Heavy Metals in Biota in Delaware Bay, NJ: Developing a Food Web Approach to Contaminants
Abstract
1. Introduction
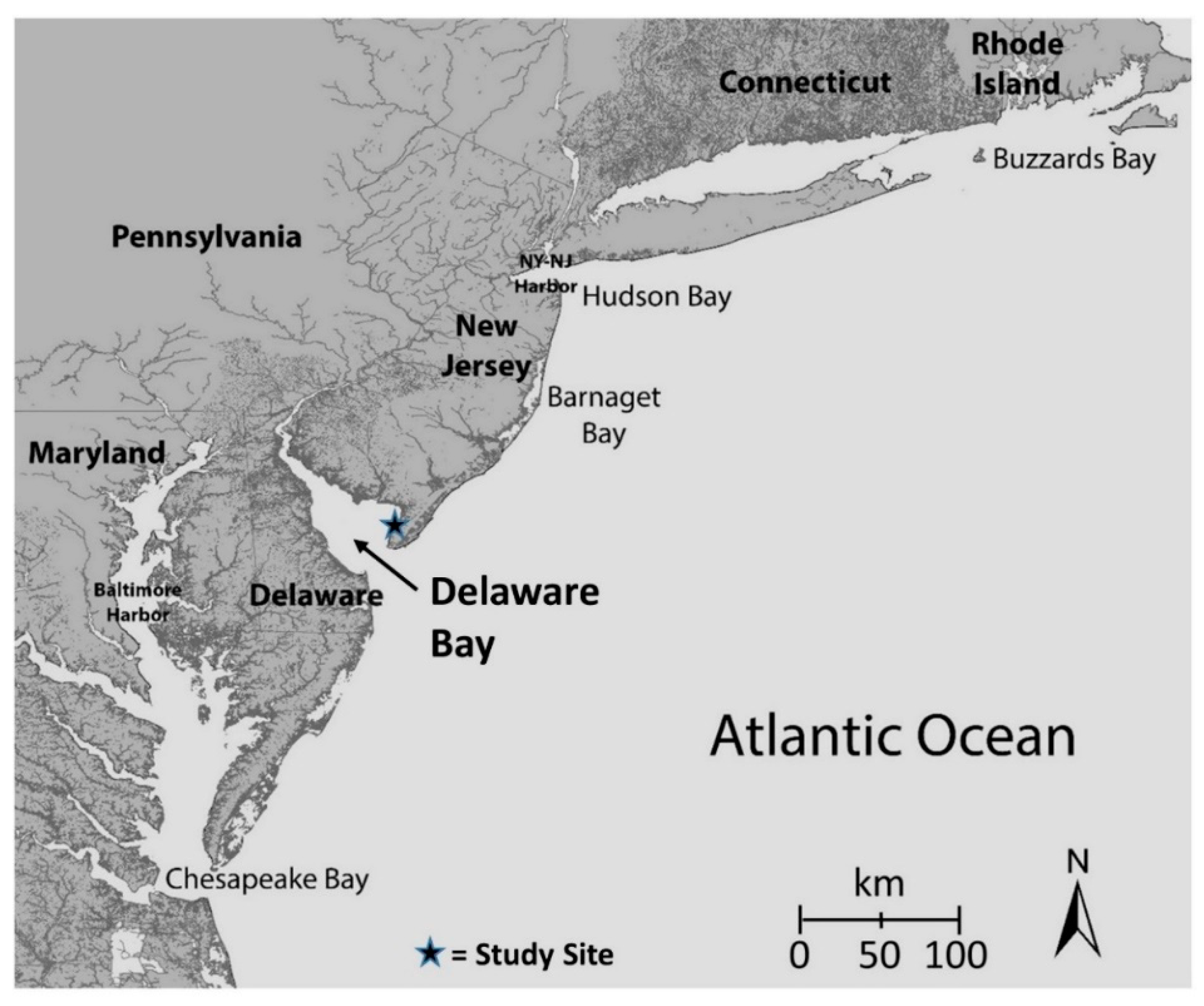
2. Materials and Methods
2.1. Collecting Methods
2.2. Chemical Analysis
3. Results
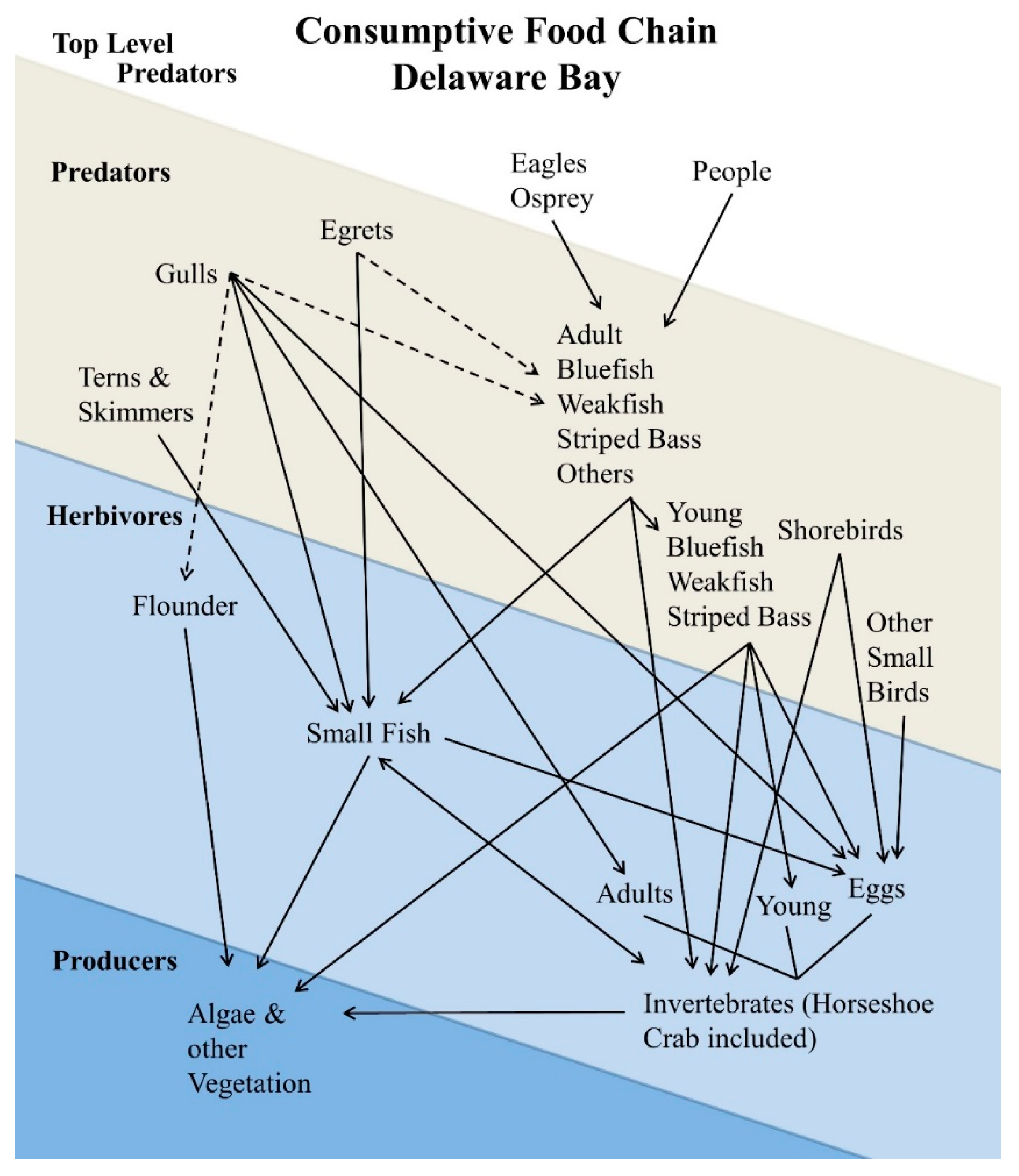
3.1. Springtime Delaware Bay Food Web
3.2. Cd, Pb, Hg, and Se Levels
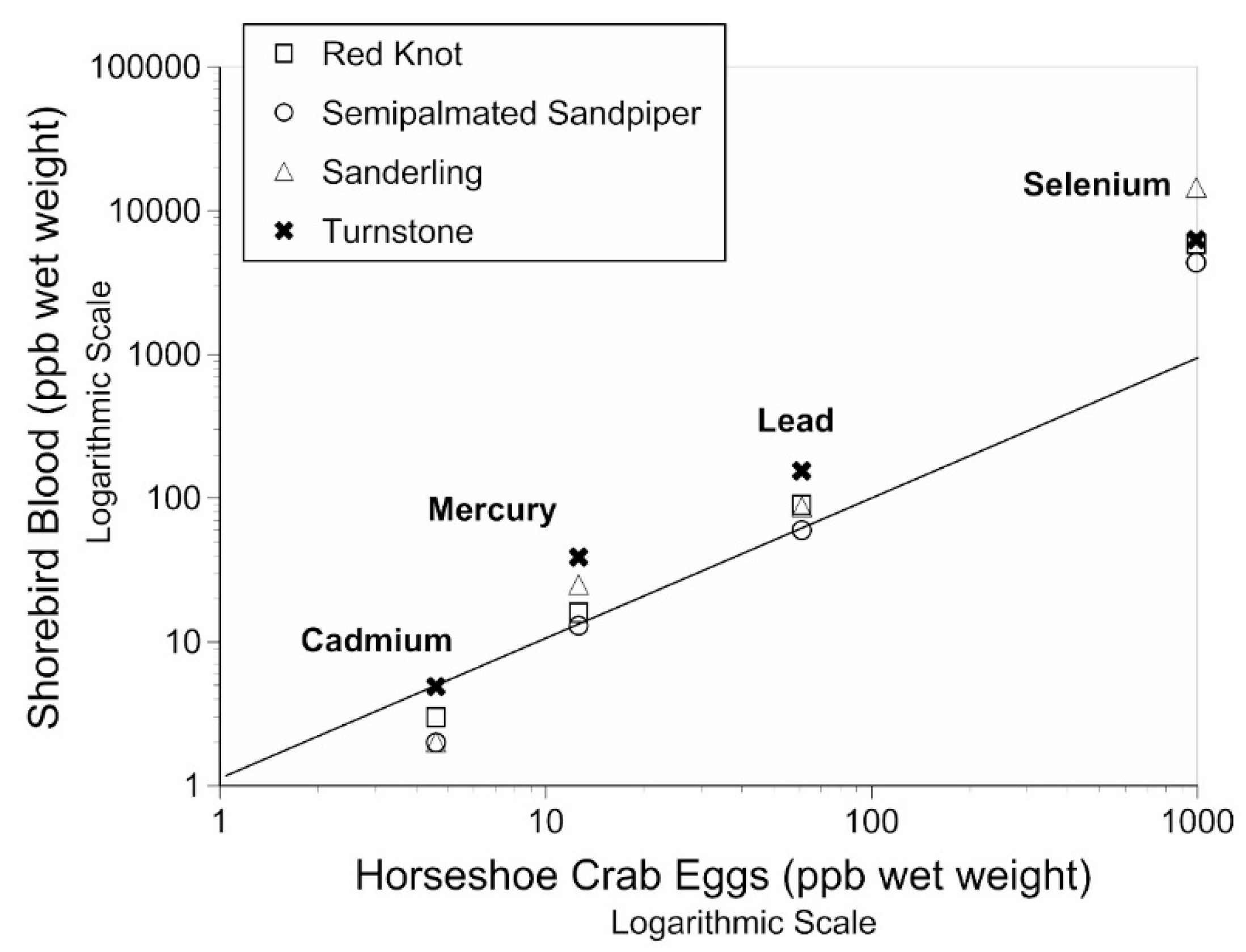
3.3. Relationship between Levels in Horseshoe Crab Eggs and Shorebird Blood and Feathers
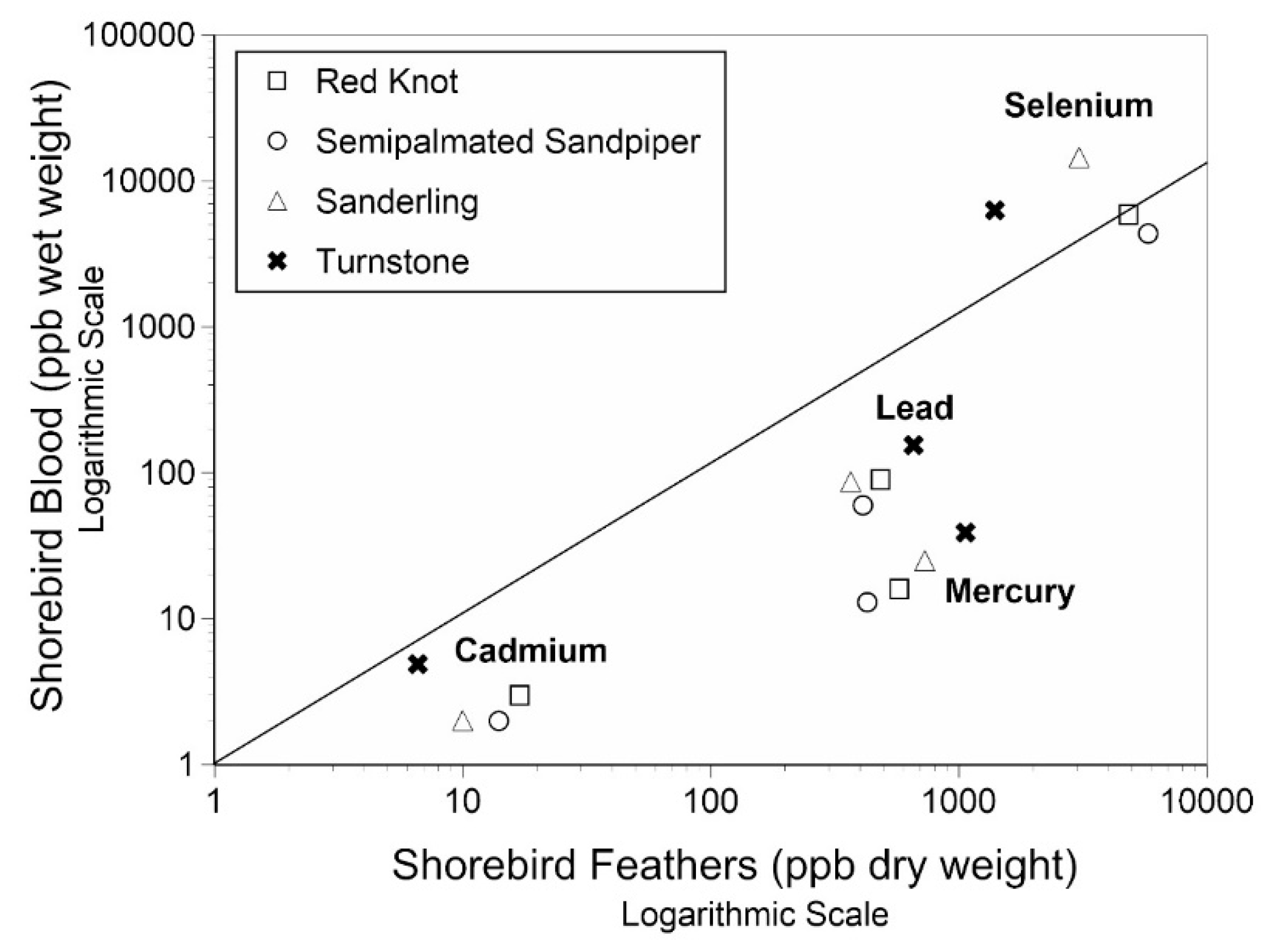
3.4. Relationship between Feathers and Blood in Shorebirds
4. Discussion
4.1. Methodological Issues
4.2. Trophic-Level Relationships
4.3. Metal Levels in the Eggs of Horseshoe Crabs and Blood of Shorebirds
4.4. Relationship of Metals in the Blood and Feathers of Shorebirds
4.5. Effects Levels of Metals for Shorebirds
4.6. Effects in Other Biota
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Endangered Species Act (ESA). Public Law 93–205, as amended, 16USC 1513 et seq.1973. Available online: https://www.epa.gov/laws-regulations/summary-endangered-species-act (accessed on 10 June 2019).
- Fairbrother, A. Federal environmental legislation in the US for protection of wildlife and regulation of environmental contaminants. Ecotoxicology 2009, 18, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Evers, D.C.; Wiener, J.G.; Basu, N.; Bodaly, R.A.; Morrison, H.A.; Williams, K.A. Mercury in the Great Lakes region: Bioaccumulation, spatiotemporal patterns, ecological risks, and policy. Ecotoxicology 2011, 20, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Piersma, T.; Lok, T.; Chen, Y.; Hassell, C.J.; Yang, H.-Y.; Boyle, A.; Slaymaker, M.; Chan, Y.-C.; Melville, D.S.; Zhang, Z.-W.; et al. Simultaneous declines in summer survival of three shorebird species signals in a flyway at risk. J. Appl. Ecol. 2016, 53, 479–490. [Google Scholar] [CrossRef]
- DeVault, T.L.; Rhodes, O.E., Jr.; Shivik, J.A. Scavenging by vertebrates: Behavioral, ecological, and evolutionary perspectives on an important energy transfer pathway in terrestrial ecosystems. Oikos 2003, 102, 225–234. [Google Scholar] [CrossRef]
- Montevecchi, W.A. Binary dietary responses of Northern Gannets (Sula bassana) indicate changing food web and oceanographic conditions. Mar. Ecol. Press Ser. 2008, 352, 213–220. [Google Scholar] [CrossRef]
- Rolfhus, K.R.; Hall, B.D.; Monson, B.A.; Paterson, M.J.; Jeremiason, J.D. Assessment of mercury bioaccumulation within the pelagic food web of lakes in the western Great Lakes region. Ecotoxicology 2011, 20, 1520–1529. [Google Scholar] [CrossRef]
- Mendoza-Carranza, M.M.; Sepulveda-Lozada, A.; Dias-Rerreira, C.; Geissen, V. Distribution and bioconcentration of heavy metals in a tropical aquatic food web: A case study of a tropical estuarine lagoon in SE Mexico. Environ. Pollut. 2016, 210, 155–165. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Habitat, Population Dynamics, and Metal Levels in Colonial Waterbirds: A Food Chain Approach; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Elnoder, L.D.; MacLeod, C.K.; Coughanowr, C. Metal and isotope analysis of bird feathers in a contaminated estuary reveals bioaccumulation, biomagnificaiton, and potential toxic effects. Archiv. Environ. Contam Toxicol. 2018, 75, 96–110. [Google Scholar] [CrossRef]
- Braune, B.M.; Gaston, A.J.; Hobson, K.A.; Gilchrist, H.G.; Mallory, M.L. Changes in trophic position affect rates of contaminant decline in two seabird colonies in the Canadian Arctic. Ecotoxicol. Environ. Saf. 2015, 115, 7–13. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Selenium; US Public Health Service: Atlanta, GA, USA, 1996.
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Lead; US Public Health Service: Atlanta, GA, USA, 2007.
- Eagles-Smith, C.A.; Ackerman, J.T.; Yee, T.; Adelsbach, T.L. Mercury demethylation in waterbird livers: Dose-response thresholds and differences among species. Environ. Toxicol. Chem. 2009, 28, 568–577. [Google Scholar] [CrossRef]
- Eagles-Smith, C.A.; Ackerman, J.T.; De La Cruz, S.E.; Takekawa, J.Y. Mercury bioaccumulation and risk to three waterbird foraging guilds is influenced by foraging ecology and breeding stage. Environ. Pollut. 2009, 157, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Min, B.Y.; Tatsukawa, R. Distribution of heavy metals and their age-related changes in the Eastern Great White Egret, Egretta alba modesta, in Korea. Arch. Environ. Contam. Toxicol. 1986, 15, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Lange, T.R.; Royals, H.E.; Connor, L.L. Mercury accumulation in largemouth bass (Micropterus salmoides) in a Florida Lake. Arch Environ. Contam. Toxicol. 1994, 27, 466–499. [Google Scholar] [CrossRef] [PubMed]
- Bidone, E.D.; Castilhos, Z.C.; Santos, T.J.S.; Souza, T.M.C.; Lacerda, L.D. Fish contamination and human exposure to mercury in Tartarugalzinho River, Northern Amazon, Brazil: A screening approach. Water Air Soil Pollut. 1997, 97, 9–15. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Metals in albatross feathers from Midway Atoll: Influence of species, age, and nest location. Environ. Res. 2000, 82, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Green, N.W.; Knutzen, J. Organohalogens and metals in marine fish and mussels and some relationships to biological variables at reference localities in Norway. Mar. Pollut. Bull. 2003, 46, 362–377. [Google Scholar] [CrossRef]
- Perkins, M.; Ferguson, L.; Lanctot, R.B.; Stenhouse, I.J.; Kendall, S.; Brown, S.; Gates, H.R.; Hall, J.O.; Regan, K.; Evers, D.C. Mercury exposure and risk in breeding and staging Alaskan shorebirds. Condor 2016, 118, 571–582. [Google Scholar] [CrossRef]
- Evers, D.C.; Burgess, N.M.; Champoux, L.; Hoskins, B.; Major, A.; Goodale, W.M.; Taylor, R.J.; Poppenga, R.; Daigle, T. Patterns and interpretation of mercury exposure in freshwater avian communities in northeastern North America. Ecotoxicology 2005, 14, 93–221. [Google Scholar] [CrossRef]
- Seewagen, C.L. Threats of environmental mercury to birds: Knowledge gaps and priorities for future research. Bird Conserv. Intern. 2010, 20, 112–123. [Google Scholar] [CrossRef]
- Clark, K.E.; Niles, L.J.; Burger, J. Abundance and distribution of migrant shorebirds in Delaware Bay. Condor 1993, 95, 694–705. [Google Scholar] [CrossRef]
- Tsipoura, N.; Burger, J. Shorebird diet during spring migration stop-over on Delaware Bay. Condor 1999, 101, 635–644. [Google Scholar] [CrossRef]
- Niles, L.; Sitters, H.; Dey, A.; Atkinson, P.; Baker, A.; Bennett, K.; Carmona, R.; Clark, K.; Clark, N.; Espoz, C.; et al. Status of the red knot (Calidris canutus rufa) in the Western Hemisphere. Stud. Avian Biol. 2008, 36, 1–185. [Google Scholar]
- Niles, L.J.; Bart, J.; Sitters, H.P.; Dey, A.D.; Clark, K.E.; Atkinson, P.W.; Baker, A.J.; Bennett, K.A.; Kalasz, K.S.; Clark, N.A. Effects of horseshoe crab harvest in Delaware Bay on Red Knots: Are harvest restrictions working? BioScience 2009, 59, 153–164. [Google Scholar] [CrossRef]
- Novcic, I.; Mizrahi, D.S.; Veit, R.R.; Symondson, W.O. Molecular analysis of the value of Horseshoe Crab eggs to migrating shorebirds. Avian Biol. Res. 2015, 8, 210–220. [Google Scholar] [CrossRef]
- Gerwing, T.G.; Kim, J.H.; Hamilton, D.J.; Barbeau, M.A.; Addison, J.A. Diet reconstruction using next-generation sequencing increases the known ecosystem usage by a shorebird. Auk Ornithol. Adv. 2016, 133, 168–177. [Google Scholar] [CrossRef]
- Jouta, J.; Dietz, M.W.; Reneerkens, J.; Piersma, T.; Rakhimberdiev, E.; Hallgrímsson, G.T.; Pen, I. Ecological forensics: Using single point stable isotope values to infer seasonal schedules of animals after two diet switches. Methods Ecol. Evol. 2017, 8, 492–500. [Google Scholar] [CrossRef]
- Goede, A.; de Bruin, M. The use of bird feather parts as a monitor for metal pollution. Environ. Pollut. 1984, 8, 281–298. [Google Scholar] [CrossRef]
- Tavares, P.C.; Kelly, A.; Maia, R.; Lopes, R.J.; Serrao-Santos, R.; Pereira, M.E.; Duarte, A.C.; Furness, R.W. Variation in the mobilization of mercury into Black-winged Stilt, Himantopus himantopus, chicks in coastal saltpans, as revealed by stable isotopes. Estuar. Coast. Shelf Sci. 2008, 77, 65–76. [Google Scholar] [CrossRef]
- Tsipoura, N.; Burger, J.; Niles, L.; Dey, A.; Gochfeld, M.; Peck, M.; Mizrahi, D. Metal levels in shorebird feathers and blood during migration through Delaware Bay. Arch. Environ. Contam. Toxicol. 2017, 72, 562–574. [Google Scholar] [CrossRef]
- Clair, C.T.S.; Baird, P.; Ydenberg, R.; Elner, R.; Bendell, L. Trace elements in Pacific Dunlin (Calidris alpina pacifica): Patterns of accumulation and concentrations in kidneys and feathers. Ecotoxicology 2015, 24, 29–44. [Google Scholar] [CrossRef]
- Burger, J.; Tsipoura, N.; Gochfeld, M. Metals levels in blood of three species of shorebirds during stopover reflect levels their food, Horseshoe Crab eggs. Toxics 2017, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.E.G.; Aubrey, Y.; Butler, R.W.; Beyersbergen, G.W.; Donaldson, G.M.; Gratto-Trevor, C.L.; Hicklin, P.W.; Johnson, W.H.; Ross, R.K. Declines in North American shorebird populations. Wader Study Group Bull. 2001, 94, 37–42. [Google Scholar]
- Andres, B.A.; Smith, P.A.; Morrison, R.G.; Gratto-Trevor, C.L.; Brown, S.C.; Friis, C.A. Population estimates of North American shorebirds, 2012. Wader Study Group Bull. 2013, 119, 178–194. [Google Scholar]
- Baker, A.J.; Gonzalez, P.M.; Piersma, T.; Niles, L.J.; deLima, I.; Nascimento, S.; Atkinson, P.W.; Collins, P.; Clark, N.A.; Minton, C.D.T.; et al. Rapid population decline in red knots: Fitness consequences of refuelling rates and late arrival in Delaware Bay. Proc. R. Soc. London/Biol. Sci. 2004, 271, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.; Gonzalez, P.; Morrison, R.I.G.; Harrington, B.A. Red Knot (Calidris canutus). In The Birds of North America Online Cornell Lab of Ornithology; Poole, A.F., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2013; Available online: https://birdsna.org/Species-Account/bna/species/redkno/introduction (accessed on 3 January 2015).
- Andres, B.A.; Gratto-Trevor, C.; Hicklin, P.; Mizrahi, D.; Morrison, R.G.; Smith, P.A. Status of the semipalmated sandpiper. Waterbirds 2012, 35, 146–149. [Google Scholar] [CrossRef]
- Morrison, R.G.; Mizrahi, D.S.; Ross, R.K.; Ottema, O.H.; de Pracontal, N.; Narine, A. Dramatic declines of Semipalmated Sandpipers on their major wintering areas in the Guianas, northern South America. Waterbirds 2012, 35, 120–134. [Google Scholar] [CrossRef]
- Brown, S.; Gratto-Trevor, C.; Porter, R.; Weiser, E.L.; Mizrahi, D.; Bentzen, R.; Boldenow, M.; Clay, R.; Freeman, S.; Giroux, M.A.; et al. Migratory connectivity of Semipalmated Sandpipers and implications for conservation. Condor Ornithol. Appl. 2017, 119, 207–224. [Google Scholar] [CrossRef]
- Burger, J.; Tsipoura, N. Metals in horseshoe crab eggs from Delaware Bay, USA: Temporal patterns from 1993 to 2012. Environ. Monit. Assess. 2014, 186, 6947–6958. [Google Scholar] [CrossRef]
- Furness, R.W.; Rainbow, P.S. Heavy Metals in the Marine Environment; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Ralston, N.V. Introduction to 2nd issue on special topic: Selenium and mercury as interactive environmental indicators. Environ. Bioindic. 2009, 4, 286–290. [Google Scholar] [CrossRef]
- Ralston, N.V.; Raymond, L.J. Dietary selenium’s protective effects against methylmercury toxicity. Toxicology 2001, 278, 112–123. [Google Scholar] [CrossRef]
- Ralston, N.V.C.; Raymond, L.J. Mercury’s neurotoxicity is characterized by its disruption of selenium biochemistry. BBA-Gen. Subject 2018, 1862, 2405–2416. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, W.F.; Engstrom, D.R.; Lamborg, C.J.; Tseng, C.M.; Balcom, P.H.; Hamerschmidt, C.R. Modern and historic atmospheric mercury fluxes in northern Alaska: Global sources and Arctic depletion. Environ. Sci. Technol. 2005, 39, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, C.F.; Fitzgerald, W.F.; Lamborg, C.H.; Balcom, P.H.; Tseng, C.M. Biogeochemical cycling of methylmercury in lakes and tundra watersheds of Arctic Alaska. Environ. Tech. Sci. 2006, 40, 1204–1211. [Google Scholar] [CrossRef]
- Burger, J. Metals in avian feathers: Bioindicators of environmental pollution. Rev. Environ. Toxicol. 1993, 5, 197–306. [Google Scholar]
- Burger, J. Risk to consumers from mercury in bluefish (Pomatomus saltatrix) from New Jersey: Size, season and geographical effects. Environ. Res. 2009, 109, 803–811. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burger, J.; Gochfeld, M. Mercury and selenium levels in 19 species of saltwater fish from New Jersey as a function of species, size, and season. Sci. Total Environ. 2011, 409, 1418–1429. [Google Scholar] [CrossRef]
- Burger, J.; Jeitner, C.; Gochfeld, M. Locational differences in mercury and selenium levels in 19 species of saltwater fish from New Jersey. J. Toxicol. Environ. Health 2011, 74, 863–874. [Google Scholar] [CrossRef]
- Gochfeld, M.; Burger, J.; Jeitner, C.; Donio, M.; Pittfield, T. Seasonal, locational and size variations in mercury and selenium levels in striped bass (Morone saxatilis) from New Jersey. Environ. Res. 2012, 112, 8–19. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M.; Niles, L.; Dey, A.; Jeitner, C.; Pittfield, T.; Tsipoura, N. Metals in tissues of semipalmated sandpipers (Calidris pusilla) from Delaware Bay, New Jersey. Environ. Res. 2014, 133, 362–379. [Google Scholar] [CrossRef]
- Burger, J.; Tsipoura, N.; Niles, L.J.; Gochfeld, M.; Dey, A.; Mizrahi, D. Mercury, lead, cadmium, arsenic, chromium and selenium in feathers of shorebirds during migrating through Delaware Bay, New Jersey: Comparing the 1990s and 2011/2012. Toxics 2015, 3, 63–74. [Google Scholar] [CrossRef]
- Burger, J.; Mizrahi, D.; Jeitnr, C.; Tsipoura, N.; Mobley, J.; Gochfeld, M. Metal and metalloid levels in blood of semipalmated sandpiper (Calidris pusilla) from Brazil, Suriname, and Delaware Bay: Sentinels of exposure to themselves, their prey, and predators that eat them. Environ. Res. (in press). [CrossRef] [PubMed]
- Orzechowsi, S.C.M.; Shipley, J.R.; Pegan, T.M.; Winkler, D.W. Negligible effects of blood sampling on reproductive performance and return rates of tree swallows. J. Field Ornithol. 2019, 90, 2–138. [Google Scholar]
- Wolfe, M.; Schwarzbach, S.; Sulaiman, R.S. Effects of mercury on wildlife: A comprehensive review. Environ. Toxicol. Chem. 1998, 17, 146–160. [Google Scholar] [CrossRef]
- Wiener, J.C.; Krabbenhoft, D.P.; Heinz, G.H.; Scheuhammer, M. Ecotoxicology of mercury. In Handbook of Ecotoxicology; Hoffman, D.J., Rattner, B.A., Burton, G.A., Jr., Cairns, J., Jr., Eds.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Burger, J.; Niles, L. Shorebirds, stakeholders, and competing claims to the beach and intertidal habitat in Delaware Bay, New Jersey, USA. Natural Sci. 2017, 9, 181–205. [Google Scholar] [CrossRef]
- Able, K.W.; Fahay, M.P. The First Year in the Life of Estuarine Fishes in the Middle Atlantic Bight; Rutgers University Press: New Brunswick, NJ, USA, 2014. [Google Scholar]
- Lazarus, R.S.; Roattner, B.S.; Brooks, B.W.; Du, B.; McGowan, P.C.; Blazer, V.W.; Ottinger, M.A. Exposure and food of transfer of pharmaceuticals in osprey (Pandion haliaetus): Predictive model and empirical data. Integr. Environ. Assess. Manag. 2015, 11, 118–129. [Google Scholar] [CrossRef]
- Ruus, A.; Øverjordet, I.B.; Braaten, H.F.V.; Evenset, A.; Christensen, G.; Heimstad, E.S.; Gabrielson, G.W.; Borgå, K. Methylmercury biomagnification in an Arctic pelagic food web. Environ. Toxicol. Chem. 2015, 34, 2636–2643. [Google Scholar] [CrossRef]
- Baudrot, V.; Fritsch, C.; Perasso, A.; Banerjee, M.; Raoul, F. Effects of contaminants and trophic cascade regulation on food chain stability: Application to cadmium soil pollution on small mammals-raptor systems. Ecol. Model. 2018, 382, 33–42. [Google Scholar] [CrossRef]
- Fort, J.; Grémillet, D.; Traisnel, G.; Amélineau, R.; Bustamante, P. Does temporal variation in mercury levels in Arctic seabirds reflect changes in global environmental contamination, or a modification of Arctic marine food web functioning? Environ. Pollut. 2016, 211, 382–388. [Google Scholar] [CrossRef]
- Nygard, T.; Lie, E.; Roy, N.; Steinnes, E. Metal dynamics in an Antarctic food chain. Mar. Pollut. Bull. 2001, 42, 598–602. [Google Scholar] [CrossRef]
- Burger, J. Assessment and management of risk to wildlife from cadmium. Sci. Total Environ. 2008, 389, 37–45. [Google Scholar] [CrossRef]
- Gochfeld, M.; Burger, J. Biological concentration of cadmium in estuarine birds of the New York Bight. Colonial Waterbirds 1982, 5, 116–123. [Google Scholar] [CrossRef]
- Eisler, R. Selenium. In Handbook of Chemical Risk Assessment: Health Hazards to Humans, Plants and Animals; CRC Press: Boca Raton, FL, USA, 2000; Volume 1. [Google Scholar]
- Vinceti, M.; Wei, E.T.; Malagoli, C.; Bergomi, M.; Vivoli, G. Adverse health effects of selenium in humans. Rev. Environ. Health 2001, 16, 233–251. [Google Scholar] [CrossRef] [PubMed]
- Eisler, R. Mercury Hazards to Fish, Wildlife and Invertebrates: A Synoptic Review; Report 10; Biological Report 85(1.10); U.S. Department of the Interior, Fish and Wildlife Service: Laurel, MD, USA, 1987.
- Agency for Toxic Substances and Disease Registry (ATSDR). Addentum to the Toxicological Profile for Mercury (Alkyl and Dialkyl Compounds); US Public Health Service: Atlanta, GA, USA, 2013.
- Church, T.M.; Sommerfield, C.K.; Velinsky, D.J.; Point, D.; Benoit, C.; Amouraux, D.; Plaa, D.; Donard, O.F.X. Marsh sediments as records of sedimentation, eutrophication and metal pollution in the urban Delaware Estuary. Mar. Chem. 2006, 102, 72–95. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Northeast National Estuary Program Coastal Condition, Partnership for the Delaware Estuary; EPA: Washington, DC, USA, 2007.
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Mercury; US Public Health Service: Atlanta, GA, USA, 1999.
- Mason, R.P. Mercury Concentrations in Fish from Tidal Waters of the Chesapeake Bay. Arch. Environ. Contam. Toxicol. 2006, 51, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Vallius, H. Heavy metal concentrations in sediment cores from the northern Baltic Sea: Declines over the last two decades. Mar. Pollut. Bull. 2013, 79, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.; Mizrahi, D.; Tsipoura, N.; Jeitner, C.; Gochfeld, M. Mercury, lead, cadmium, cobalt, arsenic and selenium in blood of semipalmated sandpipers (Calidris pusilla) from Suriname, South America: Age-related differences on a wintering site and comparisons with a stopover site in New Jersey, USA. Toxics 2018, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Eisler, R. Cadmium Hazards to Fish, Wildlife and Invertebrates: A Synoptic Review; Report 2; Biological Report 85(1.2); U.S. Department of the Interior, Fish and Wildlife Service: Laurel, MD, USA, 1985.
- Eisler, R. Lead Hazards to Fish, Wildlife and Invertebrates: A Synoptic Review; Report 14; Biological Report 85(1.14); U.S. Department of the Interior, Fish and Wildlife Service: Laurel, MD, USA, 1988.
- Burger, J.; Gochfeld, M. Effects of chemicals and pollution on seabirds. In Biology of Marine Birds; Schreiber, B.A., Burger, J., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 485–525. [Google Scholar]
- Jackson, A.K.; Evers, D.C.; Matthew, A.; Etterson, M.A.; Condon, A.N.; Folsom, S.B.; Detweiler, J.; Schmerfeld, J.; Cristol, D.S. Mercury exposure affects the reproductive success of a free-living terrestrial songbird, the Carolina Wren (Thryothorus ludovicianus). Auk 2011, 128, 759–769. [Google Scholar] [CrossRef]
- Ohlendorf, H.M.; Hothem, R.L.; Welsh, D. Nest success, cause-specific nest failure, and hatchability of aquatic birds at selenium-contaminated Kesterson Reservoir and a reference site. Condor 1989, 91, 787–796. [Google Scholar] [CrossRef]
- Heinz, G.H. Selenium in birds. In Environmental Contaminants in Wildlife: Interpreting Tissue Concentrations; Beyer, W.M., Heinz, W.M., Eds.; CRC Press: Boca Raton, FL, USA, 1996; pp. 447–458. [Google Scholar]
- Burger, J.; Gochfeld, M. Selenium/mercury molar ratios in freshwater, marine, and commercial fish from the USA: Variation, risk, and health management. Rev. Environ. Health 2013, 28, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Trull, P. Shorebirds and noodles. Am. Birds 1983, 37, 268–269. [Google Scholar]
- Ottema, O.H.; Spaans, A.L. Challenges and advances in shorebird conservation in the Guianas, with a focus on Suriname. Ornithol. Neotrop. 2008, 19, 339–346. [Google Scholar]
- Wege, D.C.; Birke, W.; Reed, E.T. Migratory Shorebirds in Barbados: Hunting, Management and Conservation. Available online: https://www.cms.int/sites/default/files/document/Shorebird Conservation Trust.pdf (accessed on 10 June 2019).
- Watts, B.D.; Reed, E.T.; Turrin, C. Estimating sustainable mortality limits for shorebirds using the Western Atlantic Flyway. Wader Study 2015, 122, 37–53. [Google Scholar] [CrossRef]
- Yeardley, R.B.; Lazorchak, J.M.; Paulsen, S.G. Elemental fish tissue contamination in northeastern US lakes: Evaluation of an approach to regional assessment. Environ. Toxicol. Chem. 1998, 17, 1875–1884. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Human Health Criteria—Methylmercury. Available online: https://www.epa.gov/wqc/human-health-criteria-methylmercury, 2010 (accessed on 10 June 2019).
- Hites, R.A.; Foran, J.A.; Carpenter, D.O.; Hamilton, M.C.; Knuth, B.A.; Schwater, S.J. Global assessment of organic contaminants in farmed salmon. Science 2004, 303, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (IOM). Seafood Choices: Balancing Benefits and Risks; National Academy of Press: Washington, DC, USA, 2006. [Google Scholar]
| Trophic Level | Cadmium | Lead | Mercury | Selenium | ||
|---|---|---|---|---|---|---|
| Algae and plants (ppb ww) b | a | 65–80 | 4–6 | a | ||
| Horseshoe Crabs | ||||||
| Eggs (ww) c | 0.4 ± 0.2 | 25 ± 5 | 1 ± 0.1 | 996 ± 137 | ||
| Muscle (ww) | 37 ± 6 | 41 ± 4 | 57 ± 5 | 876 ± 39 | ||
| Other Invertebrates | 4–30 | 22–32 | 11–32 | 160–230 | ||
| Small prey fish (whole) | 2–5 | 20–300 | 20–51 | 411–577 | ||
| Flounder | 10 ± 2 | 60 ± 10 | 150 ± 10 | 360 ± 100 | ||
| Shorebirds | ||||||
| Red Knot | ||||||
| Feather | 17 ± 2.4 | 484 ± 67 | 576 ± 105 | 4835 ± 432 | ||
| Blood | 3 ± 0.7 | 90 ± 12 | 16 ± 3.1 | 5873 ± 573 | ||
| Turnstone | ||||||
| Feather | 7 ± 1.4 | 658 ± 93 | 1065 ± 208 | 1398 ± 176 | ||
| Blood | 5 ± 0.8 | 155 ± 27 | 40 ± 6.7 | 6294 ± 785 | ||
| Sanderling | ||||||
| Feather | 10 ± 2.6 | 367 ± 52 | 730 ± 109 | 3057 ± 781 | ||
| Blood | 2 ± 0.7 | 87 ± 14 | 25 ± 5.3 | 14500 ± 2300 | ||
| Semipalmated Sandpiper | ||||||
| Feather | 14 ± 2.7 | 411 ± 46 | 428 ± 58 | 5802 ± 562 | ||
| Blood | 2 ± 0.5 | 60 ± 11 | 13 ± 3.3 | 4422 ± 470 | ||
| Bluefish | 6 ± 2 | 60 ± 10 | 300 ± 30 | 510 ± 40 | ||
| Striped Bass | 0.6 ± 0.3 | 16 ± 4 | 740 ± 20 | 290 ± 20 | ||
| Laughing Gull (feathers) | 1 ± 2 | 510 ± 35 | 650 ± 45 | 910 ± 48 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burger, J.; Tsipoura, N.; Niles, L.; Dey, A.; Jeitner, C.; Gochfeld, M. Heavy Metals in Biota in Delaware Bay, NJ: Developing a Food Web Approach to Contaminants. Toxics 2019, 7, 34. https://doi.org/10.3390/toxics7020034
Burger J, Tsipoura N, Niles L, Dey A, Jeitner C, Gochfeld M. Heavy Metals in Biota in Delaware Bay, NJ: Developing a Food Web Approach to Contaminants. Toxics. 2019; 7(2):34. https://doi.org/10.3390/toxics7020034
Chicago/Turabian StyleBurger, Joanna, Nellie Tsipoura, Larry Niles, Amanda Dey, Christian Jeitner, and Michael Gochfeld. 2019. "Heavy Metals in Biota in Delaware Bay, NJ: Developing a Food Web Approach to Contaminants" Toxics 7, no. 2: 34. https://doi.org/10.3390/toxics7020034
APA StyleBurger, J., Tsipoura, N., Niles, L., Dey, A., Jeitner, C., & Gochfeld, M. (2019). Heavy Metals in Biota in Delaware Bay, NJ: Developing a Food Web Approach to Contaminants. Toxics, 7(2), 34. https://doi.org/10.3390/toxics7020034






