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Features, Complications and General Management
3.3. AKI Onset
3.4. RTT Requirement
3.5. Additional Complications and Fatal Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bosch, X.; Poch, E.; Grau, J.M. Rhabdomyolysis and acute kidney injury. N. Engl. J. Med. 2009, 361, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Koyfman, A.; Gottlieb, M. An evidence-based narrative review of the emergency department evaluation and management of rhabdomyolysis. Am. J. Emerg. Med. 2019, 37, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.M. Factors predictive of acute renal failure in rhabdomyolysis. Arch. Intern. Med. 1988, 148, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Melli, G.; Chaudhry, V.; Cornblath, D.R. Rhabdomyolysis: An evaluation of 475 hospitalized patients. Medicine 2005, 84, 377–385. [Google Scholar] [CrossRef]
- Vivino, G.; Antonelli, M.; Moro, M.L.; Cottini, F.; Conti, G.; Bufi, M.; Cannata, F.; Gasparetto, A. Risk factors for acute renal failure in trauma patients. Intensive Care Med. 1998, 24, 808–814. [Google Scholar] [CrossRef]
- Mégarbane, B.; Donetti, L.; Blanc, T.; Chéron, G.; Jacobs, F.; Groupe d’experts de la SRLF. Treatment of acute poisoning by illicit drugs. Recommendations of the french language resuscitation society (SRLF). Rev. Prat. 2008, 58, 871–881. [Google Scholar]
- Huerta-Alardín, A.L.; Varon, J.; Marik, P.E. Bench-to-bedside review: Rhabdomyolysis—an overview for clinicians. Crit. Care 2005, 9, 158–169. [Google Scholar] [CrossRef]
- Chavez, L.O.; Leon, M.; Einav, S. Beyond muscle destruction: A systematic review of rhabdomyolysis for clinical practice. Crit. Care 2016, 20, 135. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; George, C.; Dinu, I.; Bellomo, R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol. Dial. Transplant. 2008, 23, 1203–1210. [Google Scholar] [CrossRef]
- Ostermann, M.; Chang, R.W. Acute kidney injury in the intensive care unit according to RIFLE. Crit. Care Med. 2007, 35, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Sever, M.S.; Erek, E.; Lameire, N. Rhabdomyolysis. J. Am. Soc. Nephrol. 2000, 11, 1553–1561. [Google Scholar] [PubMed]
- Gabow, P.A.; Kaehny, W.D.; Kelleher, S.P. The spectrum of rhabdomyolysis. Medicine 1982, 61, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Beetham, R. Biochemical investigation of suspected rhabdomyolysis. Ann. Clin. Biochem. 2000, 37, 581–587. [Google Scholar] [CrossRef]
- Feinfeld, D.A.; Cheng, J.T.; Beysolow, T.D.; Briscoe, A.M. A prospective study of urine and serum myoglobin levels in patients with acute rhabdomyolysis. Clin. Nephrol. 1992, 38, 193–195. [Google Scholar] [PubMed]
- Mousavi, S.R.; Vahabzadeh, M.; Mahdizadeh, A.; Vafaee, M.; Sadeghi, M.; Afshari, R.; Balali-Mood, M. Rhabdomyolysis in 114 patients with acute poisonings. J. Res. Med. Sci. 2015, 20, 239–243. [Google Scholar]
- Pajoum, A.; Fahim, F.; Akhlaghdoust, M.; Zamani, N.; Amirfirooz, Z.; Dehdehasti, M. Rhabdomyolysis and acute poisoning; a brief report. Emergency 2018, 6, e56. [Google Scholar]
- Dadpour, B.; Tajoddini, S.; Shaarbaf Eidgahi, E.; Shokouhizadeh, M.; Shafahi, A. Role of serum creatinine phosphokinase in outcome prediction of intoxicated patients; a brief report. Emergency 2017, 5, e63. [Google Scholar]
- Vanden Eede, H.; Montenij, L.J.; Touw, D.J.; Norris, E.M. Rhabdomyolysis in MDMA intoxication: A rapid and underestimated killer. “Clean” Ecstasy, a safe party drug? J. Emerg. Med. 2012, 42, 655–658. [Google Scholar] [CrossRef]
- O’Connor, A.D.; Padilla-Jones, A.; Gerkin, R.D.; Levine, M. Prevalence of rhabdomyolysis in sympathomimetic toxicity: A comparison of stimulants. J. Med. Toxicol. 2015, 11, 195–200. [Google Scholar] [CrossRef]
- Grunau, B.E.; Pourvali, R.; Wiens, M.O.; Levin, A.; Li, J.; Grafstein, E.; Joo, D.; Scheuermeyer, F.X. Characteristics and thirty-day outcomes of emergency department patients with elevated creatine kinase. Acad. Emerg. Med. 2014, 21, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Wongrakpanich, S.; Kallis, C.; Prasad, P.; Rangaswami, J.; Rosenzweig, A. The study of rhabdomyolysis in the elderly: An epidemiological study and single center experience. Aging Dis. 2018, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sułowicz, W.; Walatek, B.; Sydor, A.; Ochmański, W.; Miłkowski, A.; Szymczakiewicz-Multanowska, A.; Szumilak, D.; Kraśniak, A.; Lonak, H.; Wójcikiewicz, T. Acute renal failure in patients with rhabdomyolysis. Med. Sci. Monit. 2002, 8, CR24–CR27. [Google Scholar] [PubMed]
- Rodríguez, E.; Soler, M.J.; Rap, O.; Barrios, C.; Orfila, M.A.; Pascual, J. Risk factors for acute kidney injury in severe rhabdomyolysis. PLoS ONE 2013, 8, e82992. [Google Scholar] [CrossRef]
- McMahon, G.M.; Zeng, X.; Waikar, S.S. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern. Med. 2013, 173, 1821–1928. [Google Scholar] [CrossRef]
- Vodovar, D.; El Balkhi, S.; Curis, E.; Deye, N.; Mégarbane, B. Lithium poisoning in the intensive care unit: Predictive factors of severity and indications for extracorporeal toxin removal to improve outcome. Clin. Toxicol. 2016, 54, 615–623. [Google Scholar] [CrossRef]
- Mannix, R.; Tan, M.L.; Wright, R.; Baskin, M. Acute pediatric rhabdomyolysis: Causes and rates of renal failure. Pediatrics 2006, 118, 2119–2125. [Google Scholar] [CrossRef]
- Brown, C.V.; Rhee, P.; Chan, L.; Evans, K.; Demetriades, D.; Velmahos, G.C. Preventing renal failure in patients with rhabdomyolysis: Do bicarbonate and mannitol make a difference? J. Trauma 2004, 56, 1191–1196. [Google Scholar] [CrossRef]
- Baeza-Trinidad, R.; Brea-Hernando, A.; Morera-Rodriguez, S.; Brito-Diaz, Y.; Sanchez-Hernandez, S.; El Bikri, L.; Ramalle-Gomara, E.; Garcia-Alvarez, J.L. Creatinine as predictor value of mortality and acute kidney injury in rhabdomyolysis. Intern. Med. J. 2015, 45, 1173–1178. [Google Scholar] [CrossRef]
- Porush, J.G.; Faubert, P.F. Renal disease in elderly patients. Rev. Clin. Gerontol. 1997, 7, 299–307. [Google Scholar] [CrossRef]
- Lindeman, R.D.; Tobin, J.D.; Shock, N.W. Association between blood pressure and the rate of decline in renal function with age. Kidney Int. 1984, 26, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, W.G.; Hung, O.; Bruno, G.R.; Galea, S.; Chiang, W.K. Factors predictive of acute renal failure and need for hemodialysis among ED patients with rhabdomyolysis. Am. J. Emerg. Med. 2005, 23, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sharp, L.S.; Rozycki, G.S.; Feliciano, D.V. Rhabdomyolysis and secondary renal failure in critically ill surgical patients. Am. J. Surg. 2004, 188, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Akmal, M.; Bishop, J.E.; Telfer, N.; Norman, A.W.; Massry, S.G. Hypocalcemia and hypercalcemia in patients with rhabdomyolysis with and without acute renal failure. J. Clin. Endocrinol. Metab. 1986, 63, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Llach, F.; Felsenfeld, A.J.; Haussler, M.R. The pathophysiology of altered calcium metabolism in rhabdomyolysis-induced acute renal failure. Interactions of parathyroid hormone, 25-hydroxycholecalciferol, and 1,25-dihydroxycholecalciferol. N. Engl. J. Med. 1981, 305, 117–123. [Google Scholar] [CrossRef]
- Talaie, H.; Emam-Hadi, M.; Panahandeh, R.; Hassanian-Moghaddam, H.; Abdollahi, M. On the mechanisms underlying poisoning-induced rhabdomyolysis and acute renal failure. Toxicol. Mech. Methods 2008, 18, 585–588. [Google Scholar] [CrossRef]
- Akmal, M.; Massry, S.G. Reversible hepatic dysfunction associated with rhabdomyolysis. Am. J. Nephrol. 1990, 10, 49–52. [Google Scholar] [CrossRef]
- Regel, G.; Lobenhoffer, P.; Grotz, M.; Pape, H.C.; Lehmann, U.; Tscherne, H. Treatment results of patients with multiple trauma: An analysis of 3406 cases treated between 1972 and 1991 at a German Level I Trauma Center. J. Trauma 1995, 38, 70–78. [Google Scholar] [CrossRef]
- Vaara, S.T.; Pettilä, V.; Kaukonen, K.M.; Bendel, S.; Korhonen, A.M.; Bellomo, R.; Reinikainen, M.; Finnish Acute Kidney Injury Study Group. The attributable mortality of acute kidney injury: A sequentially matched analysis. Crit. Care Med. 2014, 42, 878–885. [Google Scholar] [CrossRef]
- El-Abdellati, E.; Eyselbergs, M.; Sirimsi, H.; Hoof, V.V.; Wouters, K.; Verbrugghe, W.; Jorens, P.G. An observational study on rhabdomyolysis in the intensive care unit. Exploring its risk factors and main complication: Acute kidney injury. Ann. Intensive Care 2013, 3, 8. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Ingwerson, J.L.; Rogers, I.R.; Hew-Butler, T.; Stuempfle, K.J. Increasing creatine kinase concentrations at the 161-km western states endurance run. Wilderness Environ. Med. 2012, 23, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.M.; Kearns, A.K.; Rouzier, P.; Rubin, R.; Thompson, P.D. Serum creatine kinase levels and renal function measures in exertional muscle damage. Med. Sci. Sports Exerc. 2006, 38, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Harmon, B.T.; Orkunoglu-Suer, E.F.; Adham, K.; Larkin, J.S.; Gordish-Dressman, H.; Clarkson, P.M.; Thompson, P.D.; Angelopoulos, T.J.; Gordon, P.M.; Moyna, N.M.; et al. CCL2 and CCR2 variants are associated with skeletal muscle strength and change in strength with resistance training. J. Appl. Physiol. 2010, 109, 1779–1785. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Delaney, K.A.; Givens, M.L.; Vohra, R.B. Use of RIFLE criteria to predict the severity and prognosis of acute kidney injury in emergency department patients with rhabdomyolysis. J. Emerg. Med. 2012, 42, 521–528. [Google Scholar] [CrossRef] [PubMed]
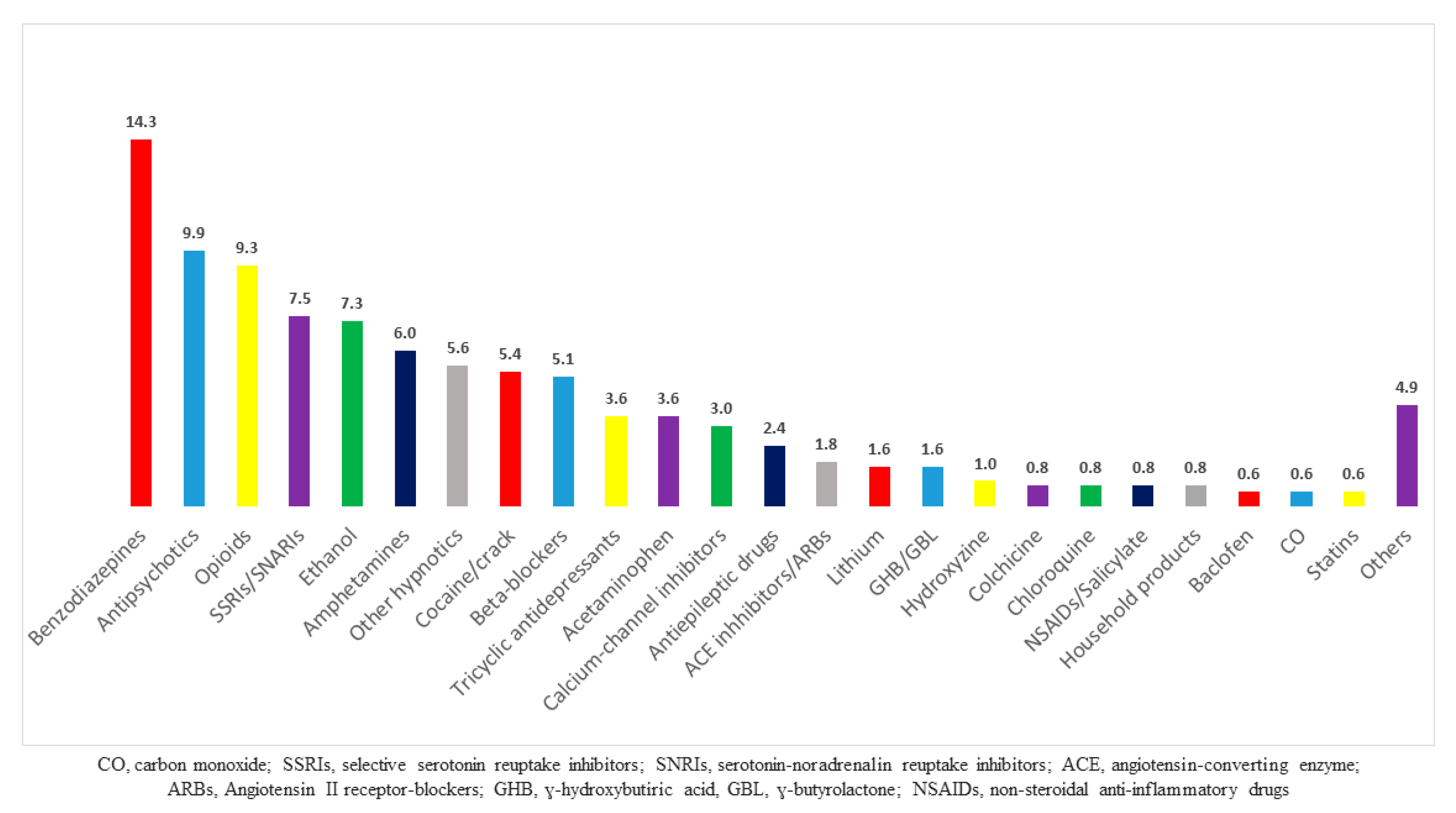
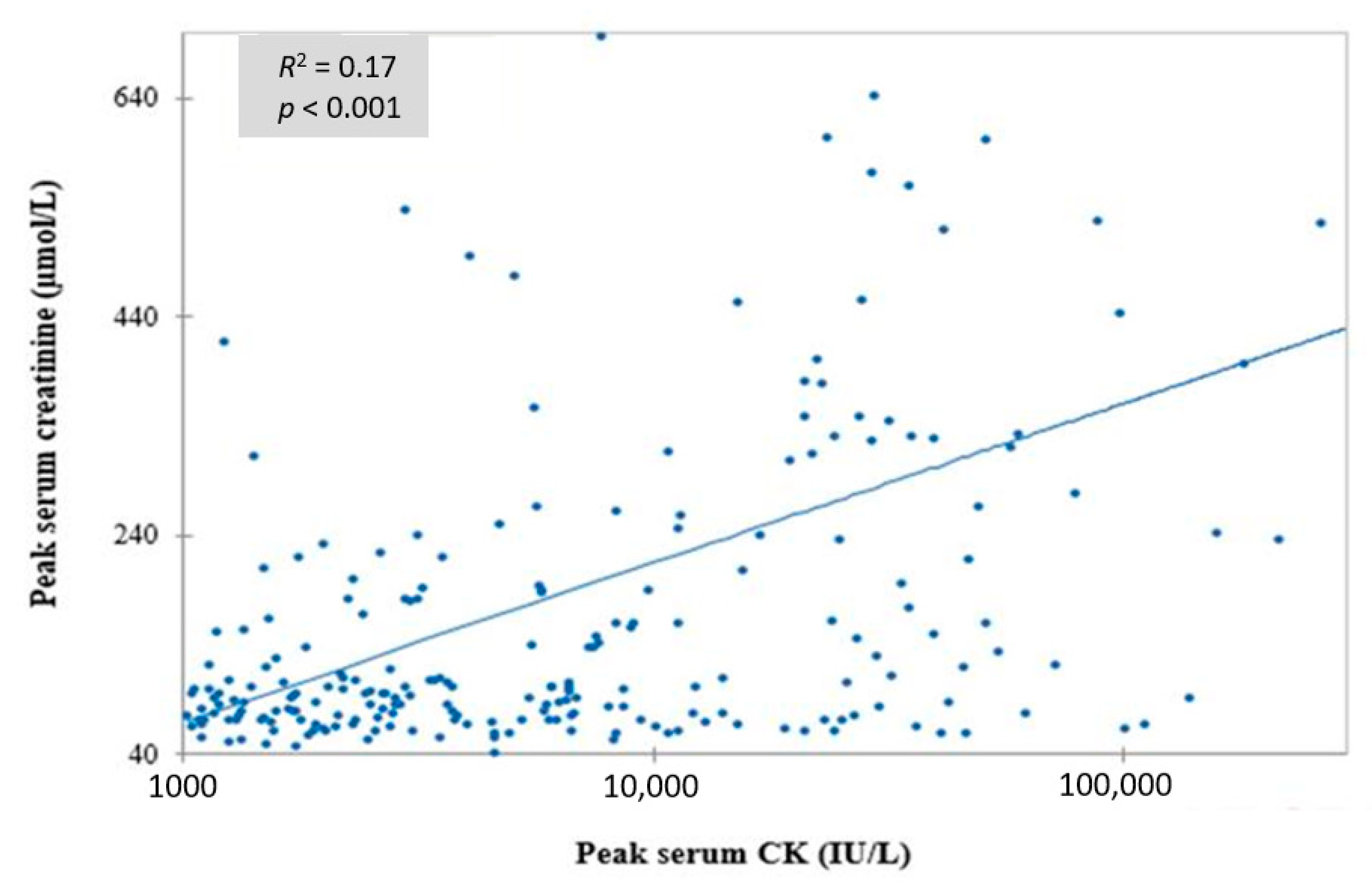
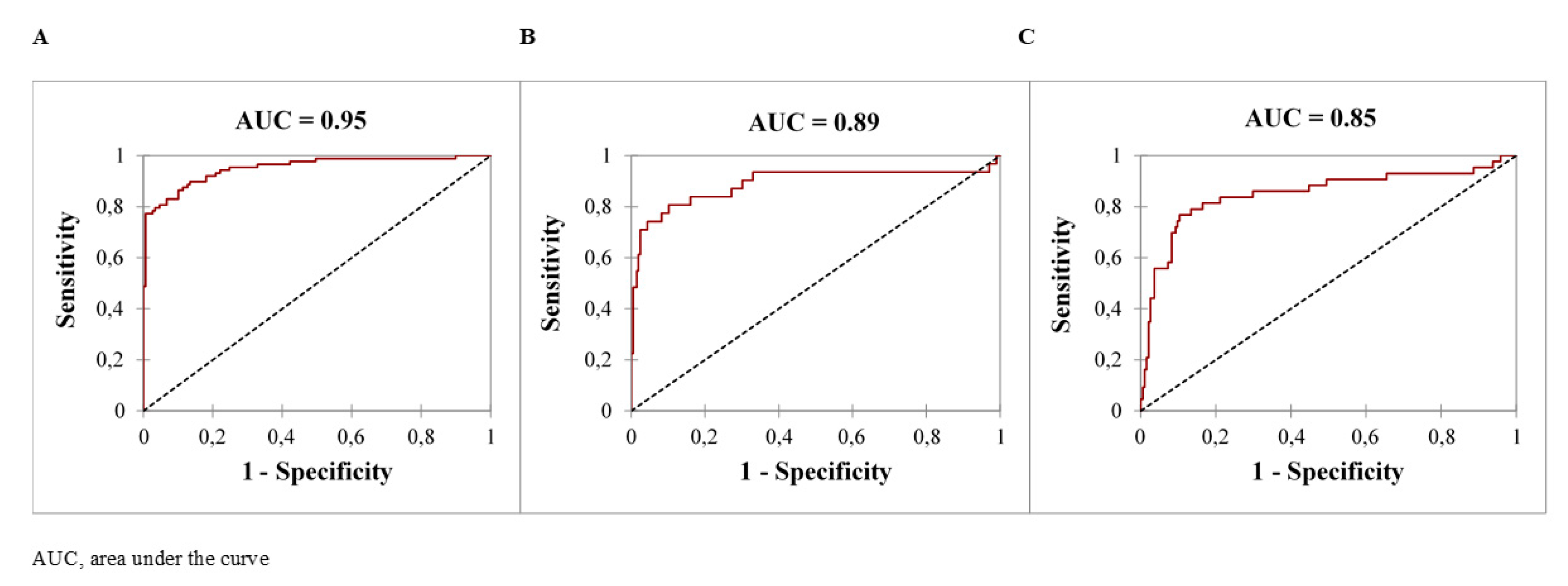
| Parameters | Median (Percentiles 25–75) or N (%) |
|---|---|
| Demographics | |
| Age (years) | 41 (31–53) |
| Body mass index (kg/m2) | 23.6 (21.0–27.2) |
| Gender (Male/Female) | 138 (58%)/99 (42%) |
| Ethnicity | |
| Caucasian | 181 (76%) |
| North-African | 30 (13%) |
| Black African | 18 (8%) |
| Asian | 5 (2%) |
| Indian | 3 (1%) |
| Exposure | |
| Intentional/Accidental | 222 (94%)/15 (6%) |
| Time from exposure to admission | |
| <4 h | 63 (29%) |
| 4–8 h | 64 (29%) |
| 8–24 h | 67 (31%) |
| >24 h | 26 (12%) |
| Medical history | |
| Past depression/suicide attempt | 192 (81%) |
| Drug addiction | 87 (37%) |
| Chronic alcoholism | 65 (27%) |
| Psychosis | 56 (24%) |
| Hypertension/coronary disease | 32 (14%) |
| Human immunodeficiency virus infection | 19 (8%) |
| Diabetes/dyslipidemia | 16 (7%) |
| Long-term statin treatment | 9 (4%) |
| Number of long-term medications | 3 (1–5) |
| Parameters | Median (Percentiles 25–75) or N (%) |
|---|---|
| Glasgow coma score | 8 (3–14) |
| Systolic blood pressure (mmHg) | 116 (101–135) |
| Diastolic blood pressure (mmHg) | 72 (58–83) |
| Heart rate (/min) | 93 (75–105) |
| Respiratory rate (cycles/min) | 17 (14–21) |
| Normopnea (12–20/min) | 134 (58%) |
| Tachypnea (>20/min) | 80 (35%) |
| Bradypnea (<12/min) | 18 (8%) |
| Agitation/confusion | 73 (31%) |
| Nausea/vomiting | 45 (19%) |
| Oliguria/anuria | 38 (16%) |
| Complications | Patients without AKI N (%) | Patients with AKI N (%) | p-Value |
|---|---|---|---|
| Infections | 77 (52%) | 67 (76%) | 0.001 |
| DIC | 3 (2%) | 26 (30%) | <0.0001 |
| Cardiac arrest | 4 (3%) | 28 (32%) | <0.0001 |
| Cardiovascular failure | 21 (14%) | 62 (71%) | <0.0001 |
| Compartment syndrome | 1 (0.7%) | 4 (5%) | 0.07 |
| Characteristics | Without AKI (N = 149) | With AKI (N = 88) | p-Value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 39 (30–50) | 49 (34–55) | 0.005 |
| Female gender | 57 (38%) | 42 (48%) | 0.2 |
| Delay exposure/admission (≥24 h) | 11 (7%) | 22 (25%) | 0.001 |
| Past history of hypertension | 10 (7%) | 22 (25%) | <0.0001 |
| Long-term treatment with statins | 3 (2%) | 6 (7%) | 0.06 |
| Involved toxicants | |||
| Benzodiazepines | 52 (34.9) | 20 (23%) | 0.05 |
| Tricyclic antidepressants | 16 (11%) | 2 (2%) | 0.02 |
| Beta-blockers | 11 (7%) | 15 (17%) | 0.02 |
| Acetaminophen | 6 (4%) | 12 (14%) | 0.007 |
| Calcium-channel inhibitors | 4 (3%) | 11 (13%) | 0.003 |
| ACE/Angiotensin II receptor-blockers | 2 (1%) | 7 (8%) | 0.01 |
| Lithium | 3 (2%) | 5 (6%) | 0.1 |
| Colchicine | 0 (0) | 4 (4%) | 0.009 |
| Clinical parameters | |||
| Glasgow coma score | 9 (4–14) | 6 (3–12) | 0.005 |
| Laboratory parameters | |||
| Serum creatinine (µmol/L) | 75 (62–95) | 160 (104–246) | <0.0001 |
| Blood urea nitrogen (mmol/L) | 5.2 (4.1–6.6) | 7.8 (6.1–13.3) | <0.0001 |
| Serum creatine kinase (IU/L) | 1935 (696–5826) | 1833 (265–8834) | 0.06 |
| Arterial pH | 7.39 (7.32–7.44) | 7.29 (7.23–7.40) | <0.0001 |
| Serum bicarbonate (mmol/L) | 22 (20–25) | 19 (14–23) | <0.0001 |
| Serum lactate (mmol/L) | 1.6 (0.9–3.0) | 4.1 (1.9–8.7) | <0.0001 |
| Serum potassium (mmol/L) | 3.9 (3.6–4.3) | 4 (3.4–4.6) | 0.08 |
| Serum calcium (mmol/L) | 2.2 (2.1–2.3) | 2.0 (1.8–2.2) | <0.0001 |
| Serum phosphate (mmol/L) | 1.1 (0.9–1.3) | 1.5 (1.1–2.4) | <0.0001 |
| ALT (IU/L) | 37 (24–71) | 88 (35–197) | <0.0001 |
| AST (IU/L) | 82 (44–164) | 198 (77–572) | 0.001 |
| Serum lactate dehydrogenase (IU/L) | 324 (240–459) | 652 (354–1851) | <0.0001 |
| Prothrombin index (%) * | 88 (75–96) | 67 (47–81) | <0.0001 |
| Platelets (G/L) | 242 (184–282) | 221 (165–283) | 0.2 |
| Serum albumin (g/L) | 35 (30–38) | 24 (20–32) | <0.0001 |
| Variables | Odds Ratio [95%-IC] | Sensitivity [95%-IC] | Specificity [95%-IC] | Positive Predictive Value [95%-IC] | Negative Predictive Value [95%-IC] | Accuracy [95%-IC] | AUC ROC Curve [95%-IC] | p-value |
|---|---|---|---|---|---|---|---|---|
| Lithium overdose | 44.4 [5.3–371.5] | 0.001 | ||||||
| Serum calcium ≤2.1 mmol/L | 14.3 [2.04–112.4] | 0.58 [0.48–0.68] | 0.79 [0.72–0.85] | 0.62 [0.52–0.72] | 0.76 [0.69–0.83] | 0.71 [0.61–0.81] | 0.70 [0.61–0.79] | 0.01 |
| Female gender | 5.5 [1.8–16.9] | 0.003 | ||||||
| Serum phosphate ≥1.5 mmol/L | 2.0 [1.0–4.2] | 0.52 [0.42–0.63] | 0.85 [0.78–0.90] | 0.67 [0.57–0.77] | 0.75 [0.66–0.82] | 0.73 [0.64–0.82] | 0.68 [0.59–0.77] | 0.05 |
| Serum lactate ≥3.3 mmol/L | 1.2 [1.1–1.4] | 0.64 [0.54–0.74] | 0.79 [0.72–0.85] | 0.65 [0.55–0.75] | 0.79 [0.70–0.88] | 0.74 [0.65–0.83] | 0.75 [0.68–0.83] | 0.002 |
| Serum creatinine ≥125 µmol/L | 1.05 [1.03–1.06] | 0.71 [0.60–0.79] | 0.92 [0.86–0.95] | 0.84 [0.76–0.92] | 0.84 [0.76–0.92] | 0.84 [0.76–0.92] | 0.87 [0.81–0.93] | <0.0001 |
| Age ≥48 years | 1.04 [1.01–1.07] | 0.52 [0.42–0.62] | 0.71 [0.63–0.77] | 0.51 [0.41–0.61] | 0.71 [0.62–0.80] | 0.64 [0.54–0.74] | 0.61 [0.53–0.70] | 0.005 |
| Characteristics | Without RRT (N = 194) | With RRT (N = 43) | p-Value | |||
|---|---|---|---|---|---|---|
| Past history of hypertension | 23 (11.9) | 9 (20.9) | 0.1 | |||
| Involved toxicants | ||||||
| Benzodiazepines | 64 (33%) | 8 (19%) | 0.06 | |||
| Antipsychotics | 45 (23%) | 5 (12%) | 0.09 | |||
| Calcium-channel inhibitors | 6 (3%) | 9 (21%) | 0.001 | |||
| Chloroquine | 2 (1%) | 2 (5%) | 0.1 | |||
| Colchicine | 0 (0%) | 4 (9%) | <0.0001 | |||
| Clinical parameters | ||||||
| Glasgow coma score | 9 (4–14) | 3 (3–11) | 0.002 | |||
| Laboratory parameters | ||||||
| Serum creatinine (µmol/L) | 86 (65–120) | 155 (95–246) | <0.0001 | |||
| Blood urea nitrogen (mmol/L) | 5.7 (4.3–7.7) | 6.9 (5.7–11.6) | 0.01 | |||
| Serum creatine kinase (IU/L) | 2070 (633–6788) | 1676 (203–4935) | 0.04 | |||
| Arterial pH | 7.38 (7.31–7.43) | 7.26 (7.18–7.40) | <0.0001 | |||
| Serum bicarbonate (mmol/L) | 22 (19–25) | 18 (14–22) | <0.0001 | |||
| Serum lactate (mmol/L) | 1.7 (1.0–3.8) | 4.5 (3.0–9.4) | <0.0001 | |||
| Serum calcium (mmol/L) | 2.2 (2.1–2.3) | 2.0 (1.7–2.2) | 0.001 | |||
| Serum phosphate (mmol/L) | 1.1 (0.9–1.4) | 1.5 (1.1–2.9) | <0.0001 | |||
| Serum ALT (IU/L) | 42 (25–90) | 99 (36–232) | 0.02 | |||
| Serum AST (IU/L) | 101 (46–213) | 207 (74–615) | 0.0057 | |||
| Serum lactate dehydrogenase (IU/L) | 363 (257–606) | 789 (353–2255) | <0.0001 | |||
| Red blood cell count (T/L) | 4.44 (4.02–4.91) | 4.36 (3.63–4.60) | 0.01 | |||
| Hemoglobin (g/dL) | 13.6 (12.6–15.1) | 13.3 (11.5–14.5) | 0.04 | |||
| Prothrombin index (%) * | 83 (71–95) | 60 (43–76) | <0.0001 | |||
| Serum albumin (g/L) | 34 (28–38) | 24 (20–32) | <0.0001 | |||
| Variables | OR [95%-IC] | Sensitivity [95%-IC] | Specificity [95%-IC] | Predictive Positive Value [95%-IC] | Predictive Negative Value [95%-IC] | Accuracy [95%-IC] | AUC ROC Curve [95%-IC] | p-Value |
|---|---|---|---|---|---|---|---|---|
| Calcium-channel blocker overdose | 14.2 [3.8–53.6] | <0.0001 | ||||||
| Serum phosphate ≥2.3 mmol/L | 1.6 [1.1–2.6] | 0.43 [0.29–0.58] | 0.95 [0.91–0.98] | 0.67 [0.53–0.81] | 0.88 [0.78–0.98] | 0.86 [0.76–0.96] | 0.67 [0.55–0.80] | 0.03 |
| Glasgow coma score ≤5 | 1.12 [1.02–1.25] | 0.63 [0.48–0.76] | 0.71 [0.64–0.77] | 0.33 [0.19–0.47] | 0.90 [0.81–0.99] | 0.70 [0.56–0.84] | 0.66 [0.55–0.76] | 0.02 |
| Prothrombin index * ≤71% | 1.03 [1.01–1.05] | 0.70 [0.55–0.81] | 0.74 [0.67–0.80] | 0.38 [0.23–0.53] | 0.92 [0.86–0.98] | 0.73 [0.60–0.86] | 0.76 [0.66–0.86] | 0.001 |
| Serum creatinine ≥125 µmol/L | 1.01 [1.00–1.01] | 0.65 [0.50–0.78] | 0.76 [0.70–0.82] | 0.38 [0.23–0.53] | 0.91 [0.82–0.99] | 0.74 [0.61–0.87] | 0.75 [0.66–0.84] | 0.02 |
| Characteristics | Survivors (N = 206) | Non-Survivors (N = 31) | p-Value |
|---|---|---|---|
| Age (years) | 41 (31–53) | 49 (34–57) | 0.2 |
| Involved toxicants | |||
| Antipsychotics | 49 (24%) | 1 (3%) | 0.001 |
| Beta blockers | 20 (10%) | 6 (19%) | 0.1 |
| Paracetamol | 13 (6%) | 5 (16%) | 0.06 |
| Calcium-channel blockers | 10 (5%) | 5 (16%) | 0.01 |
| Chloroquine | 2 (1%) | 2 (7%) | 0.03 |
| Colchicine | 1 (1%) | 3 (10%) | 0.001 |
| Clinical parameters | |||
| Glasgow coma score | 9 (3–14) | 3 (3–8) | 0.001 |
| Temperature (°C) | 36.8 (35.8–37.9) | 35.7 (34.1–36.2) | 0.1 |
| AKI (stage 3, KDIGO) | 35 (17%) | 23 (74%) | <0.0001 |
| Laboratory parameters | |||
| Serum creatinine (µmol/L) | 88 (66–127) | 147 (95–243) | <0.0001 |
| Blood urea nitrogen (mmol/L) | 5.9 (4.3–7.7) | 6.9 (5.4–11.6) | 0.001 |
| Arterial pH | 7.38 (7.30–7.43) | 7.24 (7.12–7.40) | < 0.0001 |
| Serum bicarbonate (mmol/L) | 22 (19–25) | 17 (13–21) | < 0.0001 |
| Serum lactate (mmol/L) | 1.8 (1.0–3.9) | 8.2 (3.6–12.3) | <0.0001 |
| Serum calcium (mmol/L) | 2.2 (2.1–2.3) | 1.9 (1.7–2.1) | <0.001 |
| Serum phosphate (mmol/L) | 1.1 (0.9–1.5) | 2.0 (1.2–3.1) | <0.0001 |
| Serum ALT (UI/L) | 43 (24–91) | 157 (54–531) | <0.0001 |
| Serum AST (UI/L) | 101 (47–213) | 332 (111–900) | 0.001 |
| Serum lactate dehydrogenase (UI/L) | 371 (257–630) | 859 (416–2875) | 0.001 |
| Hemoglobin (g/dL) | 13.6 (12.6–15.1) | 12.5 (11.0–14.3) | 0.003 |
| Platelets (G/L) | 236 (184–281) | 209 (149–286) | 0.1 |
| Prothrombin time (%) * | 83 (71–94) | 47 (30–63) | <0.0001 |
| Variables | OR [95%-IC] | Sensitivity [95%-IC] | Specificity [95%-IC] | Predictive Positive Value [95%-IC] | Predictive Negative Value [95%-IC] | Accuracy [95%-IC] | AUC ROC Curve [95%-IC] | p-Value |
|---|---|---|---|---|---|---|---|---|
| Acute kidney injury, stage 3 According to the KDIGO classification | 7.0 [2.5–19.8] | 0.001 | ||||||
| Hyperphosphoremia ≥3.1 mmol/L | 1.2 [1.1–1.3] | 0.84 [0.67–0.93] | 0.81 [0.75–0.86] | 0.40 [0.28–0.52] | 0.97 [0.94–0.99] | 0.81 [0.76–0.86] | 0.85 [0.80–0.92] | 0.001 |
| Prothrombin index * ≤68% | 1.04 [1.02–1.06] | 0.84 [0.67–0.93] | 0.70 [0.62–0.75] | 0.30 [0.19–0.41] | 0.97 [0.94–0.99] | 0.71 [0.65–0.77] | 0.83 [0.72–0.95] | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogliano, P.-F.; Voicu, S.; Labat, L.; Deye, N.; Malissin, I.; Laplanche, J.-L.; Vodovar, D.; Mégarbane, B. Acute Poisoning with Rhabdomyolysis in the Intensive Care Unit: Risk Factors for Acute Kidney Injury and Renal Replacement Therapy Requirement. Toxics 2020, 8, 79. https://doi.org/10.3390/toxics8040079
Rogliano P-F, Voicu S, Labat L, Deye N, Malissin I, Laplanche J-L, Vodovar D, Mégarbane B. Acute Poisoning with Rhabdomyolysis in the Intensive Care Unit: Risk Factors for Acute Kidney Injury and Renal Replacement Therapy Requirement. Toxics. 2020; 8(4):79. https://doi.org/10.3390/toxics8040079
Chicago/Turabian StyleRogliano, Pierre-François, Sebastian Voicu, Laurence Labat, Nicolas Deye, Isabelle Malissin, Jean-Louis Laplanche, Dominique Vodovar, and Bruno Mégarbane. 2020. "Acute Poisoning with Rhabdomyolysis in the Intensive Care Unit: Risk Factors for Acute Kidney Injury and Renal Replacement Therapy Requirement" Toxics 8, no. 4: 79. https://doi.org/10.3390/toxics8040079
APA StyleRogliano, P.-F., Voicu, S., Labat, L., Deye, N., Malissin, I., Laplanche, J.-L., Vodovar, D., & Mégarbane, B. (2020). Acute Poisoning with Rhabdomyolysis in the Intensive Care Unit: Risk Factors for Acute Kidney Injury and Renal Replacement Therapy Requirement. Toxics, 8(4), 79. https://doi.org/10.3390/toxics8040079






