Monosodium Glutamate (MSG)-Induced Male Reproductive Dysfunction: A Mini Review
Abstract
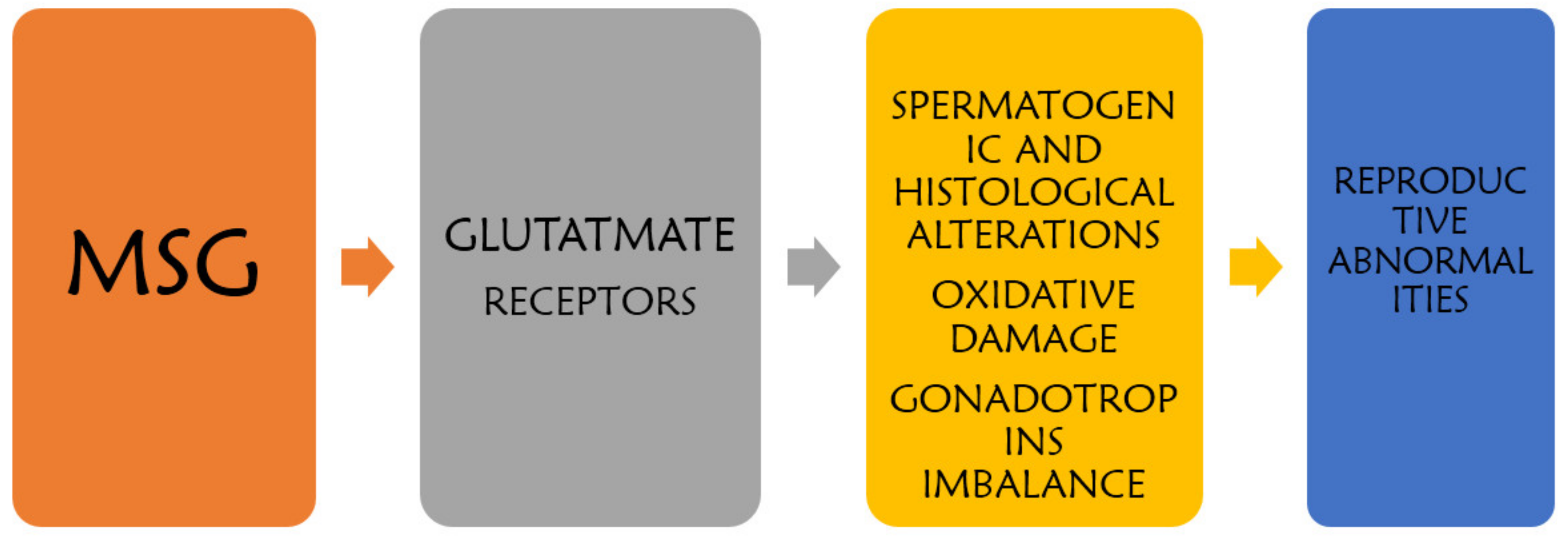
1. Introduction
2. MSG and the Induction of Reproductive Dysfunction
3. Mechanism of MSG-Induced Testicular Alteration
3.1. Oxidative Stress
3.2. Neurotoxicity
3.3. Histomorphological Alterations
3.4. Glutamate Receptor Dysfunction
3.5. Brief Clinically Observed Adverse Effect of MSG
4. Conclusions
Funding
Conflicts of Interest
References
- Guyton, A.C.; Hall, J.E. Textbook of Medical Physiology, 10th ed.; Harcourt International Edition; W.B. Saunder Company: Philadelphia, PA, USA, 2000; pp. 279–281. [Google Scholar]
- Ganong, W.F. Review of Medical Physiology, 20th ed.; Lange Medical Books/McGraw-Hill Medical Publishing Division: London, UK, 2001; p. 543. [Google Scholar]
- Kandeel, F.R.; Koussa, V.K.; Swerdloff, R.S. Male sexual function and its disorders: physiology, pathophysiology, clinical investigation, and treatment. Endocr. Rev. 2001, 22, 342. [Google Scholar] [CrossRef] [PubMed]
- Kayode, O.T.; Yakubu, M.T. Parquetina nigrescens leaves: Chemical profile and effects of its Aqueous Extract on the Physical and Biochemical Parameters of Sexual Behaviour of Male Rats. J. Integr. Med. 2017, 15, 64–76. [Google Scholar] [CrossRef]
- Harchegani, A.B.; Irandoost, A.; Mirnamniha, M.; Rahmani, H.; Tahmasbpour, E.; Shahriary, A. Possible Mechanisms for The Effects of Calcium Deficiency on Male Infertility. Int. J. Fertil. Steril. 2019, 12, 267–272. [Google Scholar]
- Bera, T.K.; Kar, S.K.; Yadav, P.K.; Mukherjee, P.; Yadav, S.; Joshi, B. Effects of monosodium glutamate on human health: A systematic review. World J. Pharm. Sci. 2017, 5, 139–144. [Google Scholar]
- Hamza, R.Z.; AL-Harbi, M.S. Monosodium glutamate induced testicular toxicity and the possible ameliorative role of vitamin E or selenium in male rats. Toxicol. Report 2014, 1, 1037–1045. [Google Scholar] [CrossRef]
- Kazmi, Z.; Fatima, I.; Perveen, S.; Malik, S.S. Monosodium glutamate: Review on clinical reports. Int. J. Food Prop. 2017, 20 (Suppl. 2), 1807–1815. [Google Scholar] [CrossRef]
- Sailo, L.; Murthy, M.K.; Pratima, K.; Roy, V.K.; Gurusubramanian, G. Monosodium Glutamate Toxicity and the Possible Protective Role of L–Carnitine. Sci. Technol. J. 2018, 6, 2321–3388. [Google Scholar]
- Abd-Elaziz, A.M.S.; Ashoush, I.S. Effect of monosodium glutamate administration on the reproductive performance in male male albino rats. Egypy J. Basic Appl. Physiol. 2007, 6, 101–110. [Google Scholar]
- Das, R.S.; Ghosh, S.K. Long term effects of monosodium glutamate on spermatogenesis following neonatal exposure in albino mice—A histological study. Nepal Med. Coll. J. 2010, 12, 149–153. [Google Scholar]
- Fernandes, G.S.A.; Arena, A.C.; Campos, K.E.; Volpato, G.T.; Anselmo-Franci, J.A.; Damasceno, D.C.; Kempinas, W.G. Glutamate-induced obesity leads to decreased sperm reserves and acceleration of transit time in the epididymis of adult male rats. Reprod. Biol. Endocrinol. 2012, 10, 105. [Google Scholar] [CrossRef]
- Hanipah, E.N.A.; Yahya, N.J.; Ajik, E.M.; Yusoff, N.A.; Taib, I.S. Monosodium Glutamate Induced Oxidative Stress in Accessory Reproductive Organs of Male Sprague-Dawley Rats. J. Sains Kesihat. Malays. 2018, 16, 67–73. [Google Scholar] [CrossRef]
- Iamsaard, S.; Sukhorum, W.; Samrid, R.; Yimdee, J.; Kanla, P.; Chaisiwamongkol, K.; Hipkaeo, W.; Fongmoon, D.; Kondo, H. The sensitivity of male rat reproductive organs to monosodium glutamate. Acta Med. Acad. 2014, 43, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Igwebuike, U.M. The effects of oral administration of monosodium glutamaste (msg) on the testicular morphology and cauda epididymal sperm reserves of young and adult male rats. Vet. Arh. 2011, 81, 525–534. [Google Scholar]
- Nosseir, N.S.; Ali, M.H.N.; Ebaid, H.M. A Histological and Morphometric Study of Monosodium Glutamate Toxic Effect on Testicular Structure and Potentiality of Recovery in Adult Albino Rats. Res. J. Biol. 2012, 2, 66–78. [Google Scholar]
- Abd-Ella, E.M.M.; Mohammed, A.M. Attenuation of Monosodium Glutamate-Induced Hepatic and Testicular Toxicity in Albino Rats by Annona Muricata Linn. (Annonaceae) Leaf Extract. IOSR J. Pharm. Biol. Sci. 2016, 11, 61–69. [Google Scholar]
- Ekaluo, U.B.; Ikpeme, E.V.; Ibiang, Y.B.; Amaechina, O.S. Attenuating role of vitamin C on sperm toxicity induced by monosodium glutamate in albino rats. J. Biol. Sci. 2013, 13, 298–301. [Google Scholar] [CrossRef]
- El-Sawy, H.B.I.; Soliman, M.M.; El-Shazly, S.A.; Ali, H.A. Protective effects of camel milk and vitamin E against monosodium glutamate induced biochemical and testicular dysfunctions. Prog. Nutr. 2018, 20, 76–85. [Google Scholar]
- Khaled, F.A.; Yousef, M.I.; Kamel, K.I. The protective role of propolis against the reproductive toxicity of mono-sodium glutamine in male rabbits. Int. J. Chem. Stud. 2016, 4, 4–9. [Google Scholar]
- Kianifard, D.; Gholamreza, S.V.; Farhad, R. Study of the protective effects of quince (Cydonia oblonga) leaf extract on fertility alterations and gonadal dysfunction induced by Monosodium glutamate in adult male wistar rats. Rom. J. Diabetes Nutr. Metab. Dis. 2015, 22, 375–384. [Google Scholar] [CrossRef]
- Sakr, S.A.; Bada, G.M. Protective Effect of Curcumin on Monosodium Glutamate-Induced Reproductive Toxicity in Male Albino Rats. Glob. J. Pharmacol. 2013, 7, 416–422. [Google Scholar]
- Yeomans, M.R.; Gould, N.J.; Mobini, S.; Prescott, J. Acquired Flavor Acceptance and Intake Facilitated by Monosodium Glutamate in Humans. Physiol. Behav. 2008, 93, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Bellisle, F.; Monneuse, M.O.; Chabert, M.; Larue-Achagiotis, C.; Lanteaume, M.T.; Louis-Sylvestre, J. Monosodium Glutamate as a Palatability Enhancer in the European Diet. Physiol. Behav. 1991, 49, 869–873. [Google Scholar] [CrossRef]
- He, K.; Zhao, L.; Daviglus, M.L.; Dyer, A.R.; Horn, L.; Garside, D.; Stamler, J. Association of Monosodium Glutamate Intake with Overweight in Chinese Adults: The INTERMAP Study. Obesity 2008, 16, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Luscombe-Marsh, N.D.; Wittert, G.A.; Yuan, B.; Dai, Y.; Pan, X.; Taylor, A.W. Monosodium Glutamate Is Not Associated with Obesity or a Greater Prevalence of Weight Gain over 5 Years: Findings from the Jiangsu Nutrition Study of Chinese Adults. Br. J. Nutar. 2010, 104, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Insawang, T.; Selmi, C.; Cha’on, U.; Pethlert, S.; Yongvanit, P.; Areejitranusorn, P.; Prasongwattana, V. Monosodium Glutamate (MSG) Intake Is Associated with the Prevalence of Metabolic Syndrome in a Rural Thai Population. Nutr. Metab. 2012, 9, 1. [Google Scholar] [CrossRef]
| Serial number | MSG Induced Alteration | Animal Type and Average Body Weight | Days of Administration and Type | Number of Animals and Dosages | Authors |
|---|---|---|---|---|---|
| 1 | Increased body weight; decreased sperm concentration, motility, and viability; Alteration in testis histological structure; Seminiferous tubules deterioration. | Albino rats 150 g | 90 days Oral | 20 rats: 4 (5/group) * MSG 1: 0 mg/kg * MSG 2: 35 mg/kg * MSG 3: 350 mg/kg * MSG 4: 500 mg/kg | [10] |
| 2 | Testicular histological changes in germinal epithelium and Leydig cells (hypertrophied); Fewer spermatogenic cells in tubules; Low serum LH and testosterone levels. | Neonate | 75 days Subcutaneous | 14 mice Control: distilled water (DW) * MSG: (2 mg/kg) | [11] |
| 3 | Decrease in weight of testis, epididymis, seminal vesicle and prostate; Decreased hormone levels: testosterone and follicle-stimulating hormone; Reduced sperm counts. | Wistar rats 180 g | 120 days Subcutaneous | 18 rats: 2(9) (Control: 2.0% NaCl) * (MSG: 4.0 mg/kg) | [12] |
| 4 | Increased SOD level in epididymis, prostate gland, and seminal vesicle; Reduced GSH level in the organs; Increased lipid peroxidation (MDA) and protein; oxidation in the organs; Histopathological alteration in organs. | Sprague Dawley Rats 170–200 g | 28 days Oral | 24 rats: 3 (8/group) Control:1 mg/kg DW * MSG 1: 60 mg/kg * MSG 2: 120 mg/kg | [13] |
| 5 | Testicular morphological alteration; Decreased testosterone level; Low sperm count. | Sprague Dawley male rats | 30 days (twice a day) | 32 rats: 4 (8/group) Control: DW MSG 1: 0.25 g/kg MSG 2: 3 g/kg * MSG 3: 6 g/kg | [14] |
| 6 | Decreased epididymal sperm count; Reduced serum testosterone level; No overt histopathological lesion. | Sprague Dawley rats 160–180g | Oral 42 days (every 48 h) | 28 rats: 4 (7/group) * MSG 1: 0 mg/kg * MSG 2: 1 mg/kg * MSG 3: 2 mg/kg * MSG 4: 4 mg/kg | [15] |
| 7 | Decrease in sperm count and abnormal sperm morphology; Decrease in testicular weight and tubular diameter; Reduction in germinal epithelium height. | Wistar male rats | 14 days intraperitoneal | 24 rats: 4 (6/group) Control group * Experimental group: 4 mL/kg | [16] |
| Serial number | Effect of MSG and Treatment | Animals Used and Body Weight | Days of Administration and Type | Number of Rats and Dosages | Authors |
|---|---|---|---|---|---|
| 1 | Histological alterations (blood hemorrhage, distorted germ cells, and few Sertoli cells) Damaged seminiferous tubules Treatment with graviola extract (GE) Improved seminiferous tubules and germ cell Restored testis configuration | Albino rats 120–150 g | 4–8 weeks Oral | 48 rats: 4 (12/group) Group 1: distilled water Group 2: GE (100 mg/kg) Group 3: MSG (4 mg/kg) Group 4: MSG (4 weeks) + GE (4 weeks) | [17] |
| 2 | Reduced testis and epididymis weight Reduced sperm motility, count, and viability Treatment with vitamin C Restored MSG effect on organ weight Repaired sperm characteristics | Albino rats | 65 days Oral: Vit C Intraperitoneal: MSG | 30 rats: 5(6/group) Control: DW Group 2: Vit. C (100 mg/kg) Group 3: MSG (2 mg/kg) Group 4: MSG + Vit C Group 5: 2 MSG + Vit C | [18] |
| 3 | Reduced testicular antioxidant activities Increased lipid peroxidation Low testosterone and luteinizing hormones Sperm motility and abnormalities Downregulation of steroidogenesis genes Treatment with camel milk and vitamin E Restored antioxidant and hormone levels Upregulated genes and restored sperm analysis | Wistar rats 170–200 g | 28 days Oral | 40 rats: 4 (10/group) Control: DW MSG (2 g/kg) MSG + Vit E (20 mg/kg) MSG + camel milk (166.6 mL/24 h/10 rats) | [19] |
| 4 | Increased oxidative stress (MDA) Reduced antioxidant activity (catalase, superoxide dismutase and glutathione peroxidase) Altered histology of the testis Treatment with vitamin E and selenium Ameliorated MSG effect on lipid peroxidation and antioxidant activities Restored testicular histological structures | Wistar rats 150–200 g | 30 days Oral | 120: 12 (10) rats Control:1 mg corn oil MSG: 6 mg/kg MSG: 17.5 mg/kg MSG: 60 mg/kg Vit. E: 150 mg/kg Vit. E: 200 mg/kg Selenium (Se): 0.25 mg/kg Se: 1.0 mg/kg MSG 60 mg/kg + Vit E 150 mg/kg MSG 60 mg/kg + Vit E 200 mg/kg MSG 60 mg/kg + Se 0.25 mg/kg MSG 60 mg/kg + Se 1.0 mg/kg | [7] |
| 5 | Decreased body and testis weight Decreased testosterone and semen quality Treatment with propolis Increased body and testis weight Elevated testosterone and semen quality | Rabbits | 12 weeks Oral | 20 rats: 4 (5/group) Group 1: control Group 2: propolis (50 mg/kg) Group 3: MSG (8 mg/kg) Group 4: propolis + MSG | [20] |
| 6 | Low testosterone and follicle-stimulating hormone (FSH) level Reduced epididymal sperm concentration No change in the luteinizing hormone (LH) Treatment with quince leaf extract Improvement in testosterone and FSH level Reduced sperm motility induced by MSG | Wistar rats 120 ± 20 g | 8 weeks Intraperitoneal | 60 rats: 6 (10/group) Control: no treatment MSG 30 mg/kg MSG 60 mg/kg MSG 30 mg/kg + quince extract (QE) 500 mg/kg MSG 60mg + QE 500 mg/kg QE 500 mg/kg | [21] |
| 7 | Decreased testis weight and sperm count Histological alteration and reduced hormone Spermatogenic loss and deformed Sertoli cells Treatment with curcumin Improved histopathological alteration Increased sperm count and sex hormones | Sprague Dawley rats 140 ± 5 g | Oral | 65 rats in total Control: 10 rats Group 2: 15 rats—150 mg/kg curcumin Group 3: 20 rats—4 mg/kg MSG Group 4: 20 rats—MSG + curcumin | [22] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayode, O.T.; Rotimi, D.E.; Kayode, A.A.A.; Olaolu, T.D.; Adeyemi, O.S. Monosodium Glutamate (MSG)-Induced Male Reproductive Dysfunction: A Mini Review. Toxics 2020, 8, 7. https://doi.org/10.3390/toxics8010007
Kayode OT, Rotimi DE, Kayode AAA, Olaolu TD, Adeyemi OS. Monosodium Glutamate (MSG)-Induced Male Reproductive Dysfunction: A Mini Review. Toxics. 2020; 8(1):7. https://doi.org/10.3390/toxics8010007
Chicago/Turabian StyleKayode, Omowumi T., Damilare E. Rotimi, Abolanle A. A. Kayode, Tomilola D. Olaolu, and Oluyomi S. Adeyemi. 2020. "Monosodium Glutamate (MSG)-Induced Male Reproductive Dysfunction: A Mini Review" Toxics 8, no. 1: 7. https://doi.org/10.3390/toxics8010007
APA StyleKayode, O. T., Rotimi, D. E., Kayode, A. A. A., Olaolu, T. D., & Adeyemi, O. S. (2020). Monosodium Glutamate (MSG)-Induced Male Reproductive Dysfunction: A Mini Review. Toxics, 8(1), 7. https://doi.org/10.3390/toxics8010007





