Abstract
Water-pipe smoking (WPS) is becoming the most popular form of tobacco use among the youth, especially in the Middle East, replacing cigarettes rapidly and becoming a major risk of tobacco addiction worldwide. Smoke from WPS contains similar toxins as those present in cigarette smoke and is linked directly with different types of cancers including lung and head and neck (HN) carcinomas. However, the underlying molecular pathways and/or target genes responsible for the carcinogenic process are still unknown. In this study, human normal oral epithelial (HNOE) cells, NanoString PanCancer Pathways panel of 770 gene transcripts and quantitative real-time polymerase chain reaction (qRT-PCR) analysis were applied to discover differentially expressed genes (DEG) modulated by WPS. In silico analysis was performed to analyze the impact of these genes in HN cancer patient’s biology and outcome. We found that WPS can induce the epithelial–mesenchymal transition (EMT: hallmark of cancer progression) of HNOE cells. More significantly, our analysis of NanoString revealed 23 genes deregulated under the effect of WPS, responsible for the modulation of cell cycle, proliferation, migration/invasion, apoptosis, signal transduction, and inflammatory response. Further analysis was performed using qRT-PCR of HNOE WPS-exposed and unexposed cells supported the reliability of our NanoString data. Moreover, we demonstrate those DEG to be upregulated in cancer compared with normal tissue. Using the Kaplan–Meier analysis, we observed a significant association between WPS-deregulated genes and relapse-free survival/overall survival in HN cancer patients. Our findings imply that WPS can modulate EMT as well as a set of genes that are directly involved in human HN carcinogenesis, thereby affecting HN cancer patients’ survival.
1. Introduction
Tobacco smoking is the most common preventable risk factor for several non-communicable diseases, such as, cardiovascular, lung, diabetes as well as cancer, and can be considered lethal [1,2]. Widespread tobacco consumption is somewhat attributed to the variation in available consumption methods, such as cigarettes, electronic cigarettes (E-cigarettes), cigars, and water-pipe smoking (WPS). Recently, global trend of tobacco smoking has started to shift towards WPS in addition to E-cigarettes [3,4,5] with approximately 100 million smokers using WPS on daily basis [6], leading to nearly 5 million deaths annually [7]. Global increase in WPS use is due to several factors including its availability in several delectable flavors and aromas along with its association with socializing, relaxation, and entertainment. Additional motives include peer-pressure, low-cost, fashion, and inquisitiveness [3,8]. Interestingly, people from the Middle East and those of Middle Eastern descent in Western countries smoke WPS, as they consider it to be a part of their culture, thus giving rise to this trend in the Western world [9].
Smoke emanating from WPS includes toxins resembling those found in cigarettes, such as carbon monoxide (CO), hydrocarbons, and carcinogenic polycyclic aromatic volatile aldehydes [10,11]. In comparison to a cigarette, which accounts for 500–600 mL of smoke inhalation per unit, a single WPS session accounts for approximately 90,000 mL of smoke inhalation; making WPS 4-times higher in CO exposure and 56-times higher in inhaled smoke volume [11]. Additionally, it has been pointed out that nicotine concentration in plasma of individuals smoking one WPS daily is similar to those smoking 10 cigarettes a day [12,13]. Out of 300 chemical compounds, which have been identified in inhaled WPS smoke, 82 have been labelled as “toxicants” [14,15] including polyaromatic hydrocarbons, heterocyclic compounds, carbonylic compounds and volatile organic compounds, tar, nicotine, carbon monoxide, nitrosamines, heavy metals, metal nanoparticles, phenolic compounds, flavoring chemicals (base propylene glycol, glycerol, vanillin, cinnamaldehyde), and free radicals which can induce head and neck (HN) as well as pulmonary toxicity [15,16]. Nevertheless, popular belief considers WPS less harmful than cigarette smoking. However, research shows that both methods of tobacco consumption lead to serious health problems including a variety of oral and systemic diseases, such as periodontal affliction, cardiovascular, and pulmonary disorders [17,18,19,20,21]. On the other hand, we have previously reported that WPS can exhibit a substantial embryotoxicity on the early stage of the normal development [22].
To date, various studies have confirmed the association between WPS and several types of human cancers, including lung, esophageal, oral, and pancreatic carcinomas [23,24,25]. Chronic human exposure to WPS smoke alters the expression of genes involved in detoxification, xenobiotic metabolism, as well as DNA stability and repair processes, hence, increasing a susceptibility to various cancers [23,26]. We also recently demonstrated that WPS exposure can induce epithelial-mesenchymal transition (EMT) and enhance a cell invasion ability of human breast cancer cells via the activation of Erk1/Erk2 pathways [27]. However, the exact role of WPS exposure on human cancer initiation, including HN, is still unclear. Therefore, in the current study, we aimed to explore the role of chronic exposure to WPS on molecular pathways and gene targets in human normal oral epithelial cells, which can increase their susceptibility to cancer.
2. Materials and Methods
2.1. Smoking Machine Protocol and WPS Preparation
A standard smoking protocol (Aleppo Method) was used as described previously by our group [22,27]. The water pipe was prepared by padding the head with 10 gr of brand tobacco mixture known as “Two Apples”, covering it with aluminum foil and perforating the foil to allow air passage. A charcoal, “Tree Kings” brand, quick-light briquette was ignited and placed on top of the head at the beginning of the smoking session. The condensate (smoking) was collected using regular laboratory filter paper. Filters were dried and weighed before and after collecting smoke. Subsequently, smoked filters were solved in phosphate buffer saline (PBS) or keratinocyte serum-free media (KSFM) (Life Technologies, Burlington, ON, Canada) with final concentration of 20 mg/mL of smoking particles; followed by filtering PBS or KSFM solutions using 0.45μm filters (Costar, Washington, DC, USA).
2.2. Cell Lines
Two human normal oral epithelial (NOE) cell lines established in our laboratory [28] were used and maintained at 37 °C in a humidified atmosphere of 5% CO2 in air. The cells were cultured in KSFM with 5 mg/100 mL of bovine pituitary extract (BPE) (Life Technologies, Burlington, ON, Canada), and 100 µg/mL penicillin–streptomycin. Cells were treated either with 100 μg/mL of WPS in PBS or KSFM solution for 48 h.
2.3. RNA Extraction and Reverse Transcriptase Real-Time PCR
Total RNA was extracted from cells using RNeasy Mini Kit spin columns (Qiagen). First strand cDNA was synthesized using 5X All-In-On MasterMix (MasterMix-LR, Diamed) per manufacturer’s protocol. Reverse transcriptase real-time polymerase chain reaction (RT-PCR) was carried out on 96-well plates using iTaq Universal SYBR Green Supermix (BioRad). Concentrations for each sample were measured using the NanoDrop ND-100 spectrophotometer 119 (NanoDrop Technologies, Wilmington, DE, USA) and Qubit (Thermo Fischer Scientific, 120 Waltham, MA, USA). The primer sequences were designed using Primer ExpressTM Software v3.0.1 (ThermoFisher Scientific, Franklin, MA, USA) (Table 1).

Table 1.
List of primers sequences used for reverse transcriptase real-time PCR.
2.4. NanoString
Gene expression was assessed using the NanoString PanCancer Pathway Panel (NanoString Technologies, Seattle, WA, USA) consisting of probes for 770 genes implicated in carcinogenic pathways, curated from The Cancer Genome Atlas (TCGA) data. All RCC files (direct outputs/raw data from NanoString runs) were normalized using nSolver analysis software (NanoString Technologies, Seattle, WA, USA) according to the manufacturer’s protocols (nSolver User Manual). In brief, a normalization factor was calculated by obtaining the geometric mean of the controls used for each sample and applied to the raw counts of the nCounter output data to eliminate variability that was unrelated to the samples. The resulting data were normalized again with the geometric mean of the housekeeping genes. Normalized data were log2-transformed and exported to Microsoft Excel for analysis.
2.5. Gene Profile and In Silica Analyses
The in silico approach used in our study helped us to support and confirm our findings (the differentially expressed genes (DEGs) were discovered by NanoString analysis as previously described). The large, publicly available database Oncomine TM consists of approximately 65 gene expression datasets and was used to explore the differential expression of our genes to compare HN cancer with respective normal tissues as well as clinico-pathological parameters. From this database we used Toruner, Ginos, Cromer, Ye, Peng, Sengupta, Estilo, Kuriakose, and TCGA datasets to evaluate mRNA expression of the discovered DEGs in normal versus malignant patient samples. In addition, TCGA Head and Neck dataset (270 patients) was used to evaluate the differences in the DNA copy numbers between smoker head and neck cancer patients compared with non-smokers with head and neck cancer. In brief, the parameters were set and the program generated the expression levels per dataset; analysis was performed, and we finally selected genes that were statistically relevant to our study. Moreover, we used a cohort of 500 HN squamous cell carcinomas (HNSCC) samples from the Pan-cancer RNA-seq dataset of the Kaplan–Meier plotter database to analyze the patients’ clinical outcome.
2.6. Network and Pathway Interaction
The Search Tool for the Retrieval of Interacting Genes (STRING v9.1) (https://string-db.org/) tool was used to investigate the network and interaction between the different WPS deregulated genes as well as biological function. This is a biological database and web resource of known and predicted protein–protein interactions. Briefly, we uploaded the obtained gene list and the software imported protein association knowledge from databases of physical interaction and databases of curated biological pathway knowledge; the program utilizes computational predictions to generate maps and connections between different proteins. We used this tool to highlight the importance of the potential connectivity network of our genes that need to be considered for the full understanding of the biological phenomena.
2.7. Statistical Analysis
In vitro assays were all performed in triplicates of at least three independent experiments. Results were shown as means ± SEM. Student’s t-test was used to evaluate the statistical significance. Statistical analyses were performed using nSolver and GraphPad Prism (version 8.4.3) analysis software. Overall survival and relapse-free survival (RFS) were performed using the Kaplan–Meier survival analysis and a p-value < 0.05 was considered significant (log-rank test).
3. Results
3.1. Identification of Differentially Expressed Genes (DEGs) Deregulated by WPS in HNOE Cells
In order to study the effect of WPS on human HN carcinogenesis, we examined the outcome of WPS on two human normal oral epithelial, 2N and 11N, which were established in our lab [28]. Our data revealed that treatment of 2N and 11N cell line with 100 μg/mL of WPS solution for 2 days slightly deregulates cell proliferation and cell cycle progression of both cell lines in comparison with untreated cells (data not shown). On the other hand, we found that WPS exposure induces EMT, where both cell lines display a more mesenchymal phenotype in comparison with their matched unexposed controls (Figure 1). The cells become more elongated in appearance and show a decrease in cell–cell contact compared with untreated ones (Figure 1).
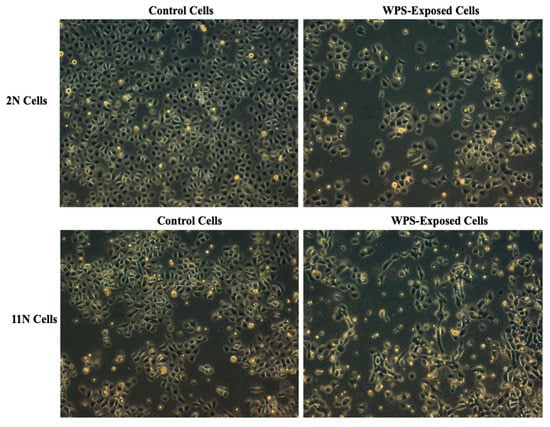
Figure 1.
Water-pipe smoking (WPS) stimulates epithelial–mesenchymal transition (EMT) of human normal oral epithelial (HNOE) cell lines, 2N and 11N. We note that treatment for 2 days with 100 μg/mL of WPS solution induces morphological changes from epithelial (control) into a “fibroblast-like” (mesenchymal) phenotype, which is known as EMT.
Similar to our study, we previously examined the expression of E-cadherin and focal adhesion kinase (FAK) proteins in cancer cells exposed to WPS [27]. Our data showed loss of E-cadherin and enhanced expression of FAK proteins in WPS-exposed cells in comparison to unexposed ones; thus, indicating WPS promotes EMT progression and enhances cell migration as well as invasion abilities [27]. Furthermore, analysis of the underlying mechanisms revealed that the expression of phosphorylated Erk1/2 was upregulated in WPS-exposed cells, thus implying that WPS promotes EMT via Erk1/2 pathways [27,29,30].
Next, gene expression was applied on both cell lines, using the NanoString PanCancer Pathway Panel consisting of probes for 770 genes implicated in carcinogenic pathways; our data showed that out of these genes, 23 were found to be differentially expressed in WPS-exposed versus unexposed 2N and 11N cells: CCL5, C1R, MMP9, IL-1B, CCL4, MASP2, OXER1, TLR3, STAT1, PPP1R12B, MX1, MX2, CCL21, IL-3, TLR9, HSH2D, CCR4, IFNγ, LT-β, IFIT1, TGF-β2, ALOX5, and LIMK1 (p < 0.05).
Following the identification of candidate genes, we validated our panel using qRT-PCR analysis. The set of genes differentially expressed corresponded with the NanoString analysis with twenty-three (IL-IB, CCL5, C1R, MMP9, LIMK1, CCR4, MASP2, OXER1, TLR3, STAT1, PPP1R12B, MX2, CCL21, IL-3, TLR9, HSH2D, CCL4, IFNγ, LT-β, IFIT1, TGF-β2, ALOX5 and MX1) upregulated genes by a factor ranging from 1.58 to 3.8 folds (p < 0.05) (Figure 2).
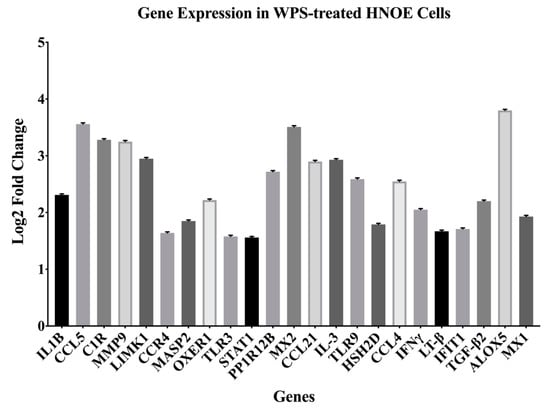
Figure 2.
Differentially expressed genes (DEGs) discovered using the NanoString PanCancer Pathway Panel. Cut-offs used were 1.5-fold change or high between the different groups and adjusted for p-value < 0.05.
Based on the molecular pathways of carcinogenesis, these 23 deregulated genes are directly involved in the modulation of cell cycle, proliferation, migration, invasion, apoptosis, angiogenesis, signal transduction, and inflammatory response (Table 2).

Table 2.
Genes based on their functional annotations.
3.2. Deregulated Genes Are Upregulated in HN Cancer Samples Compared with Normal Tissue
Using the Oncomine database, we herein initially evaluated the mRNA expression levels of the DEGs discovered in normal tissue versus head and neck tumor samples.
Using the Toruner dataset (20 patient samples), we found that the expression of IFIT1, (p = 2.76 × 10−8) and ALOX5 (p = 0.0019) genes were high in oral squamous cell carcinoma (OSCC) compared to normal squamous cells (Figure S1A). The Ginos dataset (54 patient samples) showed that IL-1B, (p = 1.77 × 10−8), STAT1 (p = 9.25 × 10−11), MX2 (p = 0.22 × 10−8), LT-β (p = 2.43 × 10−6), C1R (p = 0.005), CCL5 (p = 2.28 × 10−9), MMP9 (p = 7.07 × 10−26), ALOX5 (p = 0.050) and CCL4 (p = 3.20 × 10−15) genes were upregulated in HNSCC compared with the normal buccal mucosa (Figure S1B). Moreover, Cromer dataset (38 patient samples) reveled that IL-1B (p = 0.002), STAT1 (p = 0.001) and IFNγ (p = 3.46 × 10−4) genes were high in HNSCC compared with normal uvula tissue (Figure S1C). The dataset (38 patient samples) exhibited IL-1B (p = 2.67 × 10−6), TGF-β2 (p = 2.23 × 10−4), and MMP9 (p = 0.01) genes to be upregulated in tongue squamous cell carcinoma (SCC) compared with normal tongue tissue (Figure S1D). In addition, the Peng dataset (79 patient samples) found IL-1B (p = 4.52 × 10−10), IFIT1 (p = 1.27 × 10−17), TGF-β2 (p = 2.23 × 10−4), STAT1 (p = 7.18 × 10−20), MX2 (p = 5.11 × 10−11), OXER1 (122 patient samples, p = 0.045), LT-β (p = 0.004), CCL21 (122 patients, p = 0.005), C1R (p = 0.005), CCR4 (p = 2.50 × 10−5), HSH2D (p = 2.47 × 10−7), MASP2 (p = 5.16 × 10−5), PPP1R12B (p = 4.10 × 10−4), CCL5 (122 patients, p = 3.33 × 10−12), MX1 (p = 1.70 × 10−9), and IFNγ (p = 6.82 × 10−13) genes to be over expressed in OSCC compared with normal oral cavity (Figure S1E). Sengupta dataset (41 patient samples) evaluated that expression of IFIT1 (p = 5.38 × 10−5), CCR4 (p = 0.046), and CCL4 (p = 2.79 × 10−8) genes were increased in nasopharyngeal carcinoma compared with normal nasopharynx (Figure S1F). Estilo dataset (58 patients) showed STAT1 (p = 1.07 × 10−12), MX2 (p = 4.53 × 10−5), and MX1 (p = 1.31 × 10−10) genes to be highly expressed in tongue SCC compared with normal tongue tissue (Figure S1G). While IL-3 expression in Kuriakose dataset (20 patient samples) was predominant in lip and OSCC (20 patients, p = 0.009) (Figure S1H). Additionally, OXER1 (p = 1.38 × 10−9) expression using TCGA dataset is highly upregulated in HNSCC (364 patient samples) (Figure S1I).
3.3. Deregulated Genes Are Upregulated in Smoking HN Cancer Patients Compared to Non-Smoker Patients
Next, and to further investigate the association between our discovered DEGs and smoking as a risk factor of cancer, we investigated the DNA copy numbers of the 22 upregulated DEGs in HN cancer samples in smoker versus non-smoker HN cancer patients. For this analysis, we used TCGA HN dataset (270 patients) of the Oncomine database. Interestingly, our results confirmed that, of the 22 genes, 16 were upregulated in smoking HN cancer patients compared to those who had never smoked. These genes include CCL5, C1R, MMP9, IL-1B, CCL4, OXER1, TLR3, STAT1, PPP1R12B, MX1, MX2, HSH2D, IFNγ, LT-β, IFIT1, and TGF-β2 (p ≤ 0.05) (Figure S2).
3.4. Deregulated Genes by WPS Have a Direct Impact on HN Cancer Patient’s Prognosis
Subsequently, we explored whether the DEGs induced by WPS in oral epithelial cells could have an impact on the prognosis of HN cancer patients. To asses this point, we analyzed the association between the DEGs mRNA expression and patient’s outcome, relapse-free survival (RFS), or overall survival (OS), using a large HNSCC cohort (n = 500 patients) from the Kaplan–Meier plotter database.
Interestingly, while high expression of ALOX5 (p = 0.0091), IFNγ (p = 0.054), C1R (p = 0.0028), CCL4 (p = 0.001), MASP2 (p = 0.041), PPP1R12B (p = 0.031), TGF-β2 (p = 0.014), and CCL21 (p = 0.0062) correlates positively with poor RFS (Figure 3A), expression of TLR9 (p = 0.027), IFIT1 (p = 0.021) and IL-3 (p = 0.0031) correlates positively with poor OS (Figure 3B).
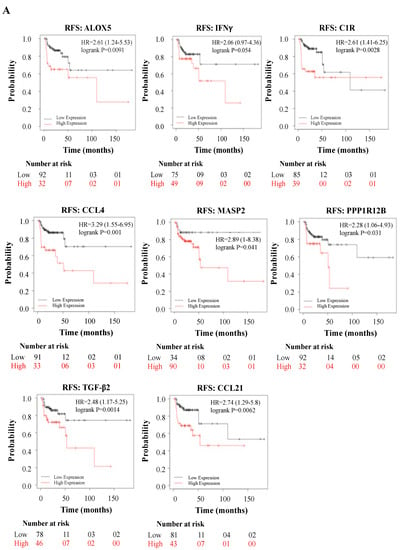
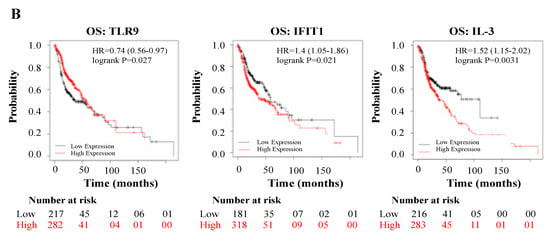
Figure 3.
(A,B): Relapse-free survival (RFS) in HN cancer patients using the Kaplan–Meier plotter database, expressed by relapse-free survival (RFS) and overall survival (OS).
3.5. WPS Deregulated Genes Are Mainly Involved in Immune Response and Cytokine/Chemokine Mediated Pathways
We investigated major gene interactions between top DEGs and possible pathway enrichment. Interestingly, our results showed a strong interaction with major biological processes including immune response, cytokine-mediated signaling pathway, and cellular response to cytokine stimulus. Moreover, the molecular functions shared between the top DEGs were also found to be related to cytokine and Cysteine-Cysteine Chemokine Receptor (CCR) chemokine binding receptors (Figure 4), (Table 3).
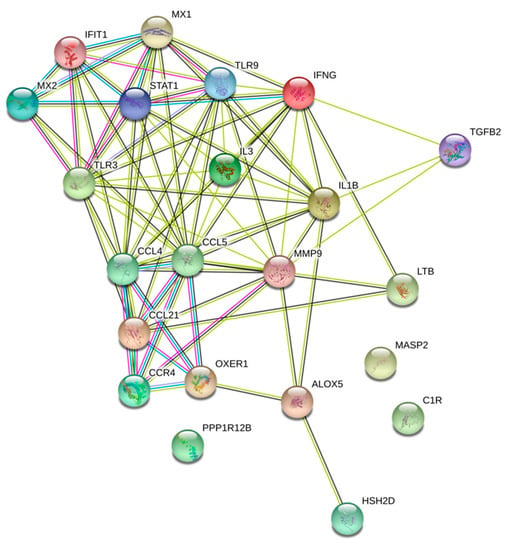
Figure 4.
Protein interaction analysis of the WPS upregulated and differentially expressed genes using the Search Tool for the Retrieval of Interacting Genes (STRING v9.1). Enriched biological process and molecular functions of those proteins are included.

Table 3.
Functional annotations of the differentially expressed genes.
4. Discussion
This investigation, to the best of our knowledge, is the first cancer genes profiling study on the effects of WPS exposure on human normal oral epithelial cells. Previously, our group has revealed that WPS can play an important role in the initiation and progression of human oral cancer, which represents the majority of HN cancer cases [24]. In this study, we found that WPS can induce EMT, which is the hallmark of cancer progression in human normal oral epithelial cells by loss of E-cadherin and upregulation of FAK protein as well as Erk1/2 pathways as previously demonstrated in our study [27,29,30]. More importantly, we used a NanoString nCounter PanCancer Pathways panel of 770 gene transcripts distributed in 13 biological pathways to determine gene targets of WPS exposure in human normal oral epithelial (HNOE) cells. Thus, we identified significant changes in the expression of 23 genes, with one gene being down-regulated and twenty-two being upregulated. In our investigation, we confirmed, both by qRT-PCR as well as the Oncomine TM database, the deregulation of the newly identified genes as targets for WPS exposure in oral cells. We also analyzed the prognostic effect of the WPS-induced deregulated genes on HNSCC survival and prognosis using Pan-cancer RNA-seq dataset of the Kaplan–Meier plotter database. More significantly, our study points out that these genes are discovered for the first time as targets of WPS exposure in human normal oral epithelial cells. The uncovered genes encode for proteins that are known for regulating cell cycle, proliferation, migration, invasion, apoptosis, angiogenesis, signal transduction, and inflammatory response. Therefore, the newly identified genes can plausibly play a role in the neoplastic transformation of normal oral epithelial cells and consequently HN cancer initiation in general.
Of the nine differentially expressed genes, four (CCL5, CCL21, CCL4, and CCR4) belong to the family of chemokines. Elevated CCL5 levels are significantly associated with oral cancer progression [31], relapse, and/or metastasis [32,33], as well as drug resistance [34], indicating its fundamental role in oral carcinogenesis [35]. Our results are concordant, suggesting CCL5 association with oral cancer progression upon WPS intake. Moreover, CCL5 is capable of upregulating the release of MMP-9 [36], a matrix-metalloproteinase that was also identified in our study. CCL5 enhances oral cancer cell migration through the increase in MMP-9 production [31]. Earlier studies have shown that overexpression of MMP9 is observed in oral cancer [37] and is associated with a poor disease-free survival (DFS) [38]. Similar data were observed in this study. Interestingly, ALOX5 acts as a mediator of invasion via MMP-9 induction [39]. ALOX5 expression is known to be involved in carcinogenesis [39] and is also involved in chronic obstructive pulmonary disease (COPD) [40]. Additionally, ALOX5 genotype was found to be linked with asthma and poorer lung function [41], while its expression in mice models showed increase in inflammation, oxidative stress as well as emphysema caused by cigarette smoke [42], indicating its possible involvement in oral cancer upon exposure to smoke from WPS. Although a previous study has shown an association between ALOX5 with poor asthma controls [41], there are no studies indicating association of ALOX5 with RFS; we herein show for the first time that ALOX5 is associated with shorter RFS.
Similar to CCL5, CCL4 has analogous role in cancer progression; CCL4 enhances susceptibility to oral cancer [43]. It has been shown that CCL4 stimulates VEGF-C expression by activating the JAK2/STAT3 signaling pathway, which is frequently linked with oral cancer cell proliferation, invasion, and angiogenesis [44]. A recent investigation showed that smoking along with CCL4 gene polymorphisms can increase risk of oral cancer [43]. Likewise, we found that WPS smoking can augment CCL4 expression resulting in enhanced inflammatory response, thus promoting tumor development and progression. Similar to our data, CCL4 expression is linked with poor prognosis in cancer [45]. On the other hand, cigarette smoking has been shown to increase blood and bronchoalveolar lavage fluid levels of the CCR7 ligands CCL19 and CCL21 [46], thereby contributing to migration of lung cancer cells [47]. Additionally, previous research has considered CCL21 in oral cancer as a candidate marker for unfavorable outcome [48]. In this study, we confirm that CCL21 is a target of WPS exposure in oral epithelial cells, implying its possible role in cell transformation and therefore HN carcinogenesis. Concordant to our data, recently it has been pointed out that CCL21/CCR7 is linked with cancer recurrence, smoking, and poor prognosis in HN cancer [49,50]. In this regard, chemokine receptors are G protein-coupled receptors and are involved in the onset and progression of several solid tumors [51,52,53]. Concordant to our data, several investigations reported CCR4 to play a role in lymph node metastasis of HNSCC as well as its progression and recurrence [50,54]. Moreover, we also identified upregulated expression of G protein coupled OXER1 in HNSCC. OXER1 has been reported to be upregulated in both prostate cancer cells as well as tumor tissues [55]. However, although, no direct role of OXER1 has been studied in HNSCC, upregulation of OXER1 in human papillomavirus (HPV)-positive tumors has been previously reported [56]. Since HPV is found to play a role in the onset and progression of HN as well as oral cancers [28,57,58], we suggest a link between OXER1 expression and HN as well as oral cancers.
Moreover, in this study, of the 23 genes, 4 (TLR3, TLR9, C1R and MASP-2) of them are involved in innate immune system. We reveal that TLR3 and TLR9 are upregulated in WPS-exposed oral epithelial cells. It has been demonstrated that HN cancer cell lines as well as OSCC tissue samples express TLR3 thereby enhancing the expression levels NF-κB and its regulated oncogene, c-myc, thus inciting cellular proliferation and migration, which is significantly associated with poorly differentiated tumor cells and perineural invasion [59,60,61,62]. Similar to data obtained in this study, upregulated expression of TLR9 in HNSCC as well OSCC was found to promote tumor cell invasion, proliferation as well as migration by enhancing MMP-2 expression [63,64,65,66]. Although C1R is known to regulate the complement pathway of the innate immune system, in this study, we found upregulated CIR expression in HNSCC. This is in concordance with a previous study that correlated the expression of C1R in cutaneous SCC (cSCC) with tumor progression, cell proliferation, and migration [67,68]. Since cSCC lesions frequently develop in the HN region [69], our data correlate with this finding. Although, the prognostic relevance of C1R has not been studied in cancer; however, it is associated with tumor progression and migration [67,68], hence our data suggest a significant correlation between C1R and shorter RFS. Furthermore, our data implicate the other member of the complement pathway, MASP-2 gene, which is upregulated in oral epithelial cells exposed to WPS. MASP-2 produced in hepatocytes is involved in innate response, and its promoter is regulated by cytokines (interleukins and TGF-β) or transcription factor (STAT) [70]; our study found both cytokines and STAT to be expressed in WPS exposed HNOE cells. Previous studies have found an association between MASP-2 expression and cancer [71,72,73]; MASP-2 expression significantly correlates with late clinical stage and nodal metastasis, thus indicating its role in cancer progression and aggressive tumor behavior in esophageal SCC [71]. Moreover, MASP-2 is significantly associated with recurrence and poor survival of colorectal as well as ovarian cancers [72,73], thus suggesting a link with poor RFS, similar to data found in this study.
The pro-inflammatory cytokine, IL-1B, is elevated in HNSCC including oral cancer [74,75]. Furthermore, similar to our data, an earlier study demonstrated the upregulation of IL-1B in tobacco and betel quid-mediated OSCC; IL-1B promotes proliferation of dysplasia of oral cells, thus triggering oncogenic cytokines as promoters of tumor aggressiveness [76]. On the other hand, cytokine IL-3, is a selective growth factor that stimulates tumor angiogenesis [77]. However, although the role of IL-3 in COPD as well as cigarette smoking is not well defined, IL-3 levels were previously detected in SCC [78]. We herein show presence of IL-3 in oral epithelial cells under the effect of WPS, thus indicating its role in oral cancer progression. Although data have shown strong correlation between IL-3 and poor survival in acute myeloid leukemia [79], research on the role of IL-3 in OSCC is scarce. Interestingly, a recent study by Almeida et al. (2019) showed an association between IL-4, IL-6, IL-8, IL-10, IL-12, and IL-13 and poor survival in OSCC [80]. In this study, we found IL-3 to significantly correlate with poor overall survival, which requires further investigation. On the other hand, transforming growth factor (TGF), a cytokine, is involved in promoting cellular invasion as well as angiogenesis in OSCC cells [81,82]. Our data revealed the presence of TGF-β2 in WPS-exposed oral epithelial cells as well as in HNSCC; previous studies have indicated the presence of TGF-β2 in cancer associated fibroblasts from OSCC [83] as well as in SCC cell lines [84]. Similar to our data, elevated TGF expression was significantly associated with shorter OS, RFS, and DFS in patients with OSCC [85]. The other type of cytokine identified in this study is IFNγ. In oral epithelial cells, a loss of IFNγ expression may be caused by the IFN-γ promoter methylation as a plausible underlying mechanism for oral cancer progression [86]. Furthermore, and in concordance with our data, low IFNγ levels are associated with poor prognosis in HNSCC including oral [87,88]. The other cytokine, LT-β, was also identified in this study; LT-β has been shown to correlate with human oral cancer [89], and additionally, it activates the NIK-IKKa-RELB/NF-κB2 pathway to stimulate HNSCC cell migration [90,91]. While STAT1 has a dual role in HNSCC [92,93,94], we report upregulation of STAT1 in OSCC as well as HNSCC, as reported previously [92], suggesting an oncogenic role of STAT1 in the pathogenesis of HNSCC.
On the other hand, we report the upregulation of an interferon-stimulated gene, IFIT-1, in WPS-exposed HNOE cells. In this context, a previous study showed that over-expression of IFIT1 in OSCC cells promote tumor growth and metastasis by activating EGFR signaling [95]. Research in OSCC has shown a distinct correlation between elevated IFIT1 expression with T-stage, lymph node metastasis, lymphovascular, perineural invasion, as well as poor overall survival in OSCC patients [95]. Moreover, increased IFIT1 expression in OSCC cells enhance resistance to several therapeutic agents (5-FU, carboplatin, cisplatin, ganetespib, and oxaliplatin) [96,97], thus indicating its role in poor RFS, which is similar to data obtained in our study. We identified the interferon-related gene, MXI along with its paralogue MX2. MX1 has a contradictory role in cancer; in one study, MX1 is upregulated in OSCC [98], but nevertheless, it is hyper methylated in HN cancer [99]. However, in our study, we found that exposure to WPS smoke in oral epithelial cells induced expression of MX1, indicating its possible oncogenic role in oral cancer.
Interestingly, we discovered two genes (HSH2D and PPP1R12B) in our cohort that were not previously reported, as possible players in OSCC or HNSCC. Traditionally, HSH2D has a role in T-cell activation and is a downstream target of CD28 costimulatory signaling pathway [100]. A previous study has reported loss of CD28 on T-cell in HNSCC [101]; thus, we postulate a role of HSH2D activation in HNSCC. Moreover, PPP1R12B, also known as MYPT2, is a subunit of MYPT [102]. Although no direct role of MYPT2 is implicated in cancer, MYPT is found to be involved in cancer [103,104], indicating a plausible role for MYPT2 in HNSCC. We found PPP1R12B to be associated with poor RFS. However, studies have shown MYPT to correlate significantly with drug resistance and poor prognosis in human carcinomas [103,104], thus postulating a plausible role of PPP1R12B in drug resistance and poor prognosis in OSCC or HNSCC.
An earlier study by our group show clearly that WPS stimulate cell invasion of human breast cancer cells [27]. While several investigations have shown a significant correlation between smoking and the onset/progression of oral cancer [27,105,106,107,108]. In addition, it has been revealed that cigarette smoking can enhance EMT of several human carcinoma cells [29,109,110,111,112]. Thus, it is apparent that smoking is an important etiological factor in the onset of numerous human cancers inducing lung, HN (especially oral) as well as breast [27,105,113,114,115]. Nevertheless, based on the number and level of toxicants and the duration of smoking session, it can be assumed that WPS is more harmful with regards to the development and progression of human cancers as well as cancer-related deaths in comparison with cigarette smoking.
5. Conclusions
We reveal for the first time, that WPS can induce EMT in human normal oral epithelial cells, which is accompanied by the deregulation of a set of genes related to oncogenesis. Thus, WPS can promote HN cancer initiation and/or progression mainly due to its effect on key regulatory genes of carcinogenesis that have a direct impact on HN cancer patients’ outcome. Nevertheless, further studies are needed to elucidate the expression of different proteins involved in EMT as well as to understand the full mechanism by which WPS can induce HN carcinogenesis.
Supplementary Materials
The following are available online at https://www.mdpi.com/2305-6304/8/3/73/s1, Figure S1: mRNA expression levels of the differentially expressed genes (DEGs) in normal tissue compared with head and neck cancer, using (A) Toruner, (B) Ginos, (C) Cromer, (D) Ye, (E) Peng, (F) Sengupta, (G) Estilo, (H) Kuriakose, and (I) TCGA datasets. Figure S2: DNA copy number of the top WPS differentially expressed genes (DEGs) in smoker versus never smoker HN cancer patients using TCGA HN dataset (270 patients) using the Oncomine database.
Author Contributions
Conceptualization, H.F.A.F. and A.-E.A.M.; methodology, V.M.L.-O., R.L.C.K., A.Y. and H.K.; software, V.M.L.-O.; validation, V.M.L.-O., R.L.C.K.; formal analysis, V.M.L.-O. and E.M.; investigation, V.M.L.-O., R.L.C.K; resources, A.E.A. and E.M.; data curation, V.L.O. and I.G.; writing—original draft preparation, V.M.L.-O. and I.G.; writing—review and editing, I.G., S.V. and H.F.A.F.; supervision, A.-E.A.M. and S.V.; funding acquisition, A.-E.A.M., S.V., H.F.A.F. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by Qatar University, grant numbers: QUCP-CMED-2019-1, QUCG-CMED-20/21-2 & QUHI-CMED-19/20-1.
Acknowledgments
We would like to thank A. Kassab for her critical reading of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- WHO. WHO Global Report on Trends in Prevalence of Tobacco Smoking 2000–2025, 2nd ed.; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Onor, I.O.; Stirling, D.L.; Williams, S.R.; Bediako, D.; Borghol, A.; Harris, M.B.; Darensburg, T.B.; Clay, S.D.; Okpechi, S.C.; Sarpong, D.F. Clinical Effects of Cigarette Smoking: Epidemiologic Impact and Review of Pharmacotherapy Options. Int. J. Environ. Res. Public Health 2017, 14, 1147. [Google Scholar] [CrossRef] [PubMed]
- Maziak, W.; Taleb, Z.B.; Bahelah, R.; Islam, F.; Jaber, R.; Auf, R.; Salloum, R.G. The global epidemiology of waterpipe smoking. Tob. Control. 2015, 24 (Suppl. S1), i3–i12. [Google Scholar] [CrossRef]
- Jawad, M.; McEwen, A.; McNeill, A.; Shahab, L. To what extent should waterpipe tobacco smoking become a public health priority? Addiction 2013, 108, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Glantz, S.A.; Bareham, D.W. E-Cigarettes: Use, Effects on Smoking, Risks, and Policy Implications. Annu. Rev. Public Health 2018, 39, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, R.M.; Chehne, F.; Oguogho, A.; Sinzinger, H. Narghile (water pipe) smoking influences platelet function and (iso-)eicosanoids. Life Sci. 2003, 74, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Neergaard, J.; Singh, P.; Job, J.; Montgomery, S. Waterpipe smoking and nicotine exposure: A review of the current evidence. Nicotine Tob. Res. 2007, 9, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Maziak, W.; Nakkash, R.; Bahelah, R.; Husseini, A.; Fanous, N.; Eissenberg, T. Tobacco in the Arab world: Old and new epidemics amidst policy paralysis. Health Policy Plan. 2014, 29, 784–794. [Google Scholar] [CrossRef]
- Akl, E.A.; Gunukula, S.K.; Aleem, S.; Obeid, R.; Jaoude, P.A.; Honeine, R.; Irani, J. The prevalence of waterpipe tobacco smoking among the general and specific populations: A systematic review. BMC Public Health 2011, 11, 244. [Google Scholar] [CrossRef]
- Eissenberg, T.; Shihadeh, A. Waterpipe tobacco and cigarette smoking: Direct comparison of toxicant exposure. Am. J. Prev. Med. 2009, 37, 518–523. [Google Scholar] [CrossRef]
- Cobb, C.O.; Shihadeh, A.; Weaver, M.F.; Eissenberg, T. Waterpipe tobacco smoking and cigarette smoking: A direct comparison of toxicant exposure and subjective effects. Nicotine Tob. Res. 2011, 13, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Maziak, W.; Ward, K.D.; Eissenberg, T. Factors related to frequency of narghile (waterpipe) use: The first insights on tobacco dependence in narghile users. Drug Alcohol Depend. 2004, 76, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Rastam, S.; Eissenberg, T.; Ibrahim, I.; Ward, K.D.; Khalil, R.; Maziak, W. Comparative analysis of waterpipe and cigarette suppression of abstinence and craving symptoms. Addict. Behav. 2011, 36, 555–559. [Google Scholar] [CrossRef]
- Shihadeh, A.; Saleh, R. Polycyclic aromatic hydrocarbons, carbon monoxide, "tar", and nicotine in the mainstream smoke aerosol of the narghile water pipe. Food Chem. Toxicol 2005, 43, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Shihadeh, A.; Schubert, J.; Klaiany, J.; El Sabban, M.; Luch, A.; Saliba, N.A. Toxicant content, physical properties and biological activity of waterpipe tobacco smoke and its tobacco-free alternatives. Tob. Control. 2015, 24 (Suppl. S1), i22–i30. [Google Scholar] [CrossRef]
- Shihadeh, A.; Salman, R.; Jaroudi, E.; Saliba, N.; Sepetdjian, E.; Blank, M.D.; Cobb, C.O.; Eissenberg, T. Does switching to a tobacco-free waterpipe product reduce toxicant intake? A crossover study comparing CO, NO, PAH, volatile aldehydes, "tar" and nicotine yields. Food Chem. Toxicol. 2012, 50, 1494–1498. [Google Scholar] [CrossRef]
- Joseph, S.; Pascale, S.; Georges, K.; Mirna, W. Cigarette and waterpipe smoking decrease respiratory quality of life in adults: Results from a national cross-sectional study. Pulm. Med. 2012, 2012, 868294. [Google Scholar] [CrossRef] [PubMed]
- Radwan, G.; Hecht, S.S.; Carmella, S.G.; Loffredo, C.A. Tobacco-specific nitrosamine exposures in smokers and nonsmokers exposed to cigarette or waterpipe tobacco smoke. Nicotine Tob. Res. 2013, 15, 130–138. [Google Scholar] [CrossRef]
- Ali, M.; Jawad, M. Health Effects of Waterpipe Tobacco Use: Getting the Public Health Message Just Right. Tob. Use Insights 2017, 10, 1179173X17696055. [Google Scholar] [CrossRef]
- Layoun, N.; Saleh, N.; Barbour, B.; Awada, S.; Rachidi, S.; Al-Hajje, A.; Bawab, W.; Waked, M.; Salameh, P. Waterpipe effects on pulmonary function and cardiovascular indices: A comparison to cigarette smoking in real life situation. Inhal. Toxicol. 2014, 26, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Al-Kheraif, A.A.; Rahman, I.; Millan-Luongo, L.T.; Feng, C.; Yunker, M.; Malmstrom, H.; Romanos, G.E. Comparison of Clinical and Radiographic Periodontal Status Between Habitual Water-Pipe Smokers and Cigarette Smokers. J. Periodontol. 2016, 87, 142–147. [Google Scholar] [CrossRef]
- Ashour, A.A.; Haik, M.Y.; Sadek, K.W.; Yalcin, H.C.; Bitharas, J.; Aboulkassim, T.; Batist, G.; Yasmeen, A.; Al Moustafa, A.E. Substantial Toxic Effect of Water-Pipe Smoking on the Early Stage of Embryonic Development. Nicotine Tob. Res. 2018, 20, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, Z.; Nyiraneza, C.; El-Katerji, H.; Little, J. Waterpipe smoking and cancer: Systematic review and meta-analysis. Tob. Control. 2017, 26, 92–97. [Google Scholar] [CrossRef]
- Rastam, S.; Li, F.M.; Fouad, F.M.; Al Kamal, H.M.; Akil, N.; Al Moustafa, A.E. Water pipe smoking and human oral cancers. Med. Hypotheses 2010, 74, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Fouad, H.; Awa, F.E.; Naga, R.A.; Emam, A.H.; Labib, S.; Palipudi, K.M.; Andes, L.J.; Asma, S.; Talley, B. Prevalence of tobacco use among adults in Egypt, 2009. Glob. Health Promot. 2016, 23, 38–47. [Google Scholar] [CrossRef]
- Waziry, R.; Jawad, M.; Ballout, R.A.; Al Akel, M.; Akl, E.A. The effects of waterpipe tobacco smoking on health outcomes: An updated systematic review and meta-analysis. Int. J. Epidemiol 2017, 46, 32–43. [Google Scholar] [CrossRef]
- Sadek, K.W.; Haik, M.Y.; Ashour, A.A.; Baloch, T.; Aboulkassim, T.; Yasmeen, A.; Vranic, S.; Zeidan, A.; Al Moustafa, A.-E. Water-pipe smoking promotes epithelial-mesenchymal transition and invasion of human breast cancer cells via ERK1/ERK2 pathways. Cancer Cell Int. 2018, 18, 180. [Google Scholar] [CrossRef] [PubMed]
- Al Moustafa, A.-E.; Foulkes, W.D.; Benlimame, N.; Wong, A.; Yen, L.; Bergeron, J.; Batist, G.; Alpert, L.; Alaoui-Jamali, M.A. E6/E7 proteins of HPV type 16 and ErbB-2 cooperate to induce neoplastic transformation of primary normal oral epithelial cells. Oncogene 2004, 23, 350–358. [Google Scholar] [CrossRef]
- Sun, X.; Deng, Q.; Liang, Z.; Liu, Z.; Geng, H.; Zhao, L.; Zhou, Q.; Liu, J.; Ma, J.; Wang, D.; et al. Cigarette smoke extract induces epithelial-mesenchymal transition of human bladder cancer T24 cells through activation of ERK1/2 pathway. Biomed. Pharmacother. 2017, 86, 457–465. [Google Scholar] [CrossRef]
- Yu, D.; Geng, H.; Liu, Z.; Zhao, L.; Liang, Z.; Zhang, Z.; Xie, D.; Wang, Y.; Zhang, T.; Min, J.; et al. Cigarette smoke induced urocystic epithelial mesenchymal transition via MAPK pathways. Oncotarget 2017, 8, 8791–8800. [Google Scholar] [CrossRef]
- Chuang, J.-Y.; Yang, W.-H.; Chen, H.-T.; Huang, C.-Y.; Tan, T.-W.; Lin, Y.-T.; Hsu, C.-J.; Fong, Y.-C.; Tang, C.-H. CCL5/CCR5 axis promotes the motility of human oral cancer cells. J. Cell. Phys. 2009, 220, 418–426. [Google Scholar] [CrossRef]
- Bièche, I.; Lerebours, F.; Tozlu, S.; Espie, M.; Marty, M.; Lidereau, R. Molecular Profiling of Inflammatory Breast Cancer: Identification of a Poor-Prognosis Gene Expression Signature. Clin. Cancer Res. 2004, 10, 6789–6795. [Google Scholar] [CrossRef] [PubMed]
- Niwa, Y.; Akamatsu, H.; Niwa, H.; Sumi, H.; Ozaki, Y.; Abe, A. Correlation of Tissue and Plasma RANTES Levels with Disease Course in Patients with Breast or Cervical Cancer. Clin. Cancer Res. 2001, 7, 285–289. [Google Scholar] [PubMed]
- Yi, E.H.; Lee, C.S.; Lee, J.-K.; Lee, Y.J.; Shin, M.K.; Cho, C.-H.; Kang, K.W.; Lee, J.W.; Han, W.; Noh, D.-Y.; et al. STAT3-RANTES autocrine signaling is essential for tamoxifen resistance in human breast cancer cells. Mol. Cancer Res. 2013, 11, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Zhang, Y.; Kim, H.-J.; Zhang, L.; Ma, X. CCL5 as a potential immunotherapeutic target in triple-negative breast cancer. Cell. Mol. Immunol. 2013, 10, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Swamydas, M.; Ricci, K.; Rego, S.L.; Dréau, D. Mesenchymal stem cell-derived CCL-9 and CCL-5 promote mammary tumor cell invasion and the activation of matrix metalloproteinases. Cell Adhes. Migr. 2013, 7, 315–324. [Google Scholar] [CrossRef]
- Lotfi, A.; Mohammadi, G.; Tavassoli, A.; Mousaviagdas, M.; Chavoshi, H.; Saniee, L. Serum Levels of MMP9 and MMP2 in Patients with Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2015, 16, 1327–1330. [Google Scholar] [CrossRef]
- Vilen, S.-T.; Salo, T.; Sorsa, T.; Nyberg, P. Fluctuating Roles of Matrix Metalloproteinase-9 in Oral Squamous Cell Carcinoma. Sci. World J. 2013, 2013, 920595. [Google Scholar] [CrossRef]
- Kummer, N.T.; Nowicki, T.S.; Azzi, J.P.; Reyes, I.; Iacob, C.; Xie, S.; Swati, I.; Darzynkiewicz, Z.; Gotlinger, K.H.; Suslina, N.; et al. Arachidonate 5 lipoxygenase expression in papillary thyroid carcinoma promotes invasion via MMP-9 induction. J. Cell. Biochem. 2012, 113, 1998–2008. [Google Scholar] [CrossRef]
- Tulah, A.S.; Parker, S.G.; Moffatt, M.F.; Wardlaw, A.J.; Connolly, M.J.; Sayers, I. The role of ALOX5AP, LTA4H and LTB4R polymorphisms in determining baseline lung function and COPD susceptibility in UK smokers. BMC Med. Genet. 2011, 12, 173. [Google Scholar] [CrossRef]
- Mougey, E.; Lang, J.E.; Allayee, H.; Teague, W.G.; Dozor, A.J.; Wise, R.A.; Lima, J.J. ALOX5 polymorphism associates with increased leukotriene production and reduced lung function and asthma control in children with poorly controlled asthma. Clin. Exp. Allergy 2013, 43, 512–520. [Google Scholar] [CrossRef]
- Kennedy-Feitosa, E.; Pinto, R.F.S.; Pires, K.M.P.; Monteiro, A.P.T.; Machado, M.N.; Santos, J.C.; Ribeiro, M.L.; Zin, W.A.; Canetti, C.A.; Romana-Souza, B.; et al. The influence of 5-lipoxygenase on cigarette smoke-induced emphysema in mice. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Lien, M.-Y.; Lin, C.-W.; Tsai, H.-C.; Chen, Y.-T.; Tsai, M.-H.; Hua, C.-H.; Yang, S.-F.; Tang, C.-H. Impact of CCL4 gene polymorphisms and environmental factors on oral cancer development and clinical characteristics. Oncotarget 2017, 8, 31424–31434. [Google Scholar] [CrossRef] [PubMed]
- Kowshik, J.; Baba, A.B.; Giri, H.; Deepak Reddy, G.; Dixit, M.; Nagini, S. Astaxanthin inhibits JAK/STAT-3 signaling to abrogate cell proliferation, invasion and angiogenesis in a hamster model of oral cancer. PLoS ONE 2014, 9, e109114. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.-D.; Zhan, Y.-T.; Zhu, Y.-H.; Li, Y.; Xie, D.; Guan, X.-Y. High levels of CCL2 or CCL4 in the tumor microenvironment predict unfavorable survival in lung adenocarcinoma. Thorac. Cancer 2018, 9, 775–784. [Google Scholar] [CrossRef]
- Zhang, J.-F.; Li, Y.; Zhang, A.-Z.; He, Q.-Q.; Du, Y.-C.; Cao, W. Expression and pathological significance of CC chemokine receptor 7 and its ligands in the airway of asthmatic rats exposed to cigarette smoke. J. Thorac. Dis. 2018, 10, 5459–5467. [Google Scholar] [CrossRef]
- Kuznar-Kaminska, B.; Mikuła-Pietrasik, J.; Sosińska, P.; Książek, K.; Batura-Gabryel, H. COPD promotes migration of A549 lung cancer cells: The role of chemokine CCL21. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Campofiorito, C.M.M.; Mangone, F.R.R.; Pasini, F.S.; Maistro, S.; Brunialti, K.C.S.; Snitcovsky, I.M.L.; Lehn, C.N.; Walder, F.; Carvalho, M.B.; Brentani, M.; et al. CCR7/CCL21 receptor ligand system may play a role in the lymph node metastasis of oral squamous cell carcinoma. Clin. Cancer Res. 2006, 12, A62. [Google Scholar]
- Domingueti, C.B.; Janini, J.B.M.; Paranaíba, L.M.R.; Lozano-Burgos, C.; Olivero, P.; González-Arriagada, W.A. Prognostic value of immunoexpression of CCR4, CCR5, CCR7 and CXCR4 in squamous cell carcinoma of tongue and floor of the mouth. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e354–e363. [Google Scholar] [CrossRef]
- González-Arriagada, W.A.; Lozano-Burgos, C.; Zúñiga-Moreta, R.; González-Díaz, P.; Coletta, R.D. Clinicopathological significance of chemokine receptor (CCR1, CCR3, CCR4, CCR5, CCR7 and CXCR4) expression in head and neck squamous cell carcinomas. J. Oral Pathol. Med. 2018, 47, 755–763. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, Y.-S.; Lee, J.Y.; Kook, M.C.; Park, J.-W.; Nam, B.-H.; Bae, J.-M. The chemokine receptor CCR4 is expressed and associated with a poor prognosis in patients with gastric cancer. Ann. Surg. 2009, 249, 933–941. [Google Scholar] [CrossRef]
- Olkhanud, P.B.; Baatar, D.; Bodogai, M.; Hakim, F.; Gress, R.; Anderson, R.L.; Deng, J.; Xu, M.; Briest, S.; Biragyn, A. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009, 69, 5996–6004. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Ou, Z.L.; Yu, S.J.; Gu, X.L.; Yang, C.; Chen, A.X.; Di, G.H.; Shen, Z.Z.; Shao, Z.M. The chemokine receptor CCR4 promotes tumor growth and lung metastasis in breast cancer. Breast Cancer Res. Treat. 2012, 131, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, T.; Yaguchi, T.; Ohmura, G.; Ohta, S.; Kobayashi, A.; Kawamura, N.; Fujita, T.; Nakano, H.; Shimada, T.; Takahashi, T.; et al. Autocrine and paracrine loops between cancer cells and macrophages promote lymph node metastasis via CCR4/CCL22 in head and neck squamous cell carcinoma. Int. J. Cancer 2013, 132, 2755–2766. [Google Scholar] [CrossRef]
- Gupta, S.; Srivastava, M.; Ahmad, N.; Sakamoto, K.; Bostwick, D.G.; Mukhtar, H. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer 2001, 91, 737–743. [Google Scholar] [CrossRef]
- Tomar, S.; Graves, C.A.; Altomare, D.; Kowli, S.; Kassler, S.; Sutkowski, N.; Gillespie, M.B.; Creek, K.E.; Pirisi, L. Human papillomavirus status and gene expression profiles of oropharyngeal and oral cancers from European American and African American patients. Head Neck 2016, 38 (Suppl. 1), E694–E704. [Google Scholar] [CrossRef]
- Kim, S.M. Human papilloma virus in oral cancer. J. Korean Assoc. Oral Maxillofac. Surg. 2016, 42, 327–336. [Google Scholar] [CrossRef]
- Sabatini, M.E.; Chiocca, S. Human papillomavirus as a driver of head and neck cancers. Br. J. Cancer 2020, 122, 306–314. [Google Scholar] [CrossRef]
- Chuang, H.C.; Huang, C.C.; Chien, C.Y.; Chuang, J.H. Toll-like receptor 3-mediated tumor invasion in head and neck cancer. Oral Oncol. 2012, 48, 226–232. [Google Scholar] [CrossRef]
- Luo, Q.; Hu, S.; Yan, M.; Sun, Z.; Chen, W.; Chen, F. Activation of Toll-like receptor 3 induces apoptosis of oral squamous carcinoma cells in vitro and in vivo. Int. J. Biochem. Cell Biol. 2012, 44, 1266–1275. [Google Scholar] [CrossRef]
- Pries, R.; Hogrefe, L.; Xie, L.; Frenzel, H.; Brocks, C.; Ditz, C.; Wollenberg, B. Induction of c-Myc-dependent cell proliferation through toll-like receptor 3 in head and neck cancer. Int. J. Mol. Med. 2008, 21, 209–215. [Google Scholar] [CrossRef][Green Version]
- Pries, R.; Wulff, S.; Wollenberg, B. Toll-like receptor modulation in head and neck cancer. Crit. Rev. Immunol. 2008, 28, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Min, R.; Zun, Z.; Siyi, L.; Wenjun, Y.; Lizheng, W.; Chenping, Z. Increased expression of Toll-like receptor-9 has close relation with tumour cell proliferation in oral squamous cell carcinoma. Arch. Oral Biol. 2011, 56, 877–884. [Google Scholar] [CrossRef]
- Min, R.; Siyi, L.; Wenjun, Y.; Shengwen, L.; Ow, A.; Lizheng, W.; Chenping, Z. Toll-like receptor-9 agonists increase cyclin D1 expression partly through activation of activator protein-1 in human oral squamous cell carcinoma cells. Cancer Sci. 2012, 103, 1938–1945. [Google Scholar] [CrossRef]
- Ruan, M.; Zhang, Z.; Li, S.; Yan, M.; Liu, S.; Yang, W.; Wang, L.; Zhang, C. Activation of Toll-like receptor-9 promotes cellular migration via up-regulating MMP-2 expression in oral squamous cell carcinoma. PLoS ONE 2014, 9, e92748. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.P.; Estadella, D.; Ribeiro, D.A. The Role of Toll Like Receptors (TLRs) in Oral Carcinogenesis. Anticancer Res. 2017, 37, 5389–5394. [Google Scholar]
- Riihilä, P.; Nissinen, L.; Farshchian, M.; Kivisaari, A.; Ala-Aho, R.; Kallajoki, M.; Grénman, R.; Meri, S.; Peltonen, S.; Peltonen, J.; et al. Complement factor I promotes progression of cutaneous squamous cell carcinoma. J. Invest. Dermatol. 2015, 135, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Riihilä, P.; Viiklepp, K.; Nissinen, L.; Farshchian, M.; Kallajoki, M.; Kivisaari, A.; Meri, S.; Peltonen, J.; Peltonen, S.; Kähäri, V.M. Tumour-cell-derived complement components C1r and C1s promote growth of cutaneous squamous cell carcinoma. Br. J. Dermatol. 2020, 182, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Toland, A.E. High risk cutaneous squamous cell carcinoma of the head and neck. World J. Otorhinolaryngol. Head Neck Surg. 2016, 2, 136–140. [Google Scholar] [CrossRef]
- Unterberger, C.; Hanson, S.; Klingenhoff, A.; Oesterle, D.; Frankenberger, M.; Endo, Y.; Matsushita, M.; Fujita, T.; Schwaeble, W.; Weiss, E.H.; et al. Stat3 is involved in control of MASP2 gene expression. Biochem. Biophys. Res. Commun. 2007, 364, 1022–1025. [Google Scholar] [CrossRef]
- Verma, A.; Matta, A.; Shukla, N.K.; Deo, S.V.; Gupta, S.D.; Ralhan, R. Clinical significance of mannose-binding lectin-associated serine protease-2 expression in esophageal squamous cell carcinoma. Int. J. Cancer 2006, 118, 2930–2935. [Google Scholar] [CrossRef]
- Ytting, H.; Jarle Christensen, I.; Thiel, S.; Jensenius, J.C.; Nielsen, H.J. Serum Mannan-Binding Lectin-Associated Serine Protease 2 Levels in Colorectal Cancer: Relation to Recurrence and Mortality. Clin. Cancer Res. 2005, 11, 1441–1446. [Google Scholar] [CrossRef][Green Version]
- Swierzko, A.S.; Szala, A.; Sawicki, S.; Szemraj, J.; Sniadecki, M.; Sokolowska, A.; Kaluzynski, A.; Wydra, D.; Cedzynski, M. Mannose-Binding Lectin (MBL) and MBL-associated serine protease-2 (MASP-2) in women with malignant and benign ovarian tumours. Cancer Immunol. Immunother. 2014, 63, 1129–1140. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Chang, C.-Y.; Kuo, Y.-Z.; Fang, W.-Y.; Kao, H.-Y.; Tsai, S.-T.; Wu, L.-W. Cancer-associated fibroblast-derived interleukin-1β activates protumor C-C motif chemokine ligand 22 signaling in head and neck cancer. Cancer Sci. 2019, 110, 2783–2793. [Google Scholar] [CrossRef]
- Jablonska, E.; Piotrowski, L.; Grabowska, Z. Serum Levels of IL-1b, IL-6, TNF-a, sTNF-RI and CRP in Patients with Oral Cavity Cancer. Pathol. Oncol. Res. POR 1997, 3, 126–129. [Google Scholar] [CrossRef]
- Lee, C.H.; Chang, J.S.; Syu, S.H.; Wong, T.S.; Chan, J.Y.; Tang, Y.C.; Yang, Z.P.; Yang, W.C.; Chen, C.T.; Lu, S.C.; et al. IL-1β promotes malignant transformation and tumor aggressiveness in oral cancer. J. Cell. Physiol. 2015, 230, 875–884. [Google Scholar] [CrossRef]
- Dentelli, P.; Rosso, A.; Calvi, C.; Ghiringhello, B.; Garbarino, G.; Camussi, G.; Pegoraro, L.; Brizzi, M.F. IL-3 affects endothelial cell-mediated smooth muscle cell recruitment by increasing TGFβ activity: Potential role in tumor vessel stabilization. Oncogene 2004, 23, 1681–1692. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yoneda, K.; Ueta, E.; Osaki, T. Serum cytokines, interleukin-2 receptor, and soluble intercellular adhesion molecule-1 in oral disorders. Oral Surg. Oral Med. Oral Pathol. 1994, 78, 727–735. [Google Scholar] [CrossRef]
- Testa, U.; Riccioni, R.; Militi, S.; Coccia, E.; Stellacci, E.; Samoggia, P.; Latagliata, R.; Mariani, G.; Rossini, A.; Battistini, A.; et al. Elevated expression of IL-3Ralpha in acute myelogenous leukemia is associated with enhanced blast proliferation, increased cellularity, and poor prognosis. Blood 2002, 100, 2980–2988. [Google Scholar] [CrossRef]
- Almeida, V.L.; Santana, I.T.S.; Santos, J.N.A.; Fontes, G.S.; Lima, I.F.P.; Matos, A.L.P.; Matos, F.R.; Paranhos, L.R. Influence of interleukins on prognosis of patients with oral squamous cells carcinoma. J. Bras. Patol. Med. Lab. 2019, 55, 550–567. [Google Scholar] [CrossRef]
- Sun, L.; Diamond, M.E.; Ottaviano, A.J.; Joseph, M.J.; Ananthanarayan, V.; Munshi, H.G. Transforming growth factor-beta 1 promotes matrix metalloproteinase-9-mediated oral cancer invasion through snail expression. Mol. Cancer Res. 2008, 6, 10–20. [Google Scholar] [CrossRef]
- Smith, A.; Teknos, T.N.; Pan, Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol. 2013, 49, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, N.; Hassona, Y.; Celentano, A.; Lim, K.P.; Manchella, S.; Parkinson, E.K.; Prime, S.S. Cancer-associated fibroblasts regulate keratinocyte cell–cell adhesion via TGF-β-dependent pathways in genotype-specific oral cancer. Carcinogenesis 2016, 38, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Prime, S.S.; Eveson, J.W.; Price, N.; Ganapathy, A.; D’Mello, A.; Paterson, I.C. Transforming growth factor-β enhances invasion and metastasis in Ras-transfected human malignant epidermal keratinocytes. Int. J. Exp. Pathol. 2012, 93, 148–156. [Google Scholar] [CrossRef]
- Lu, Z.; Ding, L.; Ding, H.; Hao, F.; Pu, Y.; Wang, Y.; Chen, S.; Yang, Y.; Zhao, X.; Huang, X.; et al. Tumor cell-derived TGF-β at tumor center independently predicts recurrence and poor survival in oral squamous cell carcinoma. J. Oral Pathol. Med. 2019, 48, 696–704. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, C.; Liu, X.; Xu, S.; Zhang, Z.; Chen, H.; Zhang, Y.; Liu, Y.; Ma, D. Hypermethylation of IFN-γ in oral cancer tissues. Clin. Oral Investig. 2017, 21, 2535–2542. [Google Scholar] [CrossRef]
- Katayama, A.; Ogino, T.; Bandoh, N.; Nonaka, S.; Harabuchi, Y. Expression of CXCR4 and Its Down-Regulation by IFN-γ in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2005, 11, 2937–2946. [Google Scholar] [CrossRef]
- Wang, S.; Sun, M.; Gu, C.; Wang, X.; Chen, D.; Zhao, E.; Jiao, X.; Zheng, J. Expression of CD163, interleukin-10, and interferon-gamma in oral squamous cell carcinoma: Mutual relationships and prognostic implications. Eur. J. Oral Sci. 2014, 122, 202–209. [Google Scholar] [CrossRef]
- Li, K.; Guo, Q.; Zhang, X.; Dong, X.; Liu, W.; Zhang, A.; Li, Y.; Yan, J.; Jia, G.; Zheng, Z.; et al. Oral cancer-associated tertiary lymphoid structures: Gene expression profile and prognostic value. Clin. Exp. Immunol. 2020, 199, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Coupar, J.; saleh, A.; Clavijo, P.E.; Chen, Z.; VanWaes, C. Abstract 358: LTB and LTBR mediates alternative Nf-kB activation through NIk and RELB/NF-kB2 to promote cell migration of HNSCC. Cancer Res. 2017, 77, 358. [Google Scholar] [CrossRef]
- Hsu, D.S.; Hwang, W.L.; Yuh, C.H.; Chu, C.H.; Ho, Y.H.; Chen, P.B.; Lin, H.S.; Lin, H.K.; Wu, S.P.; Lin, C.Y.; et al. Lymphotoxin-β Interacts with Methylated EGFR to Mediate Acquired Resistance to Cetuximab in Head and Neck Cancer. Clin. Cancer Res. 2017, 23, 4388–4401. [Google Scholar] [CrossRef]
- Buettner, R.; Mora, L.B.; Jove, R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res. 2002, 8, 945–954. [Google Scholar] [PubMed]
- Leibowitz, M.S.; Andrade Filho, P.A.; Ferrone, S.; Ferris, R.L. Deficiency of activated STAT1 in head and neck cancer cells mediates TAP1-dependent escape from cytotoxic T lymphocytes. Cancer Immunol. Immunother. CII 2011, 60, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Molavi, O.; Su, M.; Lai, R. The clinical and biological significance of STAT1 in esophageal squamous cell carcinoma. BMC Cancer 2014, 14, 791. [Google Scholar] [CrossRef]
- Pidugu, V.K.; Wu, M.M.; Yen, A.H.; Pidugu, H.B.; Chang, K.W.; Liu, C.J.; Lee, T.C. IFIT1 and IFIT3 promote oral squamous cell carcinoma metastasis and contribute to the anti-tumor effect of gefitinib via enhancing p-EGFR recycling. Oncogene 2019, 38, 3232–3247. [Google Scholar] [CrossRef]
- Pidugu, V.K.; wu, M.-M.; Pidugu, H.B.; Lee, T.-C. Abstract 2098: IFIT1 and IFIT3 modulate the drug response in human oral squamous cell carcinoma through interaction and activation of Hsp90. Cancer Res. 2019, 79, 2098. [Google Scholar] [CrossRef]
- Pidugu, V.K.; Yen, A.-H.; Chen, Y.-C.; Wu, M.-M.; Liu, C.-J.; Lee, T.-C. Abstract 3933: Characterization of oncogenic activity of interferon-induced protein with tetratricopeptide repeats 1 and 3 in human oral squamous cell carcinoma progression. Cancer Res. 2017, 77, 3933. [Google Scholar] [CrossRef]
- Chi, L.-M.; Lee, C.-W.; Chang, K.-P.; Hao, S.-P.; Lee, H.-M.; Liang, Y.; Hsueh, C.; Yu, C.-J.; Lee, I.N.; Chang, Y.-J.; et al. Enhanced interferon signaling pathway in oral cancer revealed by quantitative proteome analysis of microdissected specimens using 16O/18O labeling and integrated two-dimensional LC-ESI-MALDI tandem MS. Mol. Cell Proteom. 2009, 8, 1453–1474. [Google Scholar] [CrossRef]
- Calmon, M.F.; Rodrigues, R.V.; Kaneto, C.M.; Moura, R.P.; Silva, S.D.; Mota, L.D.C.; Pinheiro, D.G.; Torres, C.; de Carvalho, A.F.; Cury, P.M.; et al. Epigenetic Silencing of CRABP2 and MX1 in Head and Neck Tumors. Neoplasia 2009, 11, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Greene, T.; Powell, P.; Nzerem, C.; Shapiro, M.; Shapiro, V. Cloning and Characterization of ALX, an Adaptor Downstream of CD28. J. Biol. Chem. 2003, 278, 45128–45134. [Google Scholar] [CrossRef] [PubMed]
- Tsukishiro, T.; Donnenberg, A.D.; Whiteside, T.L. Rapid turnover of the CD8+CD28- T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol. Immunother. 2003, 52, 599–607. [Google Scholar] [CrossRef]
- Grassie, M.E.; Moffat, L.D.; Walsh, M.P.; MacDonald, J.A. The myosin phosphatase targeting protein (MYPT) family: A regulated mechanism for achieving substrate specificity of the catalytic subunit of protein phosphatase type 1δ. Arch. Biochem. Biophys. 2011, 510, 147–159. [Google Scholar] [CrossRef]
- Galván, S.; Felipe-Abrio, B.; Verdugo-Sivianes, E.; Jiménez-García, M.-P.; Suarez-Martinez, E.; Marco, P.; Otero Albiol, D.; Peinado-Serrano, J.; Navas, L.; Carnero, A. PO-106 Downregulation of mypt increases tumorigenesis and resistance to platin drugs in ovarian cancer. ESMO Open. 2018, 3, A62. [Google Scholar] [CrossRef]
- Liang, Y.; Zhuo, Y.; Lin, Z.; Jiang, F.; Dai, Q.; Lu, J.; Dong, W.; Zhu, X.; Han, Z.; Zhong, W. Decreased Expression of MYPT1 Contributes to Tumor Angiogenesis and Poor Patient Prognosis in Human Prostate Cancer. Curr Mol. Med. 2018, 18, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Andersen, Z.J.; Jørgensen, J.T.; Grøn, R.; Brauner, E.V.; Lynge, E. Active smoking and risk of breast cancer in a Danish nurse cohort study. BMC Cancer 2017, 17, 556. [Google Scholar] [CrossRef]
- White, A.J.; D’Aloisio, A.A.; Nichols, H.B.; DeRoo, L.A.; Sandler, D.P. Breast cancer and exposure to tobacco smoke during potential windows of susceptibility. Cancer Causes Control. 2017, 28, 667–675. [Google Scholar] [CrossRef]
- Strumylaite, L.; Kregzdyte, R.; Poskiene, L.; Bogusevicius, A.; Pranys, D.; Norkute, R. Association between lifetime exposure to passive smoking and risk of breast cancer subtypes defined by hormone receptor status among non-smoking Caucasian women. PLoS ONE 2017, 12, e0171198. [Google Scholar] [CrossRef]
- Mele, A.; Mehta, P.; Slanetz, P.J.; Brook, A.; Recht, A.; Sharma, R. Breast-Conserving Surgery Alone for Ductal Carcinoma In Situ: Factors Associated with Increased Risk of Local Recurrence. Ann. Surg. Oncol. 2017, 24, 1221–1226. [Google Scholar] [CrossRef]
- Pillai, S.; Trevino, J.; Rawal, B.; Singh, S.; Kovacs, M.; Li, X.; Schell, M.; Haura, E.; Bepler, G.; Chellappan, S. β-arrestin-1 mediates nicotine-induced metastasis through E2F1 target genes that modulate epithelial-mesenchymal transition. Cancer Res. 2015, 75, 1009–1020. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, F.; Xu, Y.; Wang, B.; Zhao, Y.; Xu, W.; Shi, L.; Lu, X.; Liu, Q. Epithelial-mesenchymal transition and cancer stem cells, mediated by a long non-coding RNA, HOTAIR, are involved in cell malignant transformation induced by cigarette smoke extract. Toxicol. Appl. Pharmacol. 2015, 282, 9–19. [Google Scholar] [CrossRef]
- Dinicola, S.; Masiello, M.G.; Proietti, S.; Coluccia, P.; Fabrizi, G.; Catizone, A.; Ricci, G.; de Toma, G.; Bizzarri, M.; Cucina, A. Nicotine increases colon cancer cell migration and invasion through epithelial to mesenchymal transition (EMT): COX-2 involvement. J. Cell. Physiol. 2018, 233, 4935–4948. [Google Scholar] [CrossRef]
- Chen, P.C.; Lee, W.Y.; Ling, H.H.; Cheng, C.H.; Chen, K.C.; Lin, C.W. Activation of fibroblasts by nicotine promotes the epithelial-mesenchymal transition and motility of breast cancer cells. J. Cell. Physiol. 2018, 233, 4972–4980. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Choi, M.; Hartge, P.; Liao, L.M.; Caporaso, N.; Freedman, N.D. Association between long-term low-intensity cigarette smoking and incidence of smoking-related cancer in the national institutes of health-AARP cohort. Int. J. Cancer 2018, 142, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.N.; Thornton, A.J.; Hamling, J.S. Epidemiological evidence on environmental tobacco smoke and cancers other than lung or breast. Regul. Toxicol. Pharmacol. 2016, 80, 134–163. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, C.; Zheng, J. Cigarette smoking impairs the response of EGFR-TKIs therapy in lung adenocarcinoma patients by promoting EGFR signaling and epithelial-mesenchymal transition. Am. J. Transl. Res. 2015, 7, 2026–2035. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).