Effects of Soil Amendments on Heavy Metal Immobilization and Accumulation by Maize Grown in a Multiple-Metal-Contaminated Soil and Their Potential for Safe Crop Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil
2.2. Plants and Soil Amendments
2.3. Experimental Design and Procedure
2.4. Sample Analysis
2.5. Data Analysis
3. Results
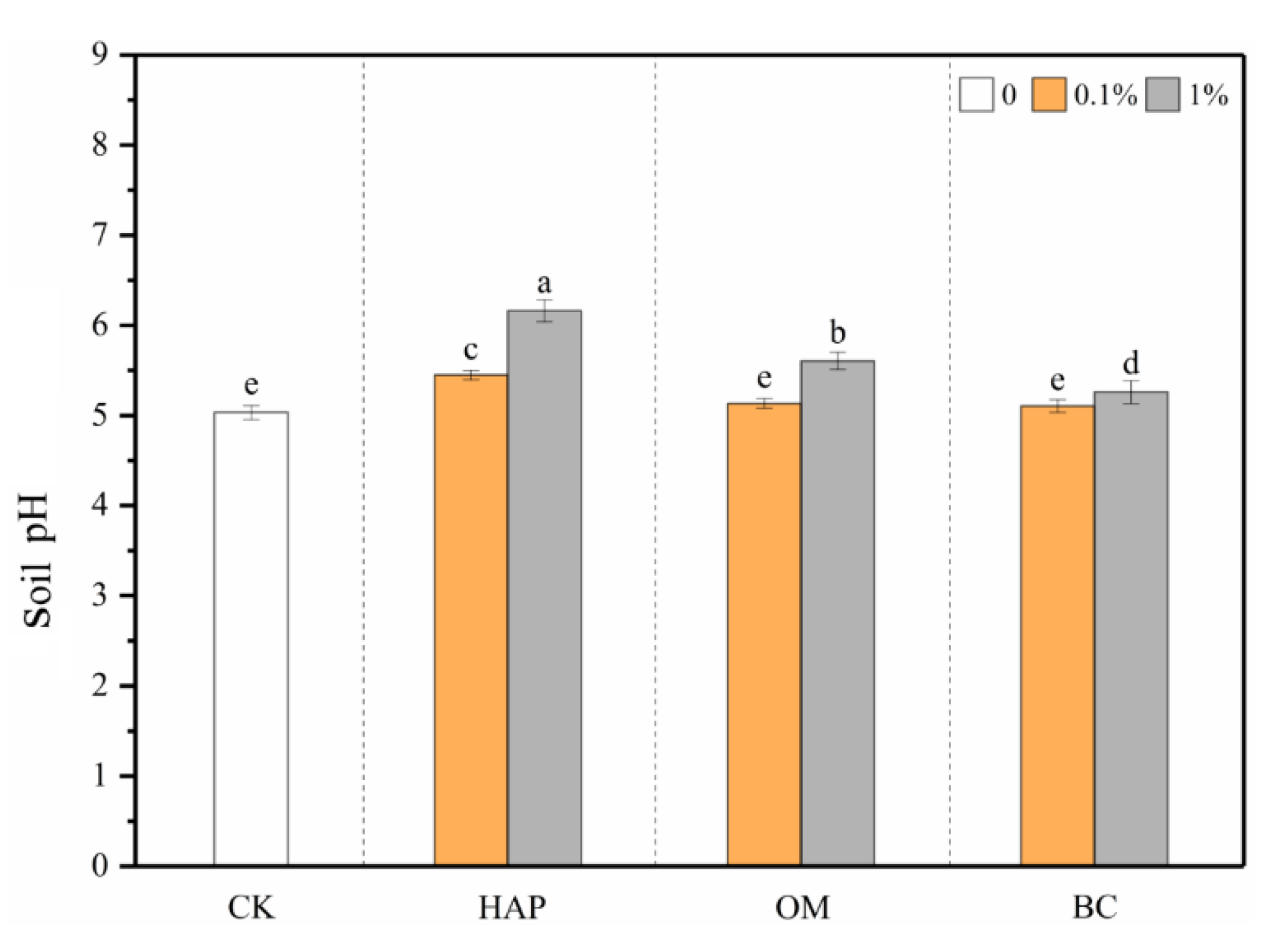
3.1. Soil pH
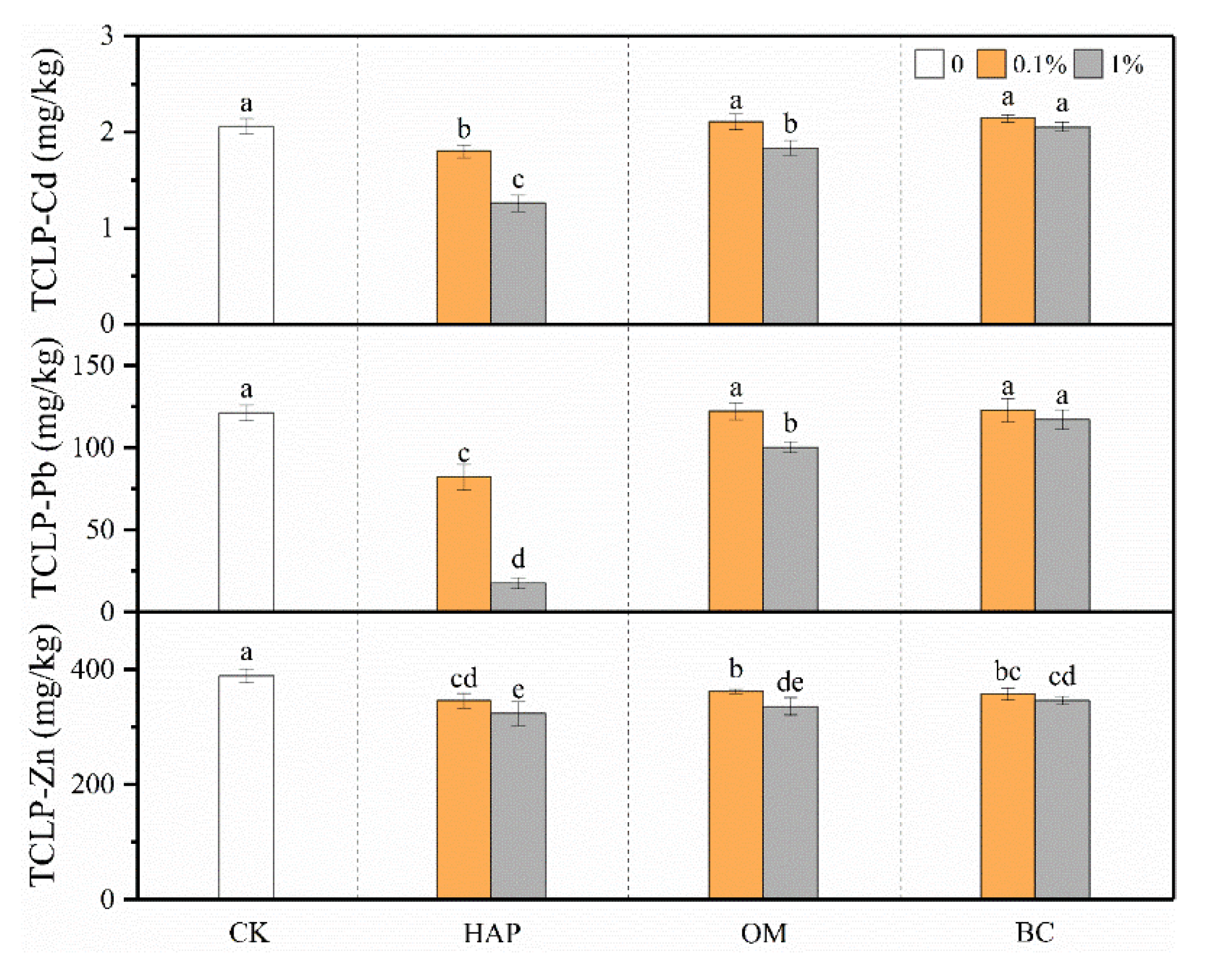
3.2. TCLP-Cd, -Pb, and -Zn in Soil
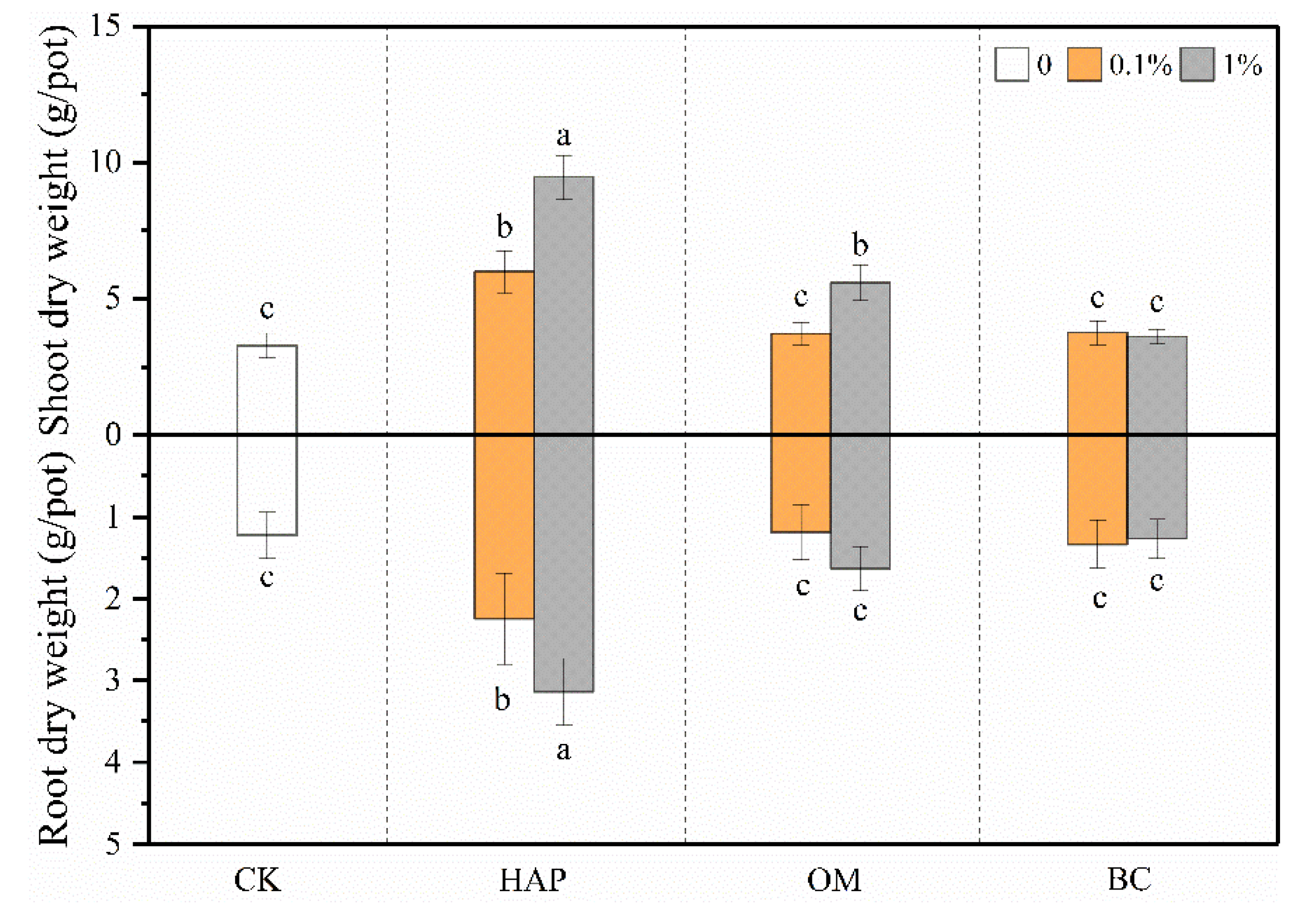
3.3. Plant Biomass
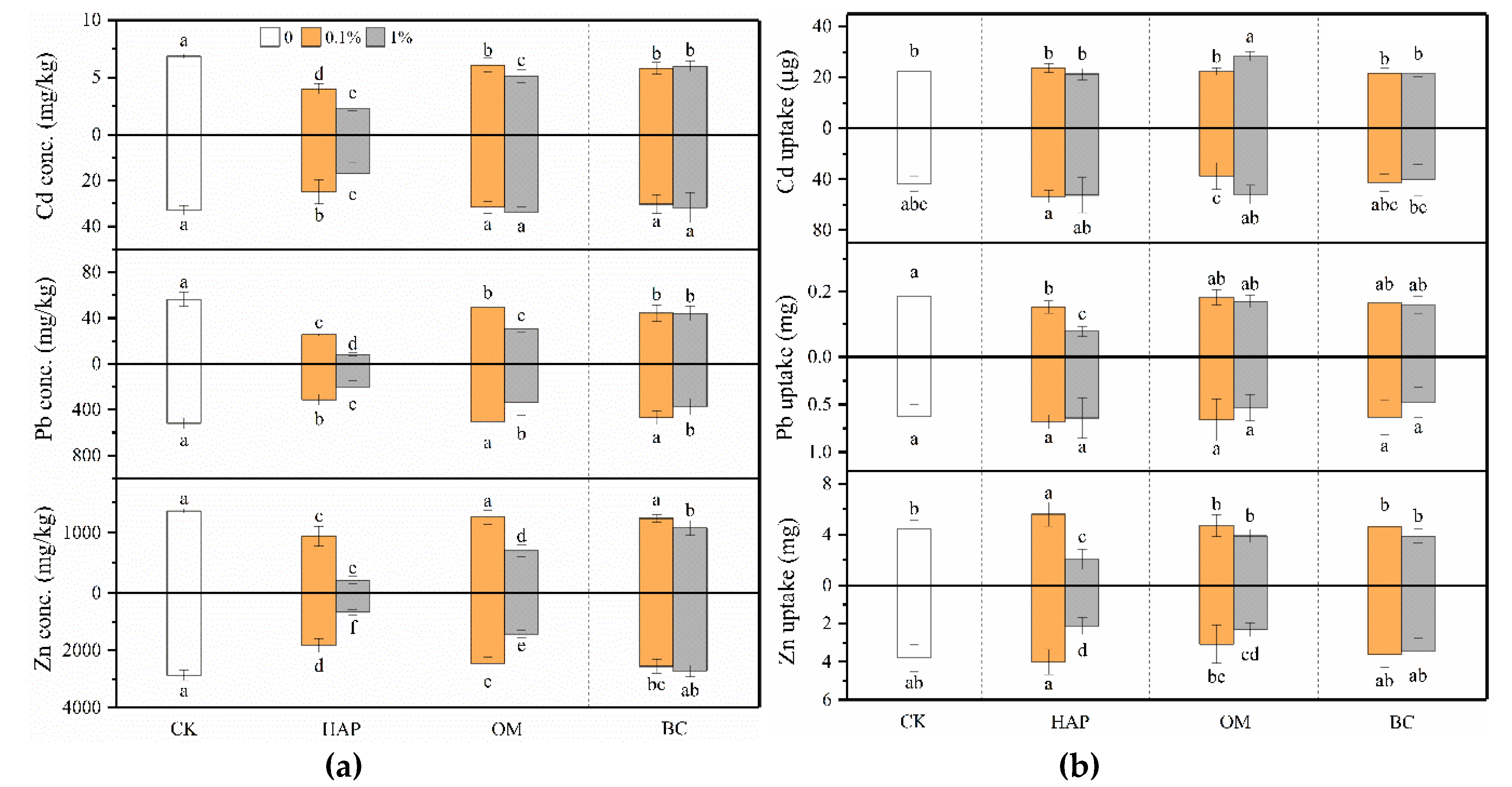
3.4. Heavy Metal Concentrations, Uptake, and TF in Plants
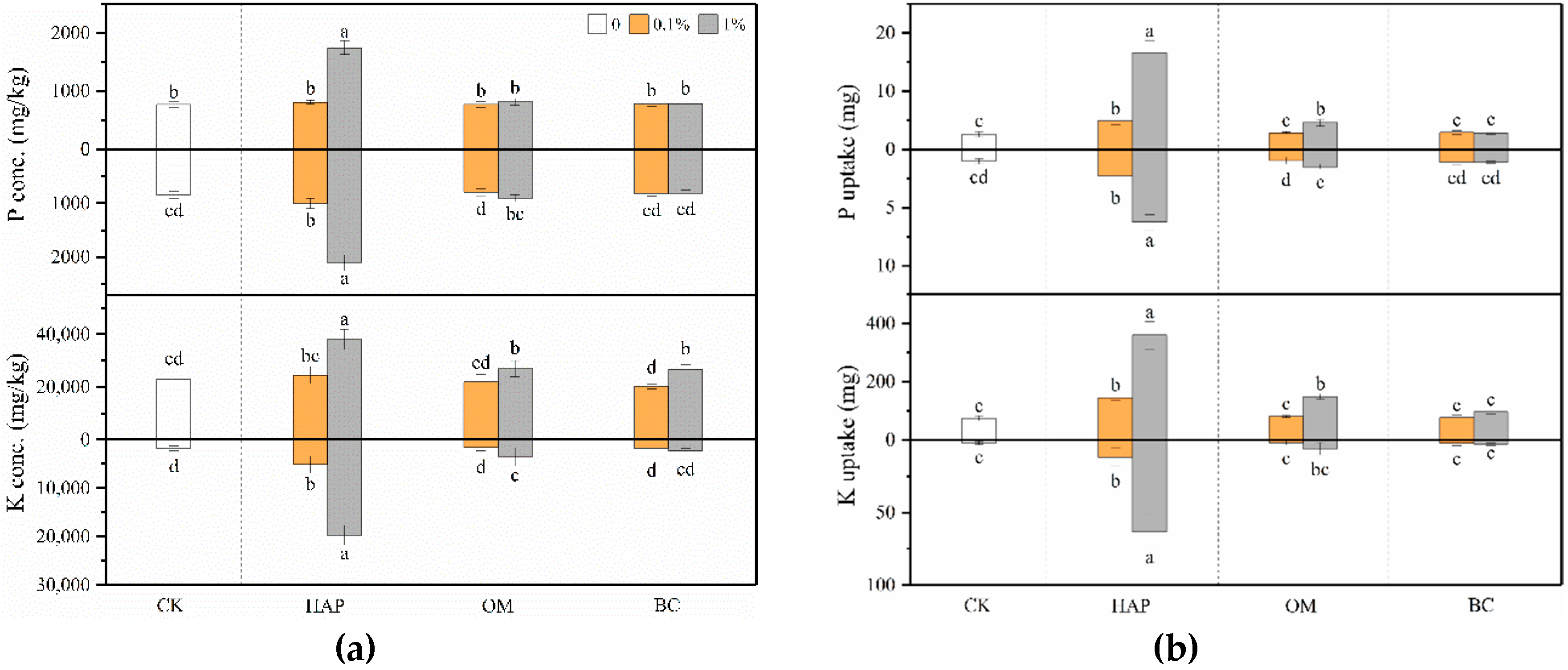
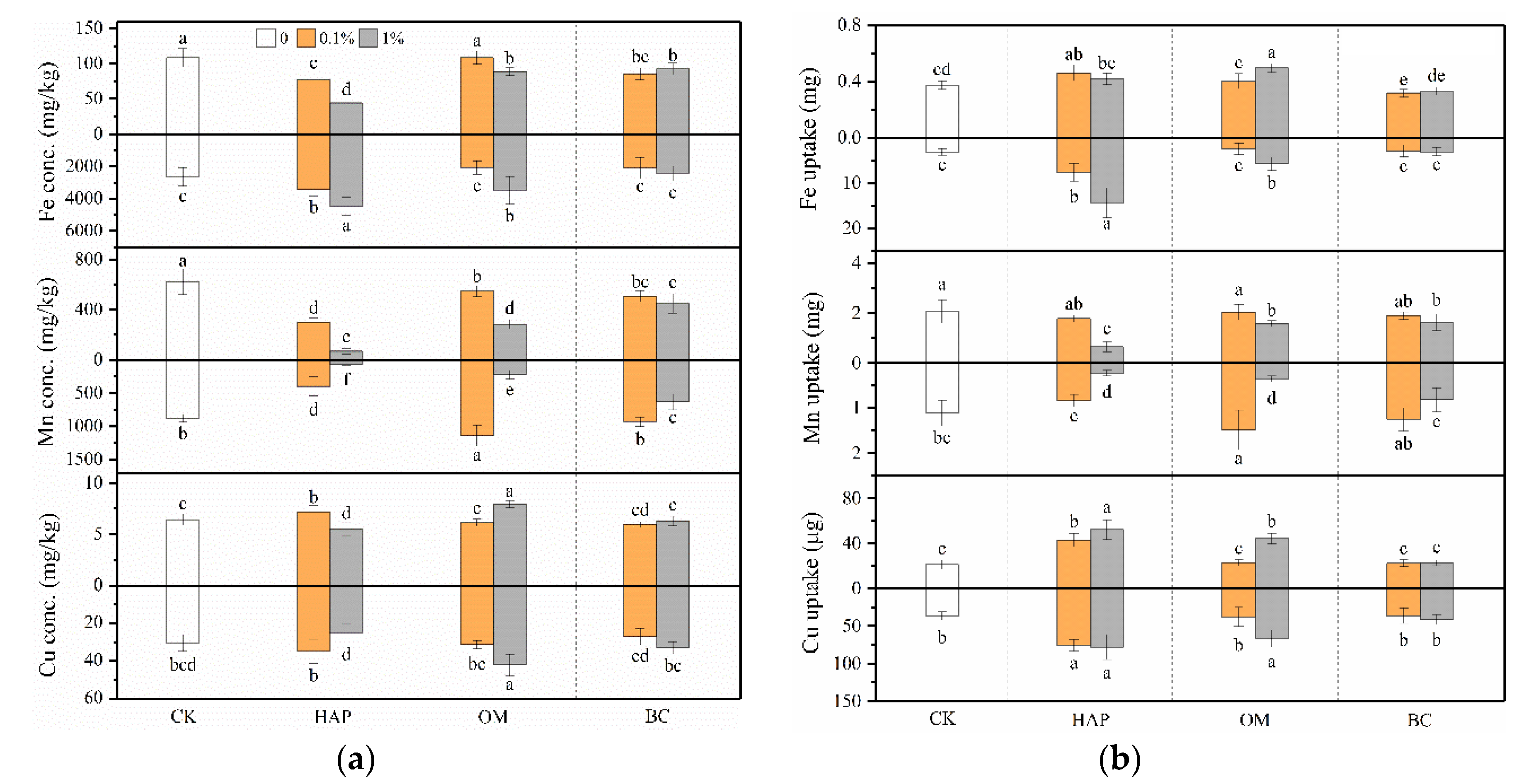
3.5. Plant Mineral Nutrition
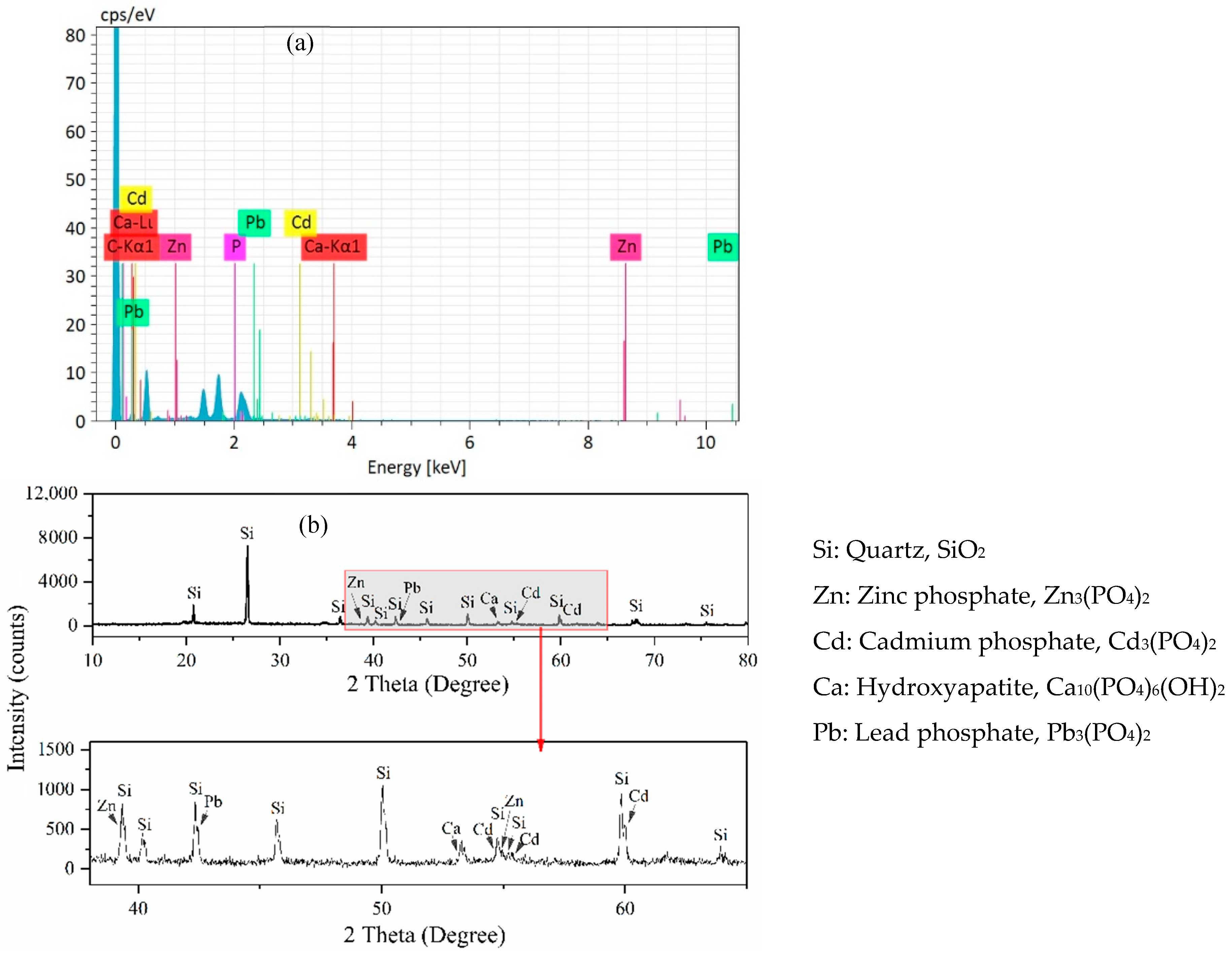
3.6. EDS and XRD Analyses
3.7. Correlations between Soil and Plant Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Chen, Y.; Weng, L.; Ma, J.; Ma, Y.; Li, Y.; Islam, S. Comparisons of heavy metal input inventory in agricultural soils in North and South China: A review. Sci. Total Environ. 2019, 660, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, F.A.; Smith, S.; Alloway, B.; Carlton-Smith, C.; Chambers, B. An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci. Total Environ. 2003, 311, 205–219. [Google Scholar] [CrossRef]
- Liu, X.; Song, Q.; Tang, Y.; Li, W.; Xu, J.; Wu, J.; Wang, F.; Brookes, P.C. Human health risk assessment of heavy metals in soil–vegetable system: A multi-medium analysis. Sci. Total Environ. 2013, 463, 530–540. [Google Scholar] [CrossRef]
- Doabi, S.A.; Karami, M.; Afyuni, M.; Yeganeh, M. Pollution and health risk assessment of heavy metals in agricultural soil, atmospheric dust and major food crops in Kermanshah province, Iran. Ecotoxicol. Environ. Saf. 2018, 163, 153–164. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Lwin, C.S.; Seo, B.-H.; Kim, H.-U.; Owens, G.; Kim, K.-R. Application of soil amendments to contaminated soils for heavy metal immobilization and improved soil quality—A critical review. Soil Sci. Plant Nutr. 2018, 64, 156–167. [Google Scholar] [CrossRef]
- Bolan, N.; Duraisamy, V.P. Role of inorganic and organic soil amendments on immobilisation and phytoavailability of heavy metals: A review involving specific case studies. Soil Res. 2003, 41, 533–555. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Li, R.; Zhang, Z. Immobilization of lead and cadmium in contaminated soil using amendments: A review. Pedosphere 2015, 25, 555–568. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Liu, W.; Cheng, L.; Jiang, Q.; Zhanga, Y. Exploratory of immobilization remediation of hydroxyapatite (HAP) on lead-contaminated soils. Environ. Sci. Pollut. Res. 2019, 26, 26674–26684. [Google Scholar] [CrossRef]
- Zeng, G.; Wan, J.; Huang, D.; Hu, L.; Huang, C.; Cheng, M.; Xue, W.; Gong, X.; Wang, R.; Jiang, D. Precipitation, adsorption and rhizosphere effect: The mechanisms for Phosphate-induced Pb immobilization in soils—A review. J. Hazard. Mater. 2017, 339, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lamb, D.; Paneerselvam, P.; Choppala, G.; Bolan, N.; Chung, J.W. Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. J. Hazard. Mater. 2011, 185, 549–574. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ. 2017, 601, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Lahori, A.H.; Guo, Z.; Zhang, Z.; Li, R.; Mahar, A.; Awasthi, M.K.; Shen, F.; Sial, T.A.; Kumbhar, F.; Wang, P.; et al. Use of biochar as an amendment for remediation of heavy metal-contaminated soils: Prospects and challenges. Pedosphere 2017, 27, 991–1014. [Google Scholar] [CrossRef]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Zhang, W. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Nagpal, A.K. Soil amendments: A tool to reduce heavy metal uptake in crops for production of safe food. Rev. Environ. Sci. Bio/Technol. 2017, 17, 187–203. [Google Scholar] [CrossRef]
- Zhu, F.; He, S.; Shang, Z. Effect of vegetables and nano-particle hydroxyapatite on the remediation of cadmium and phosphatase activity in rhizosphere soil through immobilization. Int. J. Phytoremediat. 2019, 21, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Lago-Vila, M.; Rodríguez-Seijo, A.; Vega, F.; Arenas-Lago, D. Phytotoxicity assays with hydroxyapatite nanoparticles lead the way to recover firing range soils. Sci. Total Environ. 2019, 690, 1151–1161. [Google Scholar] [CrossRef]
- Ryan, J.A.; Zhang, P.; Hesterberg, D.; Chou, J.; Sayers, D.E. Formation of chloropyromorphite in a lead-contaminated soil amended with hydroxyapatite. Environ. Sci. Technol. 2001, 35, 3798–3803. [Google Scholar] [CrossRef]
- He, M.; Shi, H.; Zhao, X.; Yu, Y.; Qu, B. Immobilization of Pb and Cd in contaminated soil using nano-crystallite hydroxyapatite. Procedia Environ. Sci. 2013, 18, 657–665. [Google Scholar] [CrossRef]
- Ding, L.; Li, J.; Liu, W.; Zuo, Q.; Liang, S.X. Influence of nano-hydroxyapatite on the metal bioavailability, plant metal accumulation and root exudates of ryegrass for phytoremediation in lead-polluted soil. Int. J. Environ. Res. Public Health 2017, 14, 532. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, W.; Li, X.-L.; Shen, S.-G.; Liang, S.X.; Liu, C.; Shan, L. Nano-hydroxyapatite immobilized lead and enhanced plant growth of ryegrass in a contaminated soil. Ecol. Eng. 2016, 95, 25–29. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Chang, R.; Sohi, S.P.; Jing, F.; Liu, Y.; Chen, J. A comparative study on biochar properties and Cd adsorption behavior under effects of ageing processes of leaching, acidification and oxidation. Environ. Pollut. 2019, 254, 113123. [Google Scholar] [CrossRef]
- Rehman, M.Z.U.; Khalid, H.; Akmal, F.; Ali, S.; Rizwan, M.; Qayyum, M.F.; Iqbal, M.; Khalid, M.U.; Azhar, M. Effect of limestone, lignite and biochar applied alone and combined on cadmium uptake in wheat and rice under rotation in an effluent irrigated field. Environ. Pollut. 2017, 227, 560–568. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Ok, Y.S.; Niazi, N.K.; Rizwan, M.; Al-Wabel, M.I.; Usman, A.R.; Moon, D.H.; Lee, S.S. Effect of corn residue biochar on the hydraulic properties of sandy loam soil. Sustainability 2017, 9, 266. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Tang, X.-Y.; Guan, Z.; Reid, B.J.; Rajapaksha, A.U.; Ok, Y.S.; Sun, H. Biochar increased water holding capacity but accelerated organic carbon leaching from a sloping farmland soil in China. Environ. Sci. Pollut. Res. 2015, 23, 995–1006. [Google Scholar] [CrossRef]
- Abel, S.; Peters, A.; Trinks, S.; Schonsky, H.; Facklam, M.; Wessolek, G. Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 2013, 183–191. [Google Scholar] [CrossRef]
- Bruun, E.W.; Petersen, C.T.; Hansen, E.H.; Holm, J.K.; Hauggaard-Nielsen, H. Biochar amendment to coarse sandy subsoil improves root growth and increases water retention. Soil Use Manag. 2014, 30, 109–118. [Google Scholar] [CrossRef]
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.; Alessi, D.S.; Shen, Z.; Bolan, N.; Hou, D. Biochar application for the remediation of heavy metal polluted land: A review of in situ field trials. Sci. Total Environ. 2018, 619, 815–826. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, B.; Shafi, M.; Ma, J.; Guo, J.; Wu, J.; Ye, Z.; Liu, D.; Jin, H. Effects of biochar on growth, and heavy metals accumulation of moso bamboo (Phyllostachy pubescens), soil physical properties, and heavy metals solubility in soil. Chemosphere 2019, 219, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, M.I.S.; Da Silva, P.S.O.; Santos, J.C.D.J.; Junior, L.F.G.D.O. Biochar increases plant water use efficiency and biomass production while reducing Cu concentration in Brassica juncea L. in a Cu-contaminated soil. Ecotoxicol. Environ. Saf. 2019, 183, 109557. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, X.; Bian, R.; Cheng, K.; Zhang, X.; Zheng, J.; Joseph, S.; Crowley, D.; Pan, G.; Li, L. Effects of biochar on availability and plant uptake of heavy metals—A meta-analysis. J. Environ. Manag. 2018, 222, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhu, Y.; Li, Z.; Huang, B.; Luo, N.; Liu, C.; Zeng, G. Compost as a soil amendment to remediate heavy metal-contaminated agricultural soil: Mechanisms, efficacy, problems, and strategies. Water Air Soil Pollut. 2016, 227, 359. [Google Scholar] [CrossRef]
- Frutos, I.; García-Delgado, C.; Gárate, A.; Eymar, E. Biosorption of heavy metals by organic carbon from spent mushroom substrates and their raw materials. Int. J. Environ. Sci. Technol. 2016, 13, 2713–2720. [Google Scholar] [CrossRef]
- Mayans, B.; Pérez-Esteban, J.; Escolástico, C.; Eymar, E.; Masaguer, A. Evaluation of commercial humic substances and other organic amendments for the immobilization of copper through 13C CPMAS NMR, FT-IR, and DSC analyses. Agronomy 2019, 9, 762. [Google Scholar] [CrossRef]
- Wei, S.; Zhou, Q.; Zhan, J.; Wu, Z.; Sun, T.; Lyubu, Y.; Prasad, M. Poultry manured Bidens tripartite L. extracting Cd from soil – potential for phytoremediating Cd contaminated soil. Bioresour. Technol. 2010, 101, 8907–8910. [Google Scholar] [CrossRef]
- Guo, D.; Ren, C.; Ali, A.; Li, R.; Du, J.; Liu, X.; Guan, W.; Zhang, Z. Streptomyces pactum combined with manure compost alters soil fertility and enzymatic activities, enhancing phytoextraction of potentially toxic metals (PTMs) in a smelter-contaminated soil. Ecotoxicol. Environ. Saf. 2019, 181, 312–320. [Google Scholar] [CrossRef]
- Wang, F.; Shi, Z.Y.; Xu, X.F.; Wang, X.G.; Li, Y.J. Contribution of AM inoculation and cattle manure to lead and cadmium phytoremediation by tobacco plants. Environ. Sci. Process. Impacts 2013, 15, 794–801. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Shi, Z.Y.; Li, Y.J.; Song, Z.M. Effects of AM inoculation and organic amendment, alone or in combination, on growth, P nutrition, and heavy-metal uptake of tobacco in Pb-Cd-contaminated soil. J. Plant Growth Regul. 2012, 31, 549–559. [Google Scholar] [CrossRef]
- Lu, R. Analytical Methods for Soils and Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Sun, Y.; Zheng, F.; Wang, W.; Zhang, S.; Wang, F. Remediation of Cr(VI)-contaminated soil by nano-zero-valent iron in combination with biochar or humic acid and the consequences for plant performance. Toxics 2020, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.H.; Hwang, I.; Koutsospyros, A.; Cheong, K.H.; Ok, Y.S.; Ji, W.H.; Park, J.H. Stabilization of lead (Pb) and zinc (Zn) in contaminated rice paddy soil using starfish: A preliminary study. Chemosphere 2018, 199, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Lee, H.H.; Owens, V.; Park, S.; Kim, J.; Hong, C.O. Adsorption and precipitation of cadmium affected by chemical form and addition rate of phosphate in soils having different levels of cadmium. Chemosphere 2018, 206, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.J.; Chen, J.H.; Fan, T.T.; Zhou, D.M.; Gao, J. Effect of nanoparticle hydroxyapatite on the immobilization of Cu and Zn in polluted soil. Environ. Sci. Pollut. Res. 2016, 25, 73–80. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments–A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Bolan, N.S.; Adriano, D.; Duraisamy, P.; Mani, A.; Arulmozhiselvan, K. Immobilization and phytoavailability of cadmium in variable charge soils. I. Effect of phosphate addition. Plant Soil 2003, 250, 83–94. [Google Scholar] [CrossRef]
- Xu, Y.; Schwartz, F.W.; Traina, S.J. Sorption of Zn2+ and Cd2+ on hydroxyapatite surfaces. Environ. Sci. Technol. 1994, 28, 1472–1480. [Google Scholar] [CrossRef]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef]
- Collins, C. Uptake of organic pollutants and potentially toxic elements (PTEs) by crops. In Persistent Organic Pollutants and Toxic Metals in Foods; Rose, M., Fernandes, A., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 129–144. [Google Scholar]
- Lim, J.E.; Ahmad, M.; Lee, S.S.; Shope, C.L.; Hashimoto, Y.; Kim, K.-R.; Usman, A.R.A.; Yang, J.E.; Ok, Y.S. Effects of lime-based waste materials on immobilization and phytoavailability of cadmium and lead in contaminated soil. Clean Soil Air Water 2013, 41, 1235–1241. [Google Scholar] [CrossRef]
- Naramabuye, F.X.; Haynes, R.J. Effect of organic amendments on soil pH and Al solubility and use of laboratory indices to predict their liming effect. Soil Sci. 2006, 171, 754–763. [Google Scholar] [CrossRef]
- Walker, D.J.; Clemente, R.; Bernal, M. Contrasting effects of manure and compost on soil pH, heavy metal availability and growth of Chenopodium album L. in a soil contaminated by pyritic mine waste. Chemosphere 2004, 57, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Pocknee, S.; Sumner, M.E. Cation and nitrogen contents of organic matter determine its soil liming potential. Soil Sci. Soc. Am. J. 1997, 61, 86–92. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.Q.; Rhue, D.R.; Appel, C.S. Mechanisms of lead, copper, and zinc retention by phosphate rock. Environ. Pollut. 2004, 131, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Impellitteri, C.A. Effects of pH and phosphate on metal distribution with emphasis on as speciation and mobilization in soils from a lead smelting site. Sci. Total Environ. 2005, 345, 175–190. [Google Scholar] [CrossRef]
- Rodríguez-Vila, A.; Asensio, V.; Forjan, R.; Covelo, E.F. Chemical fractionation of Cu, Ni, Pb and Zn in a mine soil amended with compost and biochar and vegetated with Brassica juncea L. J. Geochem. Explor. 2015, 158, 74–81. [Google Scholar] [CrossRef]
- Rodríguez-Vila, A.; Covelo, E.F.; Forján, R.; Asensio, V. Phytoremediating a copper mine soil with Brassica juncea L., compost and biochar. Environ. Sci. Pollut. Res. 2014, 21, 11293–11304. [Google Scholar] [CrossRef]
- Adriano, D.C. Trace Elements in Terrestrial Environments; Springer: New York, NY, USA, 2001. [Google Scholar]
- Lindsay, W.L. Chemical Equilibria in Soils; John Wiley and Sons: New York, NY, USA, 1979. [Google Scholar]
- Neilsen, D.; Neilsen, G.H.; Sinclair, A.H.; Linehan, D.J. Soil phosphorus status, pH and the manganese nutrition of wheat. Plant Soil 1992, 145, 45–50. [Google Scholar] [CrossRef]
- Boisson, J.; Ruttens, A.; Mench, M.; Vangronsveld, J. Evaluation of hydroxyapatite as a metal immobilizing soil additive for the remediation of polluted soils. Part 1. Influence of hydroxyapatite on metal exchangeability in soil, plant growth and plant metal accumulation. Environ. Pollut. 1999, 104, 225–233. [Google Scholar] [CrossRef]
- Barker, A.V.; Pilbeam, D.J. Handbook of Plant Nutrition, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Ghasemi-Fasaei, R.; Ronaghi, A.; Maftoun, M.; Karimian, N.A.; Soltanpour, P.N. Iron-manganese interaction in chickpea as affected by foliar and soil application of iron in a calcareous soil. Commun. Soil Sci. Plant Anal. 2005, 36, 1717–1725. [Google Scholar] [CrossRef]

| Item | Value | Screening Value * | Intervention Value * |
|---|---|---|---|
| pH (soil/water, 1:2.5, w/v) | 5.0 | ||
| Total Cd | 2.6 mg/kg | 0.3 | 1.5 |
| Total Pb | 1796 mg/kg | 70 | 400 |
| Total Zn | 1603 mg/kg | 200 | |
| TCLP-Cd | 2.25 mg/kg | ||
| TCLP-Pb | 136.8 mg/kg | ||
| TCLP-Zn | 371.6 mg/kg | ||
| Organic matter | 25.8 g/kg | ||
| Olsen P | 27 mg/kg | ||
| NH4OAc extractable K | 26.3 mg/kg | ||
| Alkali-hydrolyzable N | 118 mg/kg | ||
| DTPA-Fe | 154.6 mg/kg | ||
| DTPA-Mn | 68.5 mg/kg | ||
| DTPA-Cu | 7.5 mg/kg | ||
| Cation exchange capacity | 3.15 cmol/kg | ||
| Soil type | Paddy soil |
| Variables | Amendment Type (T) | Amendment Dose (D) | T × D |
|---|---|---|---|
| Soil pH | 151.966 *** | 221.228 *** | 28.943 *** |
| TCLP-Cd | 218.36 *** | 165.035 *** | 31.889 *** |
| TCLP-Pb | 226.041 *** | 160.43 *** | 65.231 *** |
| TCLP-Zn | 6.124 ** | 22.046 *** | 0.991 ns |
| Shoot dry weight | 165.465 *** | 83.228 *** | 30.445 *** |
| Root dry weight | 57.247 *** | 12.865 *** | 5.523 ** |
| Shoot Cd conc. | 132.490 *** | 31.075 *** | 13.495 *** |
| Shoot Pb conc. | 115.236 *** | 57.883 *** | 13.821 *** |
| Shoot Zn conc. | 101.379 *** | 200.338 *** | 24.518 *** |
| Root Cd conc. | 24.638 *** | 0.991 ns | 4.837 * |
| Root Pb conc. | 20.984 *** | 26.844 *** | 0.800ns |
| Root Zn conc. | 153.407 *** | 106.085 *** | 40.609 *** |
| TF of Cd | 7.970 ** | 5.010 * | 1.748 ns |
| TF of Pb | 30.988 *** | 1.841 ns | 14.799 *** |
| TF of Zn | 5.571 ** | 26.472 *** | 4.810 * |
| Shoot Cd uptake | 11.673 *** | 2.770 ns | 13.775 *** |
| Shoot Pb uptake | 19.292 *** | 13.781 *** | 6.631 ** |
| Shoot Zn uptake | 1.625 ns | 50.150 *** | 14.606 *** |
| Root Cd uptake | 4.499 * | 1.321 ns | 2.713 ns |
| Root Pb uptake | 1.246 ns | 3.628 ns | 0.404 ns |
| Root Zn uptake | 4.402 * | 17.688 *** | 5.024 * |
| Item | Soil pH | Dry Weight | Cd conc. | Pb conc. | Zn conc. | TF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | Shoot | Root | Shoot | Root | Cd | Pb | Zn | ||
| Soil pH | 0.934 ** | 0.804 ** | −0.877 ** | −0.687 ** | −0.928 ** | −0.831 ** | −0.956 ** | −0.941 ** | −0.624 ** | −0.727 ** | −0.487 ** | |
| TCLP-Cd | −0.936 ** | −0.955 ** | −0.877 ** | 0.907 ** | 0.717 ** | 0.679 ** | ||||||
| TCLP-Pb | −0.939 ** | −0.937 ** | −0.834 ** | 0.917 ** | 0.791 ** | 0.794 ** | ||||||
| TCLP-Zn | −0.765 ** | −0.696 ** | −0.565 ** | 0.746 ** | 0.724 ** | 0.250 ns | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Zhang, S.; Cheng, P.; Zhang, S.; Sun, Y. Effects of Soil Amendments on Heavy Metal Immobilization and Accumulation by Maize Grown in a Multiple-Metal-Contaminated Soil and Their Potential for Safe Crop Production. Toxics 2020, 8, 102. https://doi.org/10.3390/toxics8040102
Wang F, Zhang S, Cheng P, Zhang S, Sun Y. Effects of Soil Amendments on Heavy Metal Immobilization and Accumulation by Maize Grown in a Multiple-Metal-Contaminated Soil and Their Potential for Safe Crop Production. Toxics. 2020; 8(4):102. https://doi.org/10.3390/toxics8040102
Chicago/Turabian StyleWang, Fayuan, Shuqi Zhang, Peng Cheng, Shuwu Zhang, and Yuhuan Sun. 2020. "Effects of Soil Amendments on Heavy Metal Immobilization and Accumulation by Maize Grown in a Multiple-Metal-Contaminated Soil and Their Potential for Safe Crop Production" Toxics 8, no. 4: 102. https://doi.org/10.3390/toxics8040102
APA StyleWang, F., Zhang, S., Cheng, P., Zhang, S., & Sun, Y. (2020). Effects of Soil Amendments on Heavy Metal Immobilization and Accumulation by Maize Grown in a Multiple-Metal-Contaminated Soil and Their Potential for Safe Crop Production. Toxics, 8(4), 102. https://doi.org/10.3390/toxics8040102






