Urinary Concentrations of Diisoheptyl Phthalate Biomarkers in Convenience Samples of U.S. Adults in 2000 and 2018–2019
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipment
2.2. Subjects
2.3. Analytical Method
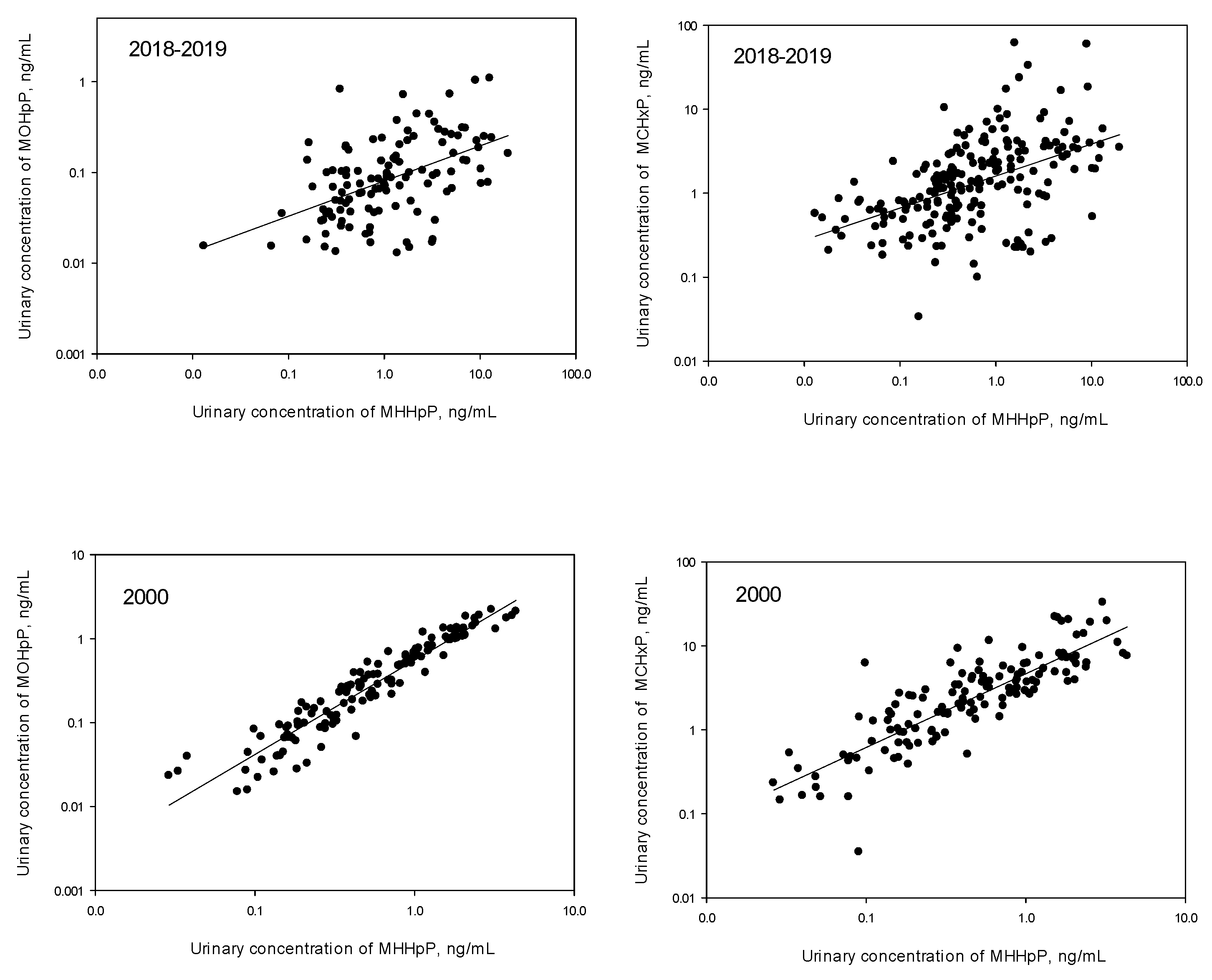
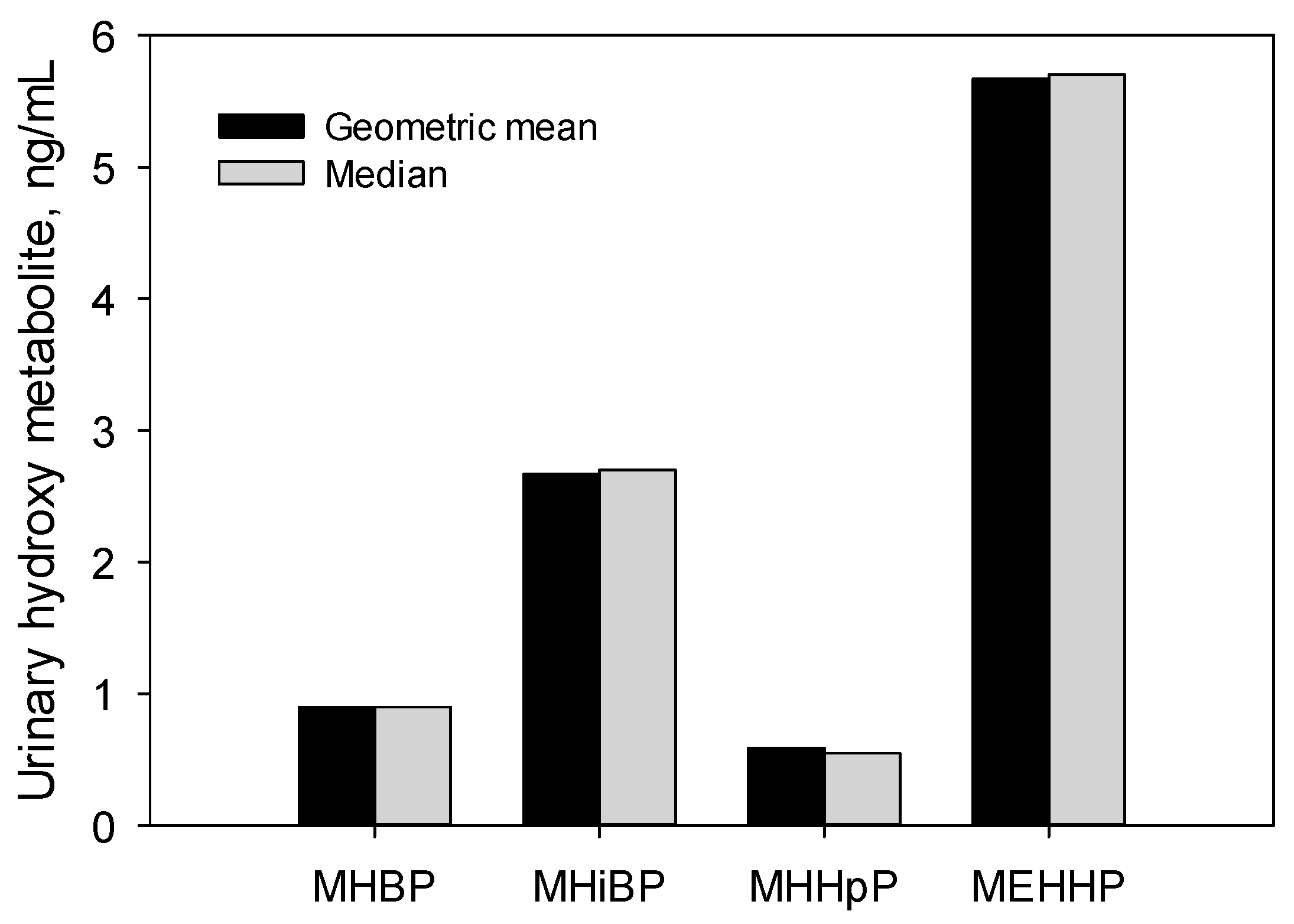
3. Results and Discussion
4. Disclaimer
Author Contributions
Funding
Conflicts of Interest
References
- CPSC. CPSC Staff Toxicity Review of 17 Phthalates for Consideration by the Chronic Hazard Advisory Panel - 2010. Available online: https://cpsc.gov/s3fs-public/CPSCStaffToxicity17Phthalates.pdf (accessed on 10 October 2019).
- CPSC. Toxicity Review of Diisoheptyl Phthalate (dihp). Available online: https://www.cpsc.gov/Global/Research-and-Statistics/Technical-Reports/Chemical/Phthalates/ToxicityReviewOfDiHP.pdf (accessed on 11 September 2019).
- Hannas, B.R.; Lambright, C.S.; Furr, J.; Howdeshell, K.L.; Wilson, V.S.; Gray, L.E. Dose-response assessment of fetal testosterone production and gene expression levels in rat testes following in utero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate, and diisononyl phthalate. Toxicol. Sci. 2011, 123, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.L.; Dewa, Y.; Kawai, M.; Nishimura, J.; Saegusa, Y.; Kemmochi, S.; Harada, T.; Shibutani, M.; Mitsumori, K. Induction of liver preneoplastic foci in f344 rats subjected to 28-day oral administration of diheptyl phthalate and its in vivo genotoxic potential. Toxicology 2009, 264, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Nakane, F.; Kunieda, M.; Shimizu, S.; Kobayashi, Y.; Akane, H.; Akie, Y.; Saito, A.; Noguchi, M.; Kadota, T.; Mitsumori, K. Twenty-six-week oral toxicity of diheptyl phthalate with special emphasis on its induction of liver proliferative lesions in male F344 rats. J. Toxicol. Sci. 2012, 37, 527–537. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lambright, C.; Howdeshell, K.; Furr, J.; Gray, L.; Wilson, V. In utero exposure to di-isoheptyl phthalate (DIHP) reduces testicular testosterone (T) production in fetal sprague dawley (SD) rats. Biol. Reprod. 2008, 547. [Google Scholar] [CrossRef]
- McKee, R.H.; Pavkov, K.L.; Trimmer, G.W.; Keller, L.H.; Stump, D.G. An assessment of the potential developmental and reproductive toxicity of di-isoheptyl phthalate in rodents. Reprod. Toxicol. 2006, 21, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, Y.; Onodera, H.; Mitsumori, K.; Maekawa, A.; Kurokawa, Y.; Takahashi, M. Twenty-eight day repeated dose toxicity test of dihepthyl phthalate in f344 rats. Eisei Shikenjo Hokoku. Bull. of Natl. Inst. Hyg. Sci. 1992, 110, 26–31. [Google Scholar]
- Koch, H.M.; Ruther, M.; Schutze, A.; Conrad, A.; Palmke, C.; Apel, P.; Bruning, T.; Kolossa-Gehring, M. Phthalate metabolites in 24-h urine samples of the german environmental specimen bank (ESB) from 1988 to 2015 and a comparison with us nhanes data from 1999 to 2012. Int. J. Hyg. Environ. Health 2016, 220, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.M.; Schutze, A.; Palmke, C.; Angerer, J.; Bruning, T. Metabolism of the plasticizer and phthalate substitute diisononyl-cyclohexane-1,2-dicarboxylate (DINCH (r)) in humans after single oral doses. Arch. Toxicol. 2013, 87, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Samandar, E.; Preau, J.L.; Needham, L.L.; Calafat, A.M. Urinary oxidative metabolites of di(2-ethylhexyl) phthalate in humans. Toxicology 2006, 219, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Wong, L.Y.; Silva, M.J.; Samandar, E.; Preau, J.L.; Jia, L.T.; Needham, L.L. Selecting adequate exposure biomarkers of diisononyl and diisodecyl phthalates: Data from the 2005–2006 national health and nutrition examination survey. Environ. Health Perspect. 2011, 119, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Schutze, A.; Gries, W.; Kolossa-Gehring, M.; Apel, P.; Schroter-Kermani, C.; Fiddicke, U.; Leng, G.; Bruning, T.; Koch, H.M. Bis-(2-propylheptyl)phthalate (DPHP) metabolites emerging in 24 h urine samples from the german environmental specimen bank (1999–2012). Int. J. Hyg. Environ. Health 2015, 218, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Hwang, M.; Baek, Y.; Jung, S.; Lee, Y.; Paek, D.; Choi, K. Urinary phthalate metabolite and bisphenol a levels in the korean adult population in association with sociodemographic and behavioral characteristics: Korean national environmental health survey (KoNEHS) 2012–2014. Int. J. Hyg. Environ. Health 2019, 222, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Saravanabhavan, G.; Guay, M.; Langlois, É.; Giroux, S.; Murray, J.; Haines, D. Biomonitoring of phthalate metabolites in the canadian population through the canadian health measures survey (2007–2009). Int. J. Hyg. Environ. Health 2013, 216, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Adachi, T.; Tanaka, A.; Yamaha, T. Biochemical-studies on phthalic esters. 4. Metabolism of diheptyl phthalate in rats. Drug Metab. Dispos. 1984, 12, 517–522. [Google Scholar] [PubMed]
- Silva, M.J.; Samandar, E.; Preau, J.L.; Reidy, J.A.; Needham, L.L.; Calafat, A.M. Quantification of 22 phthalate metabolites in human urine. J. Chromatogr. B 2007, 860, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Jia, T.; Samandar, E.; Preau, J.L.; Calafat, A.M. Environmental exposure to the plasticizer 1,2-cyclohexane dicarboxylic acid, diisononyl ester (DINCH) in us adults (2000–2012). Environ. Res. 2013, 126, 159–163. [Google Scholar] [CrossRef] [PubMed]
- National Center for Environmental Health; Division of Laboratory Sciences. National Report on Human Exposure to Environmental Chemicals; Department of Health and Human Services Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019.
- Hornung, R.W.; Reed, L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- Koch, H.M.; Muller, J.; Angerer, J. Determination of secondary, oxidised di-iso-nonylphthalate (DINP) metabolites in human urine representative for the exposure to commercial dinp plasticizers. J. Chromatogr. B 2007, 847, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Bontke, T.W.; Calafat, A.M.; Ye, X. Identification of potential biomarkers of exposure to diundecyl phthalate. Environ. Res. 2016, 148, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Silva, M.J.; Wolf, C.; Gray, L.E.; Needham, L.L.; Calafat, A.M. Urinary metabolites of diisodecyl phthalate in rats. Toxicology 2007, 236, 114–122. [Google Scholar] [CrossRef] [PubMed]
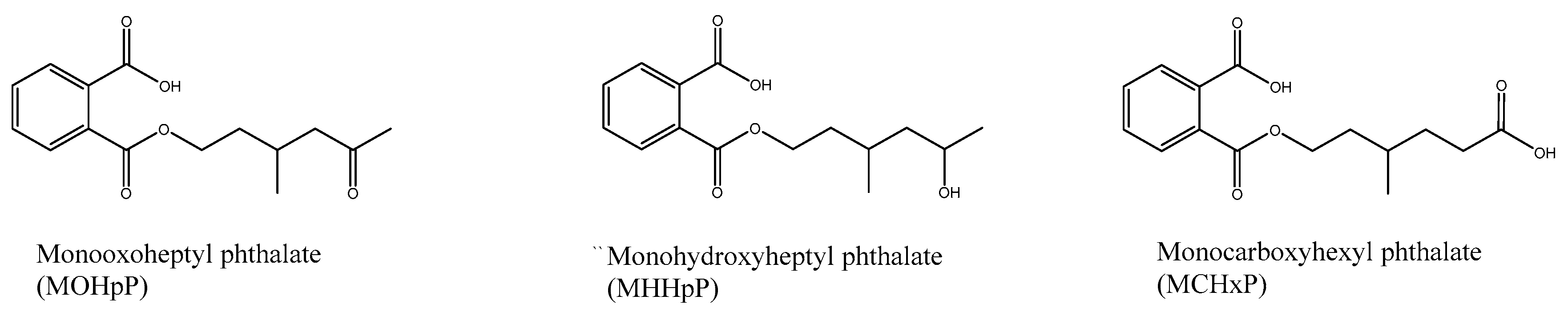
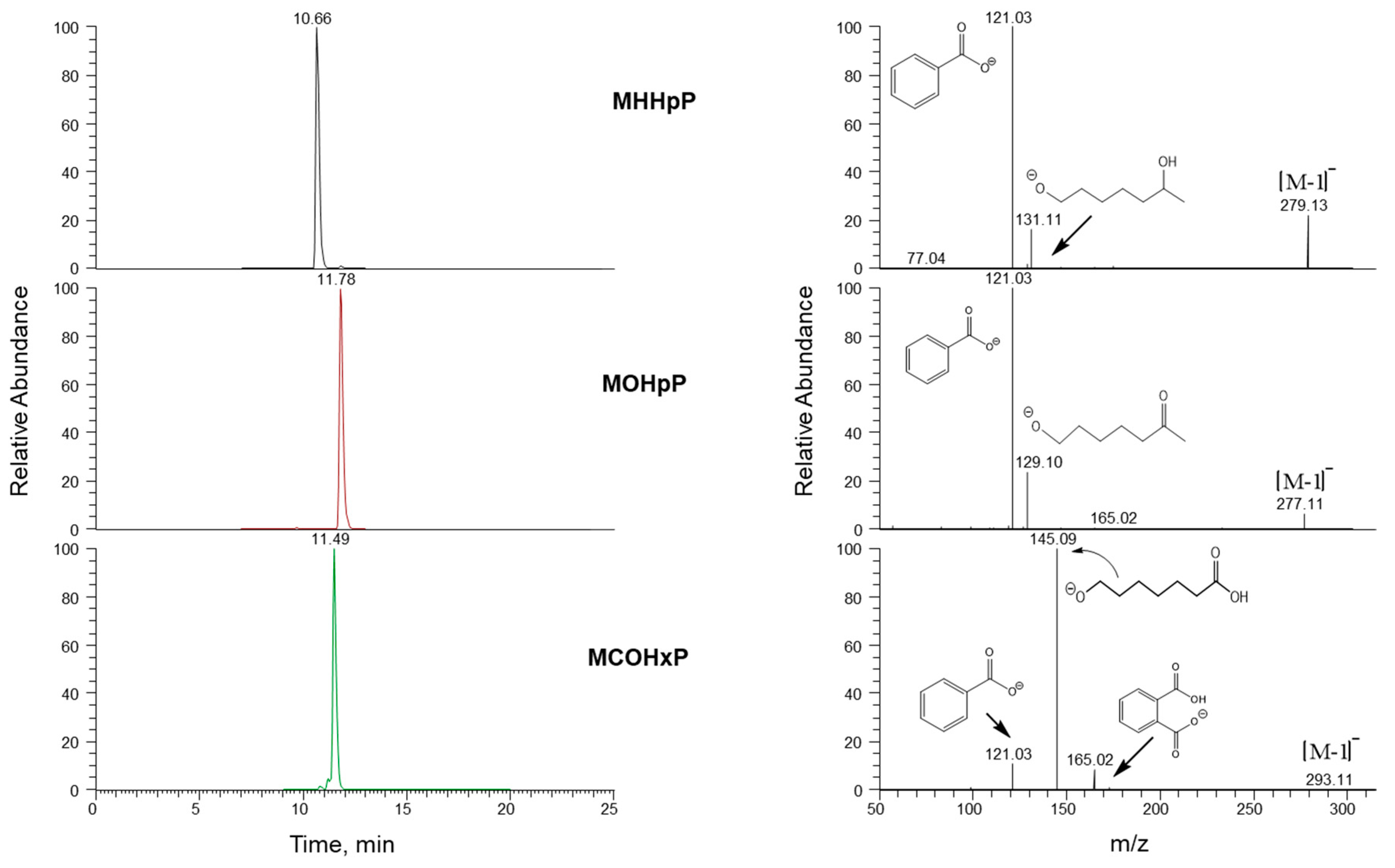
| Parent Chemical | Urinary Metabolite | Internal Standard | MS/MS Scan (Native) | Collision Energy (eV a) |
|---|---|---|---|---|
| Diisoheptyl phthalate (DHpP) | MHHpP | d4-MHHpP | 279.1/121.03 | 15 |
| MOHpP | d4-MOHpP | 277.1/121.03 | 16 | |
| MCHxP | d4-MCHxP | 293.1/145.09 | 16 |
| Urinary Metabolite | Collection Year | N | Percentile | Geometric Mean, ng/mL | Frequency of Detection (%) | |||
|---|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | 90th | |||||
| MHHpP | 2018–2019 | 205 | 0.25 (0.19, 0.29) | 0.55 (0.4, 0.72) | 1.71 (1.31, 2.2) | 4.88 (3.39, 7.07) | 0.59 (0.5, 0.7) | 100 |
| 2000 | 144 | 0.16 (0.13, 0.21) | 0.44 (0.34, 0.57) | 1.04 (0.87, 1.53) | 1.99 (1.7, 2.4) | 0.38 (0.31, 0.46) | 96 | |
| MOHpP | 2018–2019 | 205 | <LOD a | 0.02 (<LOD, 0.04) | 0.1 (0.07, 0.13) | 0.23 (0.19, 0.3) | NA b | 57 |
| 2000 | 144 | 0.07 (0.04, 0.09) | 0.24 (0.17, 0.32) | 0.64 (0.51, 0.99) | 1.29 (1.04, 1.54) | 0.19 (0.16, 0.24) | 92 | |
| MCHxP | 2018–2019 | 205 | 0.62 (0.49, 0.72) | 1.3 (1.08, 1.6) | 2.7 (2.26, 3.49) | 5.16 (4.0, 7.66) | 1.31 (1.15, 1.5) | 100 |
| 2000 | 144 | 0.93 (0.63, 1.41) | 2.63 (1.96, 3.16) | 5.11 (3.97, 6.27) | 8.1 (7.17, 13.97) | 2.01 (1.66, 2.43) | 100 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.J.; Wong, L.-Y.; Preau, J.L.; Samandar, E.; Obi, E.; Calafat, A.M.; Botelho, J.C. Urinary Concentrations of Diisoheptyl Phthalate Biomarkers in Convenience Samples of U.S. Adults in 2000 and 2018–2019. Toxics 2019, 7, 53. https://doi.org/10.3390/toxics7040053
Silva MJ, Wong L-Y, Preau JL, Samandar E, Obi E, Calafat AM, Botelho JC. Urinary Concentrations of Diisoheptyl Phthalate Biomarkers in Convenience Samples of U.S. Adults in 2000 and 2018–2019. Toxics. 2019; 7(4):53. https://doi.org/10.3390/toxics7040053
Chicago/Turabian StyleSilva, Manori J., Lee-Yang Wong, James L. Preau, Ella Samandar, Emmanuela Obi, Antonia M. Calafat, and Julianne C. Botelho. 2019. "Urinary Concentrations of Diisoheptyl Phthalate Biomarkers in Convenience Samples of U.S. Adults in 2000 and 2018–2019" Toxics 7, no. 4: 53. https://doi.org/10.3390/toxics7040053
APA StyleSilva, M. J., Wong, L.-Y., Preau, J. L., Samandar, E., Obi, E., Calafat, A. M., & Botelho, J. C. (2019). Urinary Concentrations of Diisoheptyl Phthalate Biomarkers in Convenience Samples of U.S. Adults in 2000 and 2018–2019. Toxics, 7(4), 53. https://doi.org/10.3390/toxics7040053




