Screening for Elevated Blood Lead Levels and Related Risk Factors among Thai Children Residing in a Fishing Community
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Collection and Analysis of Blood Samples
2.3. Assessment of Child Growth
2.4. Statistical Analysis
3. Results
3.1. Descriptive Characteristic of Study Children
3.2. Predictors of Blood Lead Levels ≥ 5μg/dL
3.3. Predictors of Abnormal Growth
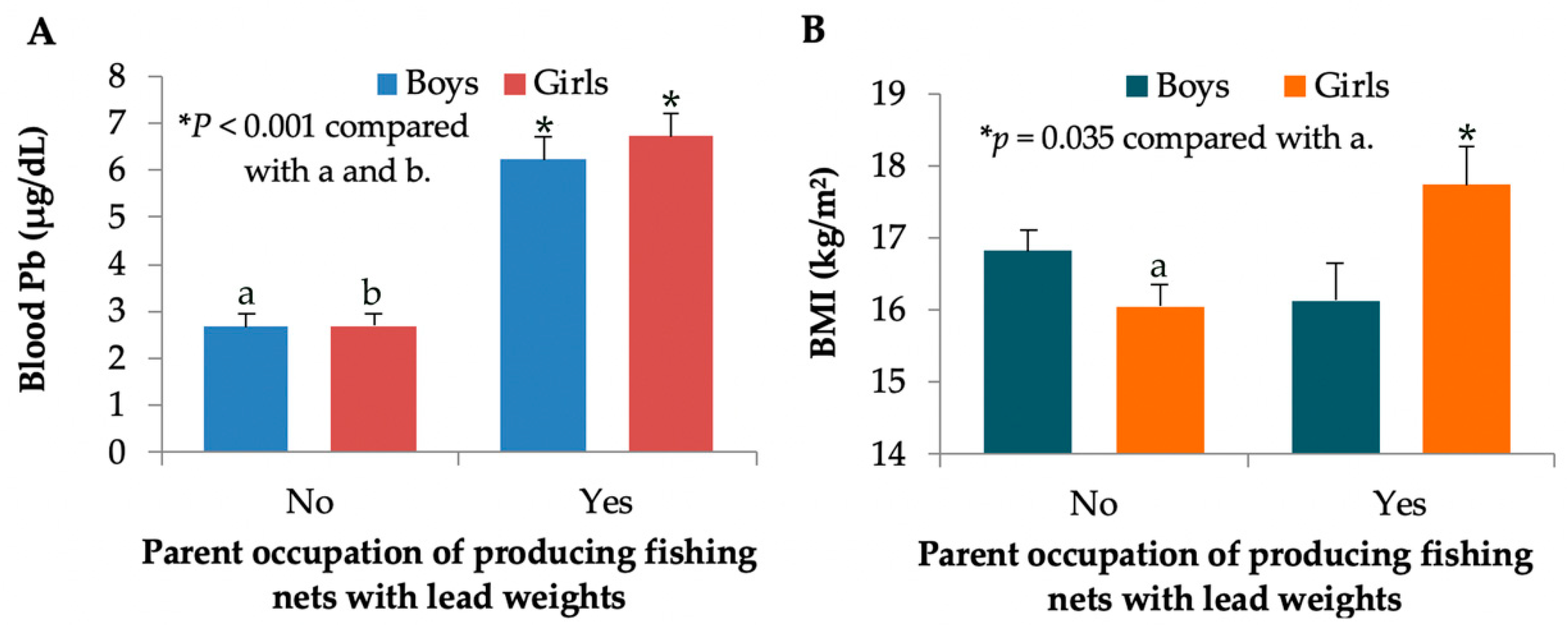
3.4. Effect-Size Estimate
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Lead. ATSDR, Division of Toxicology and Environmental Medicine/Applied Toxicology Branch: Atlanta, GA, USA. 2007. Available online: http://www.atsdr.cdc.gov/ToxProfiles/tp13.pdf (accessed on 22 September 2019).
- Cao, J.; Li, M.; Wang, Y.; Yu, G.; Yan, C. Environmental lead exposure among preschool children in Shanghai, China: Blood lead levels and risk factors. PLoS ONE 2014, 9, e113297. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Wolfe, L.; Jain, G. Impact of lead intoxication in children with iron deficiency anemia in low- and middle-income countries. Blood 2013, 122, 2288–2289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organization (WHO). Childhood Lead Poisoning. 2009. Available online: www.who.int/ceh/publications/leadguidance.pdf (accessed on 30 July 2019).
- Zhang, X.Y.; Carpenter, D.O.; Song, Y.J.; Chen, P.; Qin, Y.; Wei, N.Y.; Lin, S.C. Application of the IEUBK model for linking children’s blood lead with environmental exposure in a mining site, south China. Environ. Pollut. 2017, 231, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Taylor, M.P.; Zahran, S. The effect of contemporary mine emissions on children’s blood lead levels. Environ. Int. 2019, 122, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.P.; Isley, C.F.; Glover, J. Prevalence of childhood lead poisoning and respiratory disease associated with lead smelter emissions. Environ. Int. 2019, 127, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Ortega, N.; Caballero-Gallardo, K.; Olivero-Verbel, J. Toxicological effects in children exposed to lead: A cross-sectional study at the Colombian Caribbean coast. Environ. Int. 2019, 130, 104809. [Google Scholar] [CrossRef]
- Lin, S.; Wang, X.; Yu, I.T.; Tang, W.; Miao, J.; Li, J.; Wu, S.; Lin, X. Environmental lead pollution and elevated blood lead levels among children in a rural area of China. Am. J. Public Health 2011, 101, 834–841. [Google Scholar] [CrossRef]
- Zheng, L.; Wu, K.; Li, Y.; Qi, Z.; Han, D.; Zhang, B.; Gu, C.; Chen, G.; Liu, J.; Chen, S.; et al. Blood lead and cadmium levels and relevant factors among children from an e-waste recycling town in China. Environ. Res. 2008, 108, 15–20. [Google Scholar] [CrossRef]
- Yabe, J.; Nakayama, S.M.M.; Ikenaka, Y.; Yohannes, Y.B.; Bortey-Sam, N.; Oroszlany, B.; Muzandu, K.; Choongo, K.; Kabalo, A.N.; Ntapisha, J.; et al. Lead poisoning in children from townships in the vicinity of a lead–zinc mine in Kabwe, Zambia. Chemosphere 2015, 119, 941–947. [Google Scholar] [CrossRef]
- Mathee, A.; Khan, T.; Naicker, N.; Kootbodien, T.; Naidoo, S.; Becker, P. Lead exposure in young school children in South African subsistence fishing communities. Environ. Res. 2013, 126, 179–183. [Google Scholar] [CrossRef]
- Thanapop, C.; Geater, A.F.; Robson, M.G.; Phakthongsuk, P.; Viroonudomphol, D. Exposure to lead among boatyard workers in southern Thailand. J. Occup. Health 2007, 49, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Thanapop, C.; Geater, A.F.; Robson, M.G.; Phakthongsuk, P. Elevated lead contamination in boat-caulkers’ homes in southern Thailand. Int. J. Occup. Environ. Health 2009, 15, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Maharachpong, N.; Geater, A.; Chongsuvivatwong, V. Environmental and childhood lead contamination in the proximity of boat-repair yards in southern Thailand—I: Pattern and factors related to soil and household dust lead levels. Environ. Res. 2006, 101, 294–303. [Google Scholar] [CrossRef]
- Untimanon, O.; Geater, A.; Chongsuvivatwong, V.; Saetia, W.; Utapan, S. Skin Lead Contamination of Family Members of Boat-caulkers in Southern Thailand. Ind. Health 2011, 49, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Childhood Lead Poisoning Prevention Program. 2019. Available online: https://www.cdc.gov/nceh/lead/default.htm (accessed on 30 July 2019).
- Yamane, Taro. Statistics: An Introductory Analysis, 2nd ed.; Harper and Row Publication: New York, NY, USA, 1973; Available online: https://www.gbv.de/dms/zbw/252560191.pdf (accessed on 25 January 2015).
- Nutrition Division, Department of Health, Ministry of Public Health. The Reference Guide for the Assessment of Weight and Height Growth of Thai Children Aged 1 Day–19 Years Old; Ministry of Public Health: Nonthaburi, Thailand, 1999.
- Chambial, S.; Shukla, K.K.; Dwivedi, S.; Bhardwaj, P.; Sharma, P. Blood Lead Level (BLL) in the Adult Population of Jodhpur: A Pilot Study. Indian J. Clin. Biochem. 2015, 30, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Liu, X.; Zhu, H.; Song, A.; Jiao, J. Antioxidant and micronutrient-rich milk formula reduces lead poisoning and related oxidative damage in lead-exposed mice. Food Chem. Toxicol. 2013, 57, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Kordas, K. The “Lead Diet”: Can Dietary Approaches Prevent or Treat Lead Exposure? J. Pediatr. 2017, 185, 224–231. [Google Scholar] [CrossRef]
- Kordas, K.; Casavantes, K.M.; Mendoza, C.; Lopez, P.; Ronquillo, D.; Rosado, J.L.; Vargas, G.G.; Stoltzfus, R.J. The association between lead and micronutrient status, and children’s sleep, classroom behavior, and activity. Arch. Environ. Occup. Health 2007, 62, 105–112. [Google Scholar] [CrossRef]
- Gulson, B.; Mizon, K.; Taylor, A.; Wu, M. Dietary zinc, calcium and nickel are associated with lower childhood blood lead levels. Environ. Res. 2019, 168, 439–444. [Google Scholar] [CrossRef]
- Little, B.B.; Spalding, S.; Walsh, B.; Keyes, D.C.; Wainer, J.; Pickens, S.; Royster, M.; Villanacci, J.; Gratton, T. Blood lead levels and growth status among African–American and Hispanic children in Dallas, Texas – 1980 and 2002: Dallas Lead Project II. Ann. Hum. Biol. 2009, 36, 331–341. [Google Scholar] [CrossRef]
- Yang, H.; Huo, X.; Yekeen, A.T.; Zheng, Q.; Zheng, M.; Xu, X. Effects of lead and cadmium exposure from electronic waste on child physical growth. Environ. Sci. Pollut. Res. Int. 2013, 20, 4441–4447. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.J.; Badger, T.M.; Shema, S.J.; Roberson, P.K.; Templer, L.; Ringer, D.; Thomas, P.E. Endocrine mechanisms underlying the growth effects of developmental lead exposure in the rat. J. Toxicol. Environ. Health A 1998, 54, 101–120. [Google Scholar] [PubMed]
- Berglund, M.; Akesson, A.; Bjellerup, P.; Vahter, M. Metal-bone interactions. Toxicol. Lett. 2000, 113, 219–225. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.S.; Williams, P.L.; Lee, M.M.; Revich, B.; Sergeyev, O.; Hauser, R.; Korrick, S.A. Peripubertal blood lead levels and growth among Russian boys. Environ. Int. 2017, 106, 53–59. [Google Scholar] [CrossRef]
- Chan, H.M. Advances in methylmercury toxicology and risk Assessment. Toxics 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, Z.Y.; Yan, J.; Ying, X.L.; Tong, S.L.; Yan, C.H. Sex differences in the effects of prenatal lead exposure on birth outcomes. Environ. Pollut. 2017, 225, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Singh, V.; Wang, Z.; Voisin, G.; Lefebvre, F.; Navenot, J.-M.; Evans, B.; Verma, M.; Anderson, D.W.; Schneider, J.S. Effects of developmental lead exposure on the hippocampal methylome: Influences of sex and timing and level of exposure. Toxicol. Lett. 2018, 290, 63–72. [Google Scholar] [CrossRef]
- Cecil, K.M.; Brubaker, C.J.; Adler, C.M.; Dietrich, K.N.; Altaye, M.; Egelhoff, J.C.; Wessel, S.; Elangovan, I.; Hornung, R.; Jarvis, K.; et al. Decreased brain volume in adults with childhood lead exposure. PLoS Med. 2008, 5, e112. [Google Scholar] [CrossRef]

| Parameters/Factors | Study Children | p Values | ||
|---|---|---|---|---|
| All (n = 311) | Boys (n = 160) | Girls (n = 151) | ||
| Age (years) | 4.67 ± 1.14 | 4.67 ± 1.17 | 4.68 ± 1.11 | 0.776 |
| Age range (years) | 3−7 | 3−7 | 3−7 | - |
| Body weight (kg) | 18.28 ± 3.79 | 18.48 ±4.02 | 18.08 ±3.51 | 0.332 |
| Height (cm) | 106.0 ± 8.70 | 106.4 ± 9.50 | 105.6 ±7.80 | 0.466 |
| Body mass index (kg/m2) | 16.6 ± 3.30 | 16.7 ± 3.50 | 16.5 ± 3.00 | 0.540 |
| Blood Pb (μg/dL) | 2.81 ± 3.39 | 2.81 ± 3.37 | 2.80 ± 3.42 | 0.947 |
| Range (μg/dL) | 0.03−26.40 | 0.80−26.40 | 0.03−20.40 | - |
| Prevalence rate (%) | ||||
| Blood Pb levels ≥ 5 μg/dL | 11.9 | 10.0 | 13.9 | 0.287 |
| Birth weight < 2500 g | 14.8 | 12.5 | 17.2 | 0.241 |
| Abnormal growth a | 36.7 | 36.9 | 36.4 | 0.934 |
| Milk consumption | 64.3 | 67.5 | 60.9 | 0.227 |
| Seafood consumption | 53.7 | 51.9 | 55.6 | 0.507 |
| Living near repair boatyards | 14.5 | 14.4 | 14.6 | 0.961 |
| Parent occupation of producing fishing nets | 23.5 | 22.5 | 24.5 | 0.677 |
| Independent Variables/Factors | Blood Pb Levels ≥ 5μg/dL | ||||
|---|---|---|---|---|---|
| β Coefficients (SE) | POR | 95% CI | p Value | ||
| Lower | Upper | ||||
| Age (years) | −0.154 (0.194) | 0.857 | 0.586 | 1.255 | 0.429 |
| Gender (boy = 1, girl = 2) | 0.470 (0.426) | 1.599 | 0.694 | 3.685 | 0.270 |
| Milk consumption | −0.934 (0.440) | 0.393 | 0.166 | 0.931 | 0.034 * |
| Seafood consumption | −0.101 (0.436) | 0.904 | 0.385 | 2.124 | 0.817 |
| Symptoms of Pb toxicity | 0.490 (0.431) | 1.632 | 0.701 | 3.799 | 0.256 |
| Painted toys | 0.351 (0.459) | 1.420 | 0.578 | 3.489 | 0.444 |
| Use of painted ceramics | 0.081 (0.508) | 1.085 | 0.400 | 2.938 | 0.873 |
| Peeling of paint chips | 0.279 (0.498) | 1.322 | 0.498 | 3.504 | 0.575 |
| Living near repair boatyard | 0.093 (0.581) | 1.098 | 0.352 | 3.428 | 0.872 |
| Parent occupation of fishing net production | 2.865 (0.462) | 17.54 | 7.093 | 43.39 | <0.001 * |
| Independent Variables/Factors | Abnormal Growth a | ||||
|---|---|---|---|---|---|
| β Coefficients (SE) | POR | 95% CI | p Value | ||
| Lower | Upper | ||||
| Age (years) | −0.084 (0.113) | 0.920 | 0.737 | 1.148 | 0.459 |
| Gender (boy = 1, girl = 2) | −0.116 (0.245) | 0.891 | 0.551 | 1.440 | 0.637 |
| Blood Pb levels ≥ 5 μg/dL | 0.714 (0.365) | 2.042 | 0.999 | 4.174 | 0.050 * |
| Milk consumption | −0.556 (0.271) | 0.573 | 0.337 | 0.976 | 0.040 * |
| Seafood consumption | 0.538 (0.256) | 1.713 | 1.037 | 2.831 | 0.036 * |
| Use of painted ceramics | −0.552 (0.314) | 0.576 | 0.311 | 1.066 | 0.079 |
| Living near repair boatyards | 0.561 (0.364) | 1.753 | 0.860 | 3.574 | 0.123 |
| Painted toys | 0.277 (0.257) | 1.319 | 0.798 | 2.181 | 0.281 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yimthiang, S.; Waeyang, D.; Kuraeiad, S. Screening for Elevated Blood Lead Levels and Related Risk Factors among Thai Children Residing in a Fishing Community. Toxics 2019, 7, 54. https://doi.org/10.3390/toxics7040054
Yimthiang S, Waeyang D, Kuraeiad S. Screening for Elevated Blood Lead Levels and Related Risk Factors among Thai Children Residing in a Fishing Community. Toxics. 2019; 7(4):54. https://doi.org/10.3390/toxics7040054
Chicago/Turabian StyleYimthiang, Supabhorn, Donrawee Waeyang, and Saruda Kuraeiad. 2019. "Screening for Elevated Blood Lead Levels and Related Risk Factors among Thai Children Residing in a Fishing Community" Toxics 7, no. 4: 54. https://doi.org/10.3390/toxics7040054
APA StyleYimthiang, S., Waeyang, D., & Kuraeiad, S. (2019). Screening for Elevated Blood Lead Levels and Related Risk Factors among Thai Children Residing in a Fishing Community. Toxics, 7(4), 54. https://doi.org/10.3390/toxics7040054






