Recent Studies on DNA Adducts Resulting from Human Exposure to Tobacco Smoke
Abstract
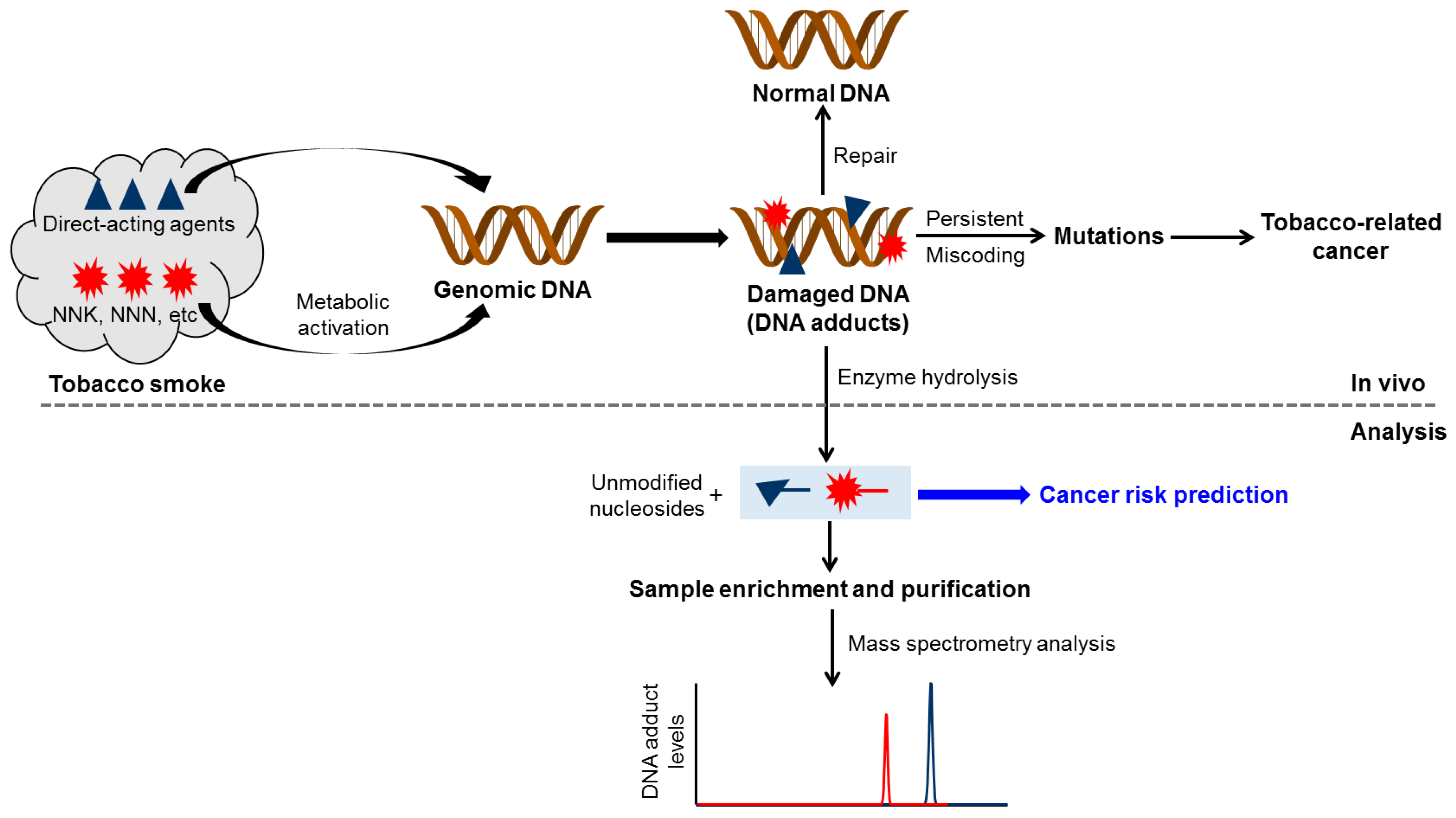
1. Introduction
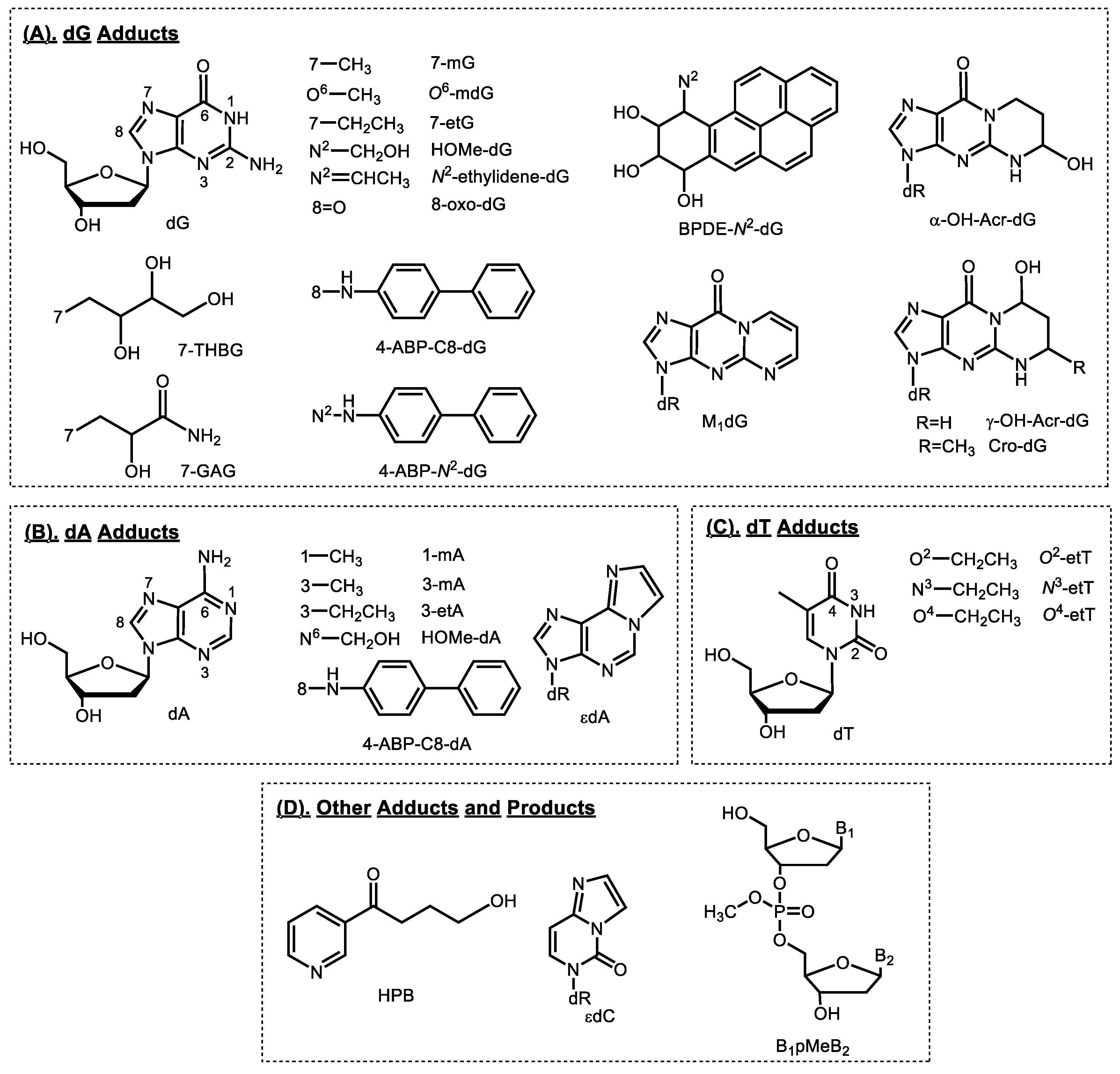
2. Smoking-Related DNA Adducts in Humans
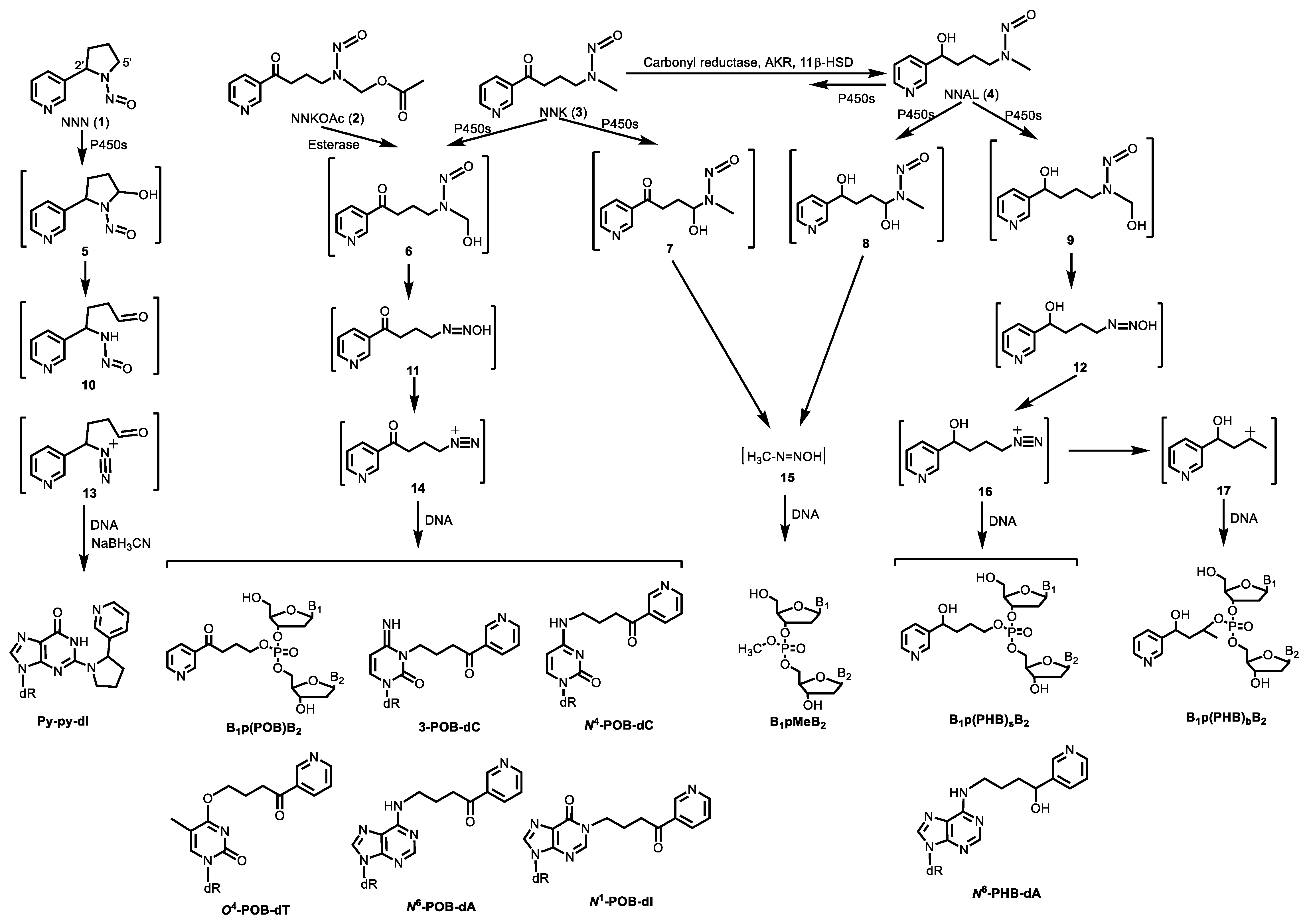
2.1. Tobacco-specific Nitrosamines (TSNA)
2.2. Bulky/Aromatic Adducts
2.3. Aromatic and Heterocyclic Aromatic Amines
2.4. Methylating Agents
2.5. Ethylating Agents
2.6. 1,3-Butadiene
2.7. Acrolein
2.8. Formaldehyde
2.9. Acetaldehyde and Crotonaldehyde
2.10. Acrylamide
2.11. Oxidative Damage
3. Newly Characterized Tobacco-Specific DNA Adducts
3.1. DNA Phosphate Adducts
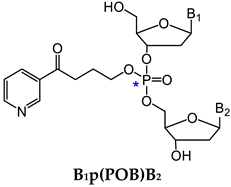
3.1.1. B1p(POB)B2
3.1.2. B1p(PHB)B2
3.1.3. B1pMeB2
3.2. DNA Base Adducts
3.2.1. Py-py-dI
3.2.2. 3-POB-dC and N4-POB-dC
3.2.3. O4-POB-dT
3.2.4. dA Adducts
4. Perspectives
4.1. Current Limitations
4.2. Analytical Methodologies
4.3. Artifactual Formation
4.4. Other Considerations
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Cancer. Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 30 January 2018).
- Centers for Disease Control and Prevention. Tobacco-Related Mortality. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/tobacco_related_mortality/index.htm (accessed on 30 January 2018).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Thun, M.J.; Henley, S.J.; Calle, E.E. Tobacco use and cancer: An epidemiologic perspective for geneticists. Oncogene 2002, 21, 7307–7325. [Google Scholar] [CrossRef]
- World Health Organization. Tobacco. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 30 January 2018).
- Yuan, J.M.; Butler, L.M.; Stepanov, I.; Hecht, S.S. Urinary tobacco smoke-constituent biomarkers for assessing risk of lung cancer. Cancer Res. 2014, 74, 401–411. [Google Scholar] [CrossRef]
- Hecht, S.S.; Stepanov, I.; Carmella, S.G. Exposure and metabolic activation biomarkers of carcinogenic tobacco-specific nitrosamines. Acc. Chem. Res. 2016, 49, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.M.; Knezevich, A.D.; Wang, R.; Gao, Y.T.; Hecht, S.S.; Stepanov, I. Urinary levels of the tobacco-specific carcinogen N′-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis 2011, 32, 1366–1371. [Google Scholar] [CrossRef]
- Yuan, J.M.; Nelson, H.H.; Carmella, S.G.; Wang, R.; Kuriger-Laber, J.; Jin, A.; Adams-Haduch, J.; Hecht, S.S.; Koh, W.P.; Murphy, S.E. CYP2A6 genetic polymorphisms and biomarkers of tobacco smoke constituents in relation to risk of lung cancer in the Singapore Chinese Health Study. Carcinogenesis 2017, 38, 411–418. [Google Scholar] [CrossRef]
- Phillips, D.H.; Venitt, S. DNA and protein adducts in human tissues resulting from exposure to tobacco smoke. Int. J. Cancer 2012, 131, 2733–2753. [Google Scholar] [CrossRef] [PubMed]
- Sabbioni, G.; Turesky, R.J. Biomonitoring human albumin adducts: The past, the present, and the future. Chem. Res. Toxicol. 2017, 30, 332–366. [Google Scholar] [CrossRef]
- Phillips, D.H. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis 2002, 23, 1979–2004. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Lung carcinogenesis by tobacco smoke. Int. J. Cancer 2012, 131, 2724–2732. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Smokeless tobacco and some tobacco-specific N-nitrosamines. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2007; Volume 89, pp. 1–592. [Google Scholar]
- Hecht, S.S. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol. 1998, 11, 559–603. [Google Scholar] [CrossRef]
- Bustamante, G.; Ma, B.; Yakovlev, G.; Yershova, K.; Le, C.; Jensen, J.; Hatsukami, D.K.; Stepanov, I. Presence of the carcinogen N′-nitrosonornicotine in saliva of e-cigarette users. Chem. Res. Toxicol. 2018, 31, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.; Adams, J.D. Carcinogenic tobacco-specific N-nitrosamines in snuff and in the saliva of snuff dippers. Cancer Res. 1981, 41, 4305–4308. [Google Scholar]
- Porubin, D.; Hecht, S.S.; Li, Z.Z.; Gonta, M.; Stepanov, I. Endogenous formation of N′-nitrosonornicotine in F344 rats in the presence of some antioxidants and grape seed extract. J. Agric. Food Chem. 2007, 55, 7199–7204. [Google Scholar] [CrossRef] [PubMed]
- Carlson, E.S.; Upadhyaya, P.; Villalta, P.W.; Ma, B.; Hecht, S.S. Analysis and identification of 2′-deoxyadenosine-derived adducts in lung and liver DNA of F-344 rats treated with the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyi)-1-butanone and enantiomers of its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem. Res. Toxicol. 2018, 31, 358–370. [Google Scholar]
- Ma, B.; Villalta, P.W.; Zarth, A.T.; Kotandeniya, D.; Upadhyaya, P.; Stepanov, I.; Hecht, S.S. Comprehensive high-resolution mass spectrometric analysis of DNA phosphate adducts formed by the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Chem. Res. Toxicol. 2015, 28, 2151–2159. [Google Scholar] [CrossRef]
- Michel, A.K.; Zarth, A.T.; Upadhyaya, P.; Hecht, S.S. Identification of 4-(3-pyridyl)-4-oxobutyl-2′-deoxycytidine adducts formed in the reaction of DNA with 4-(acetoxymethylnitrosamino)1-(3-pyridyl)-1-butanone: A chemically activated form of tobacco-specific carcinogens. ACS Omega 2017, 2, 1180–1190. [Google Scholar] [CrossRef]
- Lao, Y.; Yu, N.; Kassie, F.; Villalta, P.W.; Hecht, S.S. Analysis of pyridyloxobutyl DNA adducts in F344 rats chronically treated with (R)- and (S)-N′-nitrosonornicotine. Chem. Res. Toxicol. 2007, 20, 246–256. [Google Scholar] [CrossRef]
- Schlobe, D.; Holzle, D.; Hatz, D.; von Meyer, L.; Tricker, A.R.; Richter, E. 4-Hydroxy-1-(3-pyridyl)-1-butanone-releasing DNA adducts in lung, lower esophagus and cardia of sudden death victims. Toxicology 2008, 245, 154–161. [Google Scholar] [CrossRef]
- Hoelzle, D.; Schlobe, D.; Tricker, A.R.; Richter, E. Mass spectrometric analysis of 4-hydroxy-1-(3-pyridyl)-1-butanone-releasing DNA adducts in human lung. Toxicology 2007, 232, 277–285. [Google Scholar] [CrossRef]
- Foiles, P.G.; Akerkar, S.A.; Carmella, S.G.; Kagan, M.; Stoner, G.D.; Resau, J.H.; Hecht, S.S. Mass-spectrometric analysis of tobacco-specific nitrosamine DNA adducts in smokers and nonsmokers. Chem. Res. Toxicol. 1991, 4, 364–368. [Google Scholar] [CrossRef]
- Hecht, S.S. Oral cell DNA adducts as potential biomarkers for lung cancer susceptibility in cigarette smokers. Chem. Res. Toxicol. 2017, 30, 367–375. [Google Scholar] [CrossRef]
- Tan, D.J.; Goerlitz, D.S.; Dumitrescu, R.G.; Han, D.F.; Seillier-Moiseiwitsch, F.; Spernak, S.M.; Orden, R.A.; Chen, J.G.; Goldman, R.; Shields, P.G. Associations between cigarette smoking and mitochondrial DNA abnormalities in buccal cells. Carcinogenesis 2008, 29, 1170–1177. [Google Scholar] [CrossRef]
- Stepanov, I.; Muzic, J.; Le, C.T.; Sebero, E.; Villalta, P.; Ma, B.; Jensen, J.; Hatsukami, D.; Hecht, S.S. Analysis of 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB)-releasing DNA adducts in human exfoliated oral mucosa cells by liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem. Res. Toxicol. 2013, 26, 37–45. [Google Scholar] [CrossRef]
- Ma, B.; Ruszczak, C.; Jain, V.P.; Khariwala, S.S.; Lindgren, B.; Hatsukami, D.K.; Stepanoy, I. Optimized liquid chromatography nanoelectrospray-high-resolution tandem mass spectrometry method for the analysis of 4-hydroxy-1-(3-pyridyl)-1-butanone-releasing DNA adducts in human oral cells. Chem. Res. Toxicol. 2016, 29, 1849–1856. [Google Scholar] [CrossRef]
- Yang, J.; Villalta, P.W.; Upadhyaya, P.; Hecht, S.S. Analysis of O6-[4-(3-pyridyl)-4-oxobut-1-yl]-2′-deoxyguanosine and other DNA adducts in rats treated with enantiomeric or racemic N′-Nitrosonornicotine. Chem. Res. Toxicol. 2016, 29, 87–95. [Google Scholar] [CrossRef]
- Zarth, A.T.; Upadhyaya, P.; Yang, J.; Hecht, S.S. DNA adduct formation from metabolic 5′-hydroxylation of the tobacco-specific carcinogen N′-nitrosonornicotine in human enzyme systems and in rats. Chem. Res. Toxicol. 2016, 29, 380–389. [Google Scholar] [CrossRef]
- Villalta, P.W.; Hochalter, J.B.; Hecht, S.S. Ultrasensitive high-resolution mass spectrometric analysis of a DNA adduct of the carcinogen benzo[a]pyrene in human lung. Anal. Chem. 2017, 89, 12735–12742. [Google Scholar] [CrossRef]
- Ma, B.; Villalta, P.W.; Balbo, S.; Stepanov, I. Analysis of a malondialdehyde-deoxyguanosine adduct in human leukocyte DNA by liquid chromatography nanoelectrospray-high-resolution tandem mass spectrometry. Chem. Res. Toxicol. 2014, 27, 1829–1836. [Google Scholar] [CrossRef]
- Ma, B.; Zarth, A.T.; Carlson, E.S.; Villalta, P.W.; Upadhyaya, P.; Stepanov, I.; Hecht, S.S. Methyl DNA phosphate adduct formation in rats treated chronically with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite 4-(methylnitrosamino)-1-(3-pyridyi)-1-butanol. Chem. Res. Toxicol. 2018, 31, 48–57. [Google Scholar] [CrossRef]
- Ma, B.; Zarth, A.T.; Carlson, E.S.; Villalta, P.W.; Stepanov, I.; Hecht, S.S. Pyridylhydroxybutyl and pyridyloxobutyl DNA phosphate adduct formation in rats treated chronically with enantiomers of the tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Mutagenesis 2017, 32, 561–570. [Google Scholar]
- Ma, B.; Zarth, A.T.; Carlson, E.S.; Villalta, P.W.; Upadhyaya, P.; Stepanov, I.; Hecht, S.S. Identification of more than 100 structurally unique DNA-phosphate adducts formed during rat lung carcinogenesis by the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis 2018, 39, 232–241. [Google Scholar] [CrossRef]
- Munnia, A.; Giese, R.W.; Polvani, S.; Galli, A.; Cellai, F.; Peluso, M.E.M. Bulky DNA adducts, tobacco smoking, genetic susceptibility, and lung cancer risk. Adv. Clin. Chem. 2017, 81, 231–277. [Google Scholar]
- Poirier, M.C. Linking DNA adduct formation and human cancer risk in chemical carcinogenesis. Environ. Mol. Mutagen. 2016, 57, 499–507. [Google Scholar] [CrossRef]
- Rynning, I.; Arlt, V.M.; Vrbova, K.; Neca, J.; Rossner, P., Jr.; Klema, J.; Ulvestad, B.; Petersen, E.; Skare, O.; Haugen, A.; et al. Bulky DNA adducts, microRNA profiles, and lipid biomarkers in Norwegian tunnel finishing workers occupationally exposed to diesel exhaust. Occup. Environ. Med. 2019, 76, 10–16. [Google Scholar] [CrossRef]
- Gilberson, T.; Peluso, M.E.M.; Munia, A.; Lujan-Barroso, L.; Sanchez, M.J.; Navarro, C.; Amiano, P.; Barricarte, A.; Quiros, J.R.; Molina-Montes, E.; et al. Aromatic adducts and lung cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Spanish cohort. Carcinogenesis 2014, 35, 2047–2054. [Google Scholar]
- Pavanello, S.; Carta, A.; Mastrangelo, G.; Campisi, M.; Arici, C.; Porru, S. Relationship between telomere length, genetic traits and environmental/occupational exposures in bladder cancer risk by structural equation modelling. Int. J. Environ. Res. Public Health 2017, 15, 5. [Google Scholar] [CrossRef]
- Mastrangelo, G.; Carta, A.; Arici, C.; Pavanello, S.; Porru, S. An etiologic prediction model incorporating biomarkers to predict the bladder cancer risk associated with occupational exposure to aromatic amines: A pilot study. J. Occup. Med. Toxicol. 2017, 12, 23. [Google Scholar] [CrossRef]
- Porru, S.; Pavanello, S.; Carta, A.; Arici, C.; Simeone, C.; Izzotti, A.; Mastrangelo, G. Complex relationships between occupation, environment, DNA adducts, genetic polymorphisms and bladder cancer in a case-control study using a structural equation modeling. PLoS ONE 2014, 9, e94566. [Google Scholar]
- Agudo, A.; Peluso, M.; Munnia, A.; Lujan-Barroso, L.; Barricarte, A.; Amiano, P.; Navarro, C.; Sanchez, M.J.; Quiros, J.R.; Ardanaz, E.; et al. Aromatic DNA adducts and breast cancer risk: A case-cohort study within the EPIC-Spain. Carcinogenesis 2017, 38, 691–698. [Google Scholar]
- Molina, E.; Perez-Morales, R.; Rubio, J.; Petrosyan, P.; Cadena, L.H.; Arlt, V.M.; Phillips, D.H.; Gonsebatt, M.E. The GSTM1null (deletion) and MGMT84 rs12917 (Phe/Phe) haplotype are associated with bulky DNA adduct levels in human leukocytes. Mutat. Res. 2013, 758, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Schoket, B.; Godschalk, R.W.; Wright, J.; von Stedingk, H.; Tornqvist, M.; Sunyer, J.; Nielsen, J.K.; Merlo, D.F.; Mendez, M.A.; et al. Bulky DNA adducts in cord blood, maternal fruit-and-vegetable consumption, and birth weight in a European Mother-Child Study (NewGeneris). Environ. Health Perspect. 2013, 121, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.; Antic, R.; Berni, A.; Dallner, G.; Dettbarn, G.; Gromadzinska, J.; Joksic, G.; Lundin, C.; Palitti, F.; Prochazka, G.; et al. Exposure to polycyclic aromatic hydrocarbons in women from Poland, Serbia and Italy—Relation between PAH metabolite excretion, DNA damage, diet and genotype (the EU DIEPHY project). Biomarkers 2013, 18, 165–173. [Google Scholar] [CrossRef]
- Peluso, M.; Bollati, V.; Munnia, A.; Srivatanakul, P.; Jedpiyawongse, A.; Sangrajrang, S.; Piro, S.; Ceppi, M.; Bertazzi, P.A.; Boffetta, P.; et al. DNA methylation differences in exposed workers and nearby residents of the Ma Ta Phut industrial estate, Rayong, Thailand. Int. J. Epidemiol. 2012, 41, 1753–1760. [Google Scholar] [CrossRef]
- Pedersen, M.; Halldorsson, T.I.; Autrup, H.; Brouwer, A.; Besselink, H.; Loft, S.; Knudsen, L.E. Maternal diet and dioxin-like activity, bulky DNA adducts and micronuclei in mother-newborns. Mutat. Res. 2012, 734, 12–19. [Google Scholar] [CrossRef]
- Agudo, A.; Peluso, M.; Munnia, A.; Lujan-Barroso, L.; Sanchez, M.J.; Molina-Montes, E.; Sanchez-Cantalejo, E.; Navarro, C.; Tormo, M.J.; Chirlaque, M.D.; et al. Aromatic DNA adducts and risk of gastrointestinal cancers: A case-cohort study within the EPIC-Spain. Cancer Epidemiol. Prev. Biomark. 2012, 21, 685–692. [Google Scholar] [CrossRef]
- Gu, D.; Turesky, R.J.; Tao, Y.Q.; Langouet, S.A.; Nauwelaers, G.C.; Yuan, J.M.; Yee, D.; Yu, M.M.C. DNA adducts of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and 4-aminobiphenyl are infrequently detected in human mammary tissue by liquid chromatography/tandem mass spectrometry. Carcinogenesis 2012, 33, 124–130. [Google Scholar] [CrossRef]
- Lee, M.S.; Asomaning, K.; Su, L.; Wain, J.C.; Mark, E.J.; Christiani, D.C. MTHFR polymorphisms, folate intake and carcinogen DNA adducts in the lung. Int. J. Cancer 2012, 131, 1203–1209. [Google Scholar] [CrossRef]
- Weng, M.W.; Lee, H.W.; Park, S.H.; Hu, Y.; Wang, H.T.; Chen, L.C.; Rom, W.N.; Huang, W.C.; Lepor, H.; Wu, X.R.; et al. Aldehydes are the predominant forces inducing DNA damage and inhibiting DNA repair in tobacco smoke carcinogenesis. Proc. Natl. Acad. Sci. USA 2018, 115, E6152–E6161. [Google Scholar] [CrossRef]
- Jin, Y.T.; Xu, P.W.; Liu, X.N.; Zhang, C.Y.; Tan, C.; Chen, C.M.; Sun, X.Y.; Xu, Y.C. Cigarette smoking, BPDE-DNA adducts, and aberrant promoter methylations of tumor suppressor genes (TSGs) in NSCLC from Chinese population. Cancer Investig. 2016, 34, 173–180. [Google Scholar] [CrossRef]
- Niehoff, N.; White, A.J.; McCullough, L.E.; Steck, S.E.; Beyea, J.; Mordukhovich, I.; Shen, J.; Neugut, A.I.; Conway, K.; Santella, R.M.; et al. Polycyclic aromatic hydrocarbons and postmenopausal breast cancer: An evaluation of effect measure modification by body mass index and weight change. Environ. Res. 2017, 152, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, R.N.; Makwela, M.H.; Chuturgoon, A.; Tiloke, C.; Ramkaran, P.; Phulukdaree, A. Petrol exposure and DNA integrity of peripheral lymphocytes. Int. Arch. Occup. Environ Health 2016, 89, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.Y.; Tung, J.N.; Su, M.C.; Wu, B.C.; Hsin, C.H.; Chen, Y.J.; Yeh, K.T.; Lee, H.; Cheng, Y.W. BPDE-like DNA adduct level in oral tissue may act as a risk biomarker of oral cancer. Arch. Oral Biol. 2013, 58, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Rundle, A.; Richards, C.; Neslund-Dudas, C.; Tang, D.L.; Rybicki, B.A. Neighborhood socioeconomic status modifies the association between individual smoking status and PAH-DNA adduct levels in prostate tissue. Environ. Mol. Mutagen. 2012, 53, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Pavanello, S.; Favretto, D.; Brugnone, F.; Mastrangelo, G.; Dal Pra, G.; Clonfero, E. HPLC/fluorescence determination of anti-BPDE-DNA adducts in mononuclear white blood cells from PAH-exposed humans. Carcinogenesis 1999, 20, 431–435. [Google Scholar] [CrossRef]
- Ewa, B.; Danuta, M.S. Polycyclic aromatic hydrocarbons and PAH-related DNA adducts. J. Appl. Genet. 2017, 58, 321–330. [Google Scholar] [CrossRef]
- Monien, B.H.; Schumacher, F.; Herrmann, K.; Glatt, H.; Turesky, R.J.; Chesne, C. Simultaneous detection of multiple DNA adducts in human lung samples by isotope-dilution UPLC-MS/MS. Anal. Chem. 2015, 87, 641–648. [Google Scholar] [CrossRef]
- Beland, F.A.; Churchwell, M.I.; Von Tungeln, L.S.; Chen, S.J.; Fu, P.P.; Culp, S.J.; Schoket, B.; Gyorffy, E.; Minarovits, J.; Poirier, M.C.; et al. High-performance liquid chromatography electrospray ionization tandem mass spectrometry for the detection and quantitation of benzo[a]pyrene-DNA adducts. Chem. Res. Toxicol. 2005, 18, 1306–1315. [Google Scholar] [CrossRef]
- Bessette, E.E.; Spivack, S.D.; Goodenough, A.K.; Wang, T.; Pinto, S.; Kadlubar, F.F.; Turesky, R.J. Identification of carcinogen DNA adducts in human saliva by linear quadrupole ion trap/multistage tandem mass spectrometry. Chem. Res. Toxicol. 2010, 23, 1234–1244. [Google Scholar] [CrossRef]
- Bessette, E.E.; Goodenough, A.K.; Langouet, S.; Yasa, I.; Kozekov, I.D.; Spivack, S.D.; Turesky, R.J. Screening for DNA adducts by data-dependent constant neutral loss-triple stage mass spectrometry with a linear quadrupole ion trap mass spectrometer. Anal. Chem. 2009, 81, 809–819. [Google Scholar] [CrossRef]
- Xiao, S.; Guo, J.; Yun, B.H.; Villalta, P.W.; Krishna, S.; Tejpaul, R.; Murugan, P.; Weight, C.J.; Turesky, R.J. Biomonitoring DNA adducts of cooked meat carcinogens in human prostate by nano liquid chromatography-high resolution tandem mass spectrometry: Identification of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine DNA adduct. Anal. Chem. 2016, 88, 12508–12515. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Some aromatic amines, organic dyes, and related exposures. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2010; Volume 99, pp. 1–658. [Google Scholar]
- Guo, J.; Villalta, P.W.; Weight, C.J.; Bonala, R.; Johnson, F.; Rosenquist, T.A.; Turesky, R.J. Targeted and untargeted detection of DNA adducts of aromatic amine carcinogens in human bladder by ultra-performance liquid chromatography-high-resolution mass spectrometry. Chem. Res. Toxicol. 2018, 31, 1382–1397. [Google Scholar] [CrossRef]
- Lee, H.W.; Wang, H.T.; Weng, M.W.; Hu, Y.; Chen, W.S.; Chou, D.; Liu, Y.; Donin, N.; Huang, W.C.; Lepor, H.; et al. Acrolein- and 4-aminobiphenyl-DNA adducts in human bladder mucosa and tumor tissue and their mutagenicity in human urothelial cells. Oncotarget 2014, 5, 3526–3540. [Google Scholar] [CrossRef] [PubMed]
- Faraglia, B.; Chen, S.Y.; Gammon, M.D.; Zhang, Y.J.; Teitelbaum, S.L.; Neugut, A.I.; Ahsan, H.; Garbowski, G.C.; Hibshoosh, H.; Lin, D.X.; et al. Evaluation of 4-aminobiphenyl-DNA adducts in human breast cancer: The influence of tobacco smoke. Carcinogenesis 2003, 24, 719–725. [Google Scholar] [CrossRef]
- Peterson, L.A. Context matters: Contribution of specific DNA adducts to the genotoxic properties of the tobacco-specific nitrosamine NNK. Chem. Res. Toxicol. 2017, 30, 420–433. [Google Scholar] [CrossRef]
- Wu, J.; Wang, P.; Wang, Y. Cytotoxic and mutagenic properties of alkyl phosphotriester lesions in Escherichia coli cells. Nucleic Acids Res. 2018, 46, 4013–4021. [Google Scholar] [CrossRef]
- Marushige, K.; Marushige, Y. Template properties of DNA alkylated with N-methyl-N-nitrosourea and N-ethyl-N-nitrosourea. Chem. Biol. Interact. 1983, 46, 179–188. [Google Scholar] [CrossRef]
- He, C.; Hus, J.C.; Sun, L.J.; Zhou, P.; Norman, D.P.; Dotsch, V.; Wei, H.; Gross, J.D.; Lane, W.S.; Wagner, G.; et al. A methylation-dependent electrostatic switch controls DNA repair and transcriptional activation by E. coli ada. Mol. Cell 2005, 20, 117–129. [Google Scholar] [CrossRef]
- Crosbie, P.A.J.; Harrison, K.; Shah, R.; Watson, A.J.; Agius, R.; Barber, P.V.; Margison, G.P.; Povey, A.C. Topographical study of O6-alkylguanine DNA alkyltransferase repair activity and N7-methylguanine levels in resected lung tissue. Chem.-Biol. Interact. 2013, 204, 98–104. [Google Scholar] [CrossRef]
- Wang, Y.; Narayanapillai, S.; Hu, Q.; Fujioka, N.; Xing, C.G. Contribution of tobacco use and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone to three methyl DNA adducts in urine. Chem. Res. Toxicol. 2018, 31, 836–838. [Google Scholar] [CrossRef]
- Hu, K.; Zhao, G.; Liu, J.W.; Jia, L.Z.; Xie, F.W.; Zhang, S.S.; Liu, H.M.; Liu, M.Y. Simultaneous quantification of three alkylated-purine adducts in human urine using sulfonic acid poly(glycidyl methacrylate-divinylbenzene)-based microspheres as sorbent combined with LC-MS/MS. J. Chromatogr. B 2018, 1081, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Hou, H.; Zhang, X.; Wang, A.; Liu, Y.; Hu, Q. New validated LC-MS/MS method for the determination of three alkylated adenines in human urine and its application to the monitoring of alkylating agents in cigarette smoke. Anal. Bioanal. Chem. 2014, 406, 5293–5302. [Google Scholar] [CrossRef]
- Hu, C.W.; Hsu, Y.W.; Chen, J.L.; Tam, L.M.; Chao, M.R. Direct analysis of tobacco-specific nitrosamine NNK and its metabolite NNAL in human urine by LC-MS/MS: Evidence of linkage to methylated DNA lesions. Arch. Toxicol. 2014, 88, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Villalta, P.W.; Hochalter, J.B.; Stepanov, I.; Hecht, S.S. Methyl DNA phosphate adduct formation in lung tumor tissue and adjacent normal tissue of lung cancer patients. Carcinogenesis 2019. [Google Scholar] [CrossRef]
- Singh, R.; Kaur, B.; Farmer, P.B. Detection of DNA damage derived from a direct acting ethylating agent present in cigarette smoke by use of liquid chromatography-tandem mass spectrometry. Chem. Res. Toxicol. 2005, 18, 249–256. [Google Scholar] [CrossRef]
- Hu, C.W.; Cooke, M.S.; Chang, Y.J.; Chao, M.R. Direct-acting DNA ethylating agents associated with tobacco use primarily originate from the tobacco itself, not combustion. J. Hazard. Mater. 2018, 358, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Shooter, K.V.; Slade, T.A. The stability of methyl and ethyl phosphotriesters in DNA in vivo. Chem. Biol. Interact. 1977, 19, 353–361. [Google Scholar] [CrossRef]
- Chen, H.J.C.; Lee, C.R. Detection and simultaneous quantification of three smoking-related ethylthymidine adducts in human salivary DNA by liquid chromatography tandem mass spectrometry. Toxicol. Lett. 2014, 224, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.C.; Liu, Y.F. Simultaneous quantitative analysis of N3-ethyladenine and N7-ethylguanine in human leukocyte deoxyribonucleic acid by stable isotope dilution capillary liquid chromatography-nanospray ionization tandem mass spectrometry. J. Chromatogr. A 2013, 1271, 86–94. [Google Scholar] [CrossRef]
- Swenberg, J.A.; Dyroff, M.C.; Bedell, M.A.; Popp, J.A.; Huh, N.; Kirstein, U.; Rajewsky, M.F. O4-Ethyldeoxythymidine, but not O6-ethyldeoxyguanosine, accumulates in hepatocyte DNA of rats exposed continuously to diethylnitrosamine. Proc. Natl. Acad. Sci. USA 1984, 81, 1692–1695. [Google Scholar] [CrossRef]
- Chen, H.J.C.; Wang, Y.C.; Lin, W.P. Analysis of ethylated thymidine adducts in human leukocyte DNA by stable isotope dilution nanoflow liquid chromatography-nanospray ionization tandem mass spectrometry. Anal. Chem. 2012, 84, 2521–2527. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.C.; Lin, C.R. Noninvasive measurement of smoking-associated N3-ethyladenine and N7-ethylguanine in human salivary DNA by stable isotope dilution nanoflow liquid chromatography-nanospray ionization tandem mass spectrometry. Toxicol. Lett. 2014, 225, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.C.; Lin, C.R. Simultaneous quantification of ethylpurine adducts in human urine by stable isotope dilution nanoflow liquid chromatography nanospray ionization tandem mass spectrometry. J. Chromatogr. A 2013, 1322, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Tretyakova, N.Y.; Chiang, S.Y.; Walker, V.E.; Swenberg, J.A. Quantitative analysis of 1,3-butadiene-induced DNA adducts in vivo and in vitro using liquid chromatography electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 1998, 33, 363–376. [Google Scholar] [CrossRef]
- Sangaraju, D.; Villalta, P.; Goggin, M.; Agunsoye, M.O.; Campbell, C.; Tretyakova, N. Capillary HPLC-accurate mass ms/ms quantitation of N7-(2,3,4-trihydroxybut-1-yl)-guanine adducts of 1,3-butadiene in human leukocyte DNA. Chem. Res. Toxicol. 2013, 26, 1486–1497. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Personal habits and indoor combustions. A review of human carcinogens. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100, pp. 1–538. [Google Scholar]
- Zhang, S.Y.; Balbo, S.; Wang, M.Y.; Hecht, S.S. Analysis of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human leukocyte DNA from smokers and nonsmokers. Chem. Res. Toxicol. 2011, 24, 119–124. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Villalta, P.W.; Wang, M.Y.; Hecht, S.S. Detection and quantitation of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human lung by liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem. Res. Toxicol. 2007, 20, 565–571. [Google Scholar] [CrossRef]
- Yang, J.; Balbo, S.; Villalta, P.W.; Hecht, S.S. Analysis of acrolein-derived 1, N2-propanodeoxyguanosine adducts in human lung DNA from smokers and non-smokers. Chem. Res. Toxicol. 2019, 32, 318–325. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, L.J.; Wang, H.J.; Chen, J.; Zhu, B.B.; Chen, H.; Hou, H.W.; Hu, Q.Y. Simultaneous analysis of six aldehyde-DNA adducts in salivary DNA of nonsmokers and smokers using stable isotope dilution liquid chromatography electrospray ionization-tandem mass spectrometry. J. Chromatogr. B 2017, 1060, 451–459. [Google Scholar] [CrossRef]
- Chung, F.L.; Wu, M.Y.; Basudan, A.; Dyba, M.; Nath, R.G. Regioselective formation of acrolein-derived cyclic 1,N2-propanodeoxyguanosine adducts mediated by amino acids, proteins, and cell lysates. Chem. Res. Toxicol. 2012, 25, 1921–1928. [Google Scholar] [CrossRef]
- Wang, M.Y.; Cheng, G.; Balbo, S.; Carmella, S.G.; Villalta, P.W.; Hecht, S.S. Clear differences in levels of a formaldehyde-DNA adduct in leukocytes of smokers and nonsmokers. Cancer Res. 2009, 69, 7170–7174. [Google Scholar] [CrossRef]
- Zhang, S.; Villalta, P.W.; Wang, M.Y.; Hecht, S.S. Analysis of crotonaldehyde- and acetaldehyde-derived 1,N2-propanodeoxyguanosine adducts in DNA from human tissues using liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem. Res. Toxicol. 2006, 19, 1386–1392. [Google Scholar] [CrossRef]
- Wang, M.; Yu, N.; Chen, L.; Villalta, P.W.; Hochalter, J.B.; Hecht, S.S. Identification of an acetaldehyde adduct in human liver DNA and quantitation as N2-ethyldeoxyguanosine. Chem. Res. Toxicol. 2006, 19, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.C.M.; Freitas, F.P.; Sanchez, A.B.; Di Mascio, P.; Medeiros, M.H.G. Elevated alpha-methyl-γ-hydroxy-1,N2-propano-2′-deoxyguanosine levels in urinary samples from individuals exposed to urban air pollution. Chem. Res. Toxicol. 2013, 26, 1602–1604. [Google Scholar] [CrossRef] [PubMed]
- Balbo, S.; Meng, L.; Bliss, R.L.; Jensen, J.A.; Hatsukami, D.K.; Hecht, S.S. Time course of DNA adduct formation in peripheral blood granulocytes and lymphocytes after drinking alcohol. Mutagenesis 2012, 27, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Balbo, S.; Meng, L.; Bliss, R.L.; Jensen, J.A.; Hatsukami, D.K.; Hecht, S.S. Kinetics of DNA adduct formation in the oral cavity after drinking alcohol. Cancer Epidemiol. Prev. Biomark. 2012, 21, 601–608. [Google Scholar] [CrossRef]
- Singh, R.; Gromadzinska, J.; Mistry, Y.; Cordell, R.; Juren, T.; Segerback, D.; Farmer, P.B. Detection of acetaldehyde derived N2-ethyl-2′-deoxyguanosine in human leukocyte DNA following alcohol consumption. Mutat. Res. 2012, 737, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.J.; Wu, C.F.; Shih, W.C.; Luo, Y.S.; Chen, M.F.; Li, C.M.; Liou, S.H.; Chung, W.S.; Chiang, S.Y.; Wu, K.Y. Potential association of urinary N7-(2-carbamoyl-2-hydroxyethyl) guanine with dietary acrylamide intake of smokers and nonsmokers. Chem. Res. Toxicol. 2015, 28, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Huang, C.C.J.; Lu, C.A.; Chen, M.L.; Liou, S.H.; Chiang, S.Y.; Wu, K.Y. Feasibility of using urinary N7-(2-carbamoy1-2-hydroxyethyl) Guanine as a biomarker for acrylamide exposed workers. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cui, Y.X.; Niedernhofer, L.J.; Wang, Y.S. Occurrence, biological consequences, and human health relevance of oxidative stress-induced DNA damage. Chem. Res. Toxicol. 2016, 29, 2008–2039. [Google Scholar] [CrossRef]
- Marnett, L.J. Lipid peroxidation-DNA damage by malondialdehyde. Mutat. Res. 1999, 424, 83–95. [Google Scholar] [CrossRef]
- Bonassi, S.; Cellai, F.; Munnia, A.; Ugolini, D.; Cristaudo, A.; Neri, M.; Milic, M.; Bonotti, A.; Giese, R.W.; Peluso, M.E.M. 3-(2-deoxy-β-d-erythro-pentafuranosyl)pyrimido[1,2-alpha]purin-10 (3H)-one deoxyguanosine adducts of workers exposed to asbestos fibers. Toxicol. Lett. 2017, 270, 1–7. [Google Scholar] [CrossRef]
- Saieva, C.; Peluso, M.; Palli, D.; Cellai, F.; Ceroti, M.; Selvi, V.; Bendinelli, B.; Assedi, M.; Munnia, A.; Masala, G. Dietary and lifestyle determinants of malondialdehyde DNA adducts in a representative sample of the Florence City population. Mutagenesis 2016, 31, 475–480. [Google Scholar] [CrossRef]
- Peluso, M.; Munnia, A.; Piro, S.; Jedpiyawongse, A.; Sangrajrang, S.; Giese, R.W.; Ceppi, M.; Boffetta, P.; Srivatanakul, P. Fruit and vegetable and fried food consumption and 3-(2-deoxy-β-d-erythro-pentafuranosyl)pyrimido[1,2-alpha] purin-10(3H)-one deoxyguanosine adduct formation. Free Radic. Res. 2012, 46, 85–92. [Google Scholar] [CrossRef]
- Peluso, M.E.M.; Munnia, A.; Bollati, V.; Srivatanakul, P.; Jedpiyawongse, A.; Sangrajrang, S.; Ceppi, M.; Giese, R.W.; Boffetta, P.; Baccarelli, A.A. Aberrant methylation of hypermethylated-in-cancer-1 and exocyclic DNA adducts in tobacco smokers. Toxicol. Sci. 2014, 137, 47–54. [Google Scholar] [CrossRef]
- Peluso, M.E.M.; Munnia, A.; Giese, R.W.; Chellini, E.; Ceppi, M.; Capacci, F. Oxidatively damaged DNA in the nasal epithelium of workers occupationally exposed to silica dust in Tuscany region, Italy. Mutagenesis 2015, 30, 519–525. [Google Scholar] [CrossRef]
- Bono, R.; Munnia, A.; Romanazzi, V.; Bellisario, V.; Cellai, F.; Peluso, M.E.M. Formaldehyde-induced toxicity in the nasal epithelia of workers of a plastic laminate plant. Toxicol. Res. 2016, 5, 752–760. [Google Scholar] [CrossRef]
- Micillo, A.; Vassallo, M.R.C.; Cordeschi, G.; D’Andrea, S.; Necozione, S.; Francavilla, F.; Francavilla, S.; Barbonetti, A. Semen leukocytes and oxidative-dependent DNA damage of spermatozoa in male partners of subfertile couples with no symptoms of genital tract infection. Andrology 2016, 4, 808–815. [Google Scholar] [CrossRef]
- Kumar, S.B.; Chawla, B.; Bisht, S.; Yadav, R.K.; Dada, R. Tobacco use increases oxidative DNA damage in sperm—Possible etiology of childhood cancer. Asian Pac. J. Cancer Prev. 2015, 16, 6967–6972. [Google Scholar] [CrossRef]
- Ma, B.; Jing, M.; Villalta, P.W.; Kapphahn, R.J.; Montezuma, S.R.; Ferrington, D.A.; Stepanov, I. Simultaneous determination of 8-oxo-2′-deoxyguanosine and 8-oxo-2′-deoxyadenosine in human retinal DNA by liquid chromatography nanoelectrospray-tandem mass spectrometry. Sci. Rep. 2016, 6, 22375. [Google Scholar] [CrossRef]
- De Carvalho, A.M.; Carioca, A.A.F.; Fisberg, R.M.; Qi, L.; Marchioni, D.M. Joint association of fruit, vegetable, and heterocyclic amine intake with DNA damage levels in a general population. Nutrition 2016, 32, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Kendzia, B.; Pesch, B.; Marczynski, B.; Lotz, A.; Welge, P.; Rihs, H.P.; Bruning, T.; Raulf-Heimsoth, M. Pre- and postshift levels of inflammatory biomarkers and DNA damage in non-bitumen-exposed construction workers-subpopulation of the German Human Bitumen Study. J. Toxicol. Environ. Health A 2012, 75, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Kafferlein, H.U.; Marczynski, B.; Simon, P.; Angerer, J.; Rihs, H.P.; Wilhelm, M.; Straif, K.; Pesch, B.; Bruning, T. Internal exposure to carcinogenic polycyclic aromatic hydrocarbons and DNA damage: A null result in brief. Arch. Toxicol. 2012, 86, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pant, M.C.; Singh, H.S.; Khandelwal, S. Assessment of the redox profile and oxidative DNA damage (8-OHdG) in squamous cell carcinoma of head and neck. J. Cancer Res. Ther. 2012, 8, 254–259. [Google Scholar] [PubMed]
- Deng, Q.F.; Dai, X.Y.; Guo, H.; Huang, S.L.; Kuang, D.; Feng, J.; Wang, T.; Zhang, W.Z.; Huang, K.; Hu, D.; et al. Polycyclic aromatic hydrocarbons-associated microRNAs and their interactions with the environment: Influences on oxidative DNA damage and lipid peroxidation in coke oven workers. Environ. Sci. Technol. 2014, 48, 4120–4128. [Google Scholar] [CrossRef]
- Bin, P.; Shen, M.L.; Li, H.B.; Sun, X.; Niu, Y.; Meng, T.; Yu, T.; Zhang, X.; Dai, Y.F.; Gao, W.M.; et al. Increased levels of urinary biomarkers of lipid peroxidation products among workers occupationally exposed to diesel engine exhaust. Free Radic. Res. 2016, 50, 820–830. [Google Scholar] [CrossRef]
- Leng, J.; Wang, Y. Liquid chromatography-tandem mass spectrometry for the quantification of tobacco-specific nitrosamine-induced DNA adducts in mammalian cells. Anal. Chem. 2017, 89, 9124–9130. [Google Scholar] [CrossRef]
- Li, Y.; Ma, B.; Cao, Q.; Balbo, S.; Zhao, L.; Upadhyaya, P.; Hecht, S.S. Mass spectrometric quantitation of pyridyloxobutyl DNA phosphate adducts in rats chronically treated with N′-nitrosonornicotine. Chem. Res. Toxicol. 2019. [Google Scholar] [CrossRef]
- Jones, G.D.D.; Le Pla, R.C.; Farmer, P.B. Phosphotriester adducts (PTEs): DNA’s overlooked lesion. Mutagenesis 2010, 25, 3–16. [Google Scholar] [CrossRef]
- Den Engelse, L.; De Graaf, A.; De Brij, R.J.; Menkveld, G.J. O2- and O4-ethylthymine and the ethylphosphotriester dTp(Et)dT are highly persistent DNA modifications in slowly dividing tissues of the ethylnitrosourea-treated rat. Carcinogenesis 1987, 8, 751–757. [Google Scholar] [CrossRef]
- Singer, B. In vivo formation and persistence of modified nucleosides resulting from alkylating agents. Environ. Health Perspect. 1985, 62, 41–48. [Google Scholar] [CrossRef]
- Haglund, J.; Henderson, A.P.; Golding, B.T.; Tornqvist, M. Evidence for phosphate adducts in DNA from mice treated with 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Chem. Res. Toxicol. 2002, 15, 773–779. [Google Scholar] [CrossRef]
- Upadhyaya, P.; McIntee, E.J.; Villalta, P.W.; Hecht, S.S. Identification of adducts formed in the reaction of 5′-acetoxy-N′-nitrosonornicotine with deoxyguanosine and DNA. Chem. Res. Toxicol. 2006, 19, 426–435. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, P.; Cui, Y.; Wang, Y. Chemical analysis of DNA damage. Anal. Chem. 2018, 90, 556–576. [Google Scholar] [CrossRef]
- Tretyakova, N.; Villalta, P.W.; Kotapati, S. Mass spectrometry of structurally modified DNA. Chem. Rev. 2013, 113, 2395–2436. [Google Scholar] [CrossRef]
- Villalta, P.W.; Balbo, S. The future of DNA adductomic analysis. Int. J. Mol. Sci. 2017, 18, 1870. [Google Scholar] [CrossRef]
- Hofer, T.; Moller, L. Reduction of oxidation during the preparation of DNA and analysis of 8-hydroxy-2′-deoxyguanosine. Chem. Res. Toxicol. 1998, 11, 882–887. [Google Scholar] [CrossRef]
- Bhutani, M.; Pathak, A.K.; Fan, Y.H.; Liu, D.D.; Lee, J.J.; Tang, H.L.; Kurie, J.M.; Morice, R.C.; Kim, E.S.; Hong, W.K.; et al. Oral epithelium as a surrogate tissue for assessing smoking-induced molecular alterations in the lungs. Cancer Prev. Res. 2008, 1, 39–44. [Google Scholar] [CrossRef]
- Wilson, K.A.; Garden, J.L.; Wetmore, N.T.; Wetmore, S.D. Computational insights into the mutagenicity of two tobacco-derived carcinogenic DNA lesions. Nucleic Acids Res. 2018, 46, 11858–11868. [Google Scholar] [CrossRef]
- Manderville, R.A.; Wetmore, S.D. Understanding the mutagenicity of O-linked and C-linked guanine DNA adducts: A combined experimental and computational approach. Chem. Res. Toxicol. 2017, 30, 177–188. [Google Scholar] [CrossRef]
- Millen, A.L.; Sharma, P.; Wetmore, S.D. C8-linked bulky guanosine DNA adducts: Experimental and computational insights into adduct conformational preferences and resulting mutagenicity. Future Med. Chem. 2012, 4, 1981–2007. [Google Scholar] [CrossRef]
| DNA Adduct | Source | Tissue | Method of Detection | Reference |
|---|---|---|---|---|
| HPB-releasing DNA adducts | NNK, NNN | Buccal cells | LC-NSI-HRMS/MS, LC-MS/MS | [28,29] |
| Bulky adducts | PAH, other aromatic and nonpolar chemicals (?) | Leukocytes, lung | 32P-postlabelling | [39,40,41,42,43,44,45,46,47,48,49,50,51,52] |
| BPDE-N2-dG | Benzo[a]pyrene | Buccal cells, sputum, lung, leukocytes, lymphocytes, tonsil, oropharynx, gingiva, prostate | Immunochemical, LC-NSI-HRMS/MS | [32,53,54,55,56,57,58] |
| 4-ABP-C8-dG | 4-aminobiphenyl (4-ABP) | Bladder, breast | LC-NSI-HRMS/MS, 32P-postlabelling, LC-ESI-MS/MS3 | [51,67,68] |
| 4-ABP-C8-dA | 4-ABP | Bladder | 32P-postlabelling | [68] |
| 4-ABP-N2-dG | 4-ABP | Bladder | 32P-postlabelling | [68] |
| O6-methyldeoxyguanosine (O6-mdG) | NNK, NDMA, other methylating agents | Lung | Immunochemical | [53] |
| 7-Methylguanine (7-mG) | NNK, NDMA, other methylating agents | Lung, urine, | Capillary LC-HRMS/MS, Immunochemical | [74,75,78] |
| 3-Methyladenine (3-mA) | NNK, other methylating agents | Urine | Capillary LC-HRMS/MS, LC-ESI-MS/MS | [75,76,77,78] |
| 1-Methyladenine (1-mA) | NNK, other methylating agents | Urine | Capillary LC-HRMS/MS | [75] |
| Methyl DNA phosphate adducts (B1pMeB2) | NNK, other methylating agents | Lung | LC-NSI-HRMS/MS | [79] |
| 3-Ethyladenine (3-etA) | Unknown ethylating agents | Urine, saliva | LC-ESI-MS/MS, LC-NSI-MS/MS | [76,77,87] |
| 7-Ethylguanine (7-etG) | Unknown ethylating agents | Urine, leukocytes, saliva | LC-ESI-MS/MS, LC-NSI-MS/MS | [76,84,87,88] |
| O2-ethylthymidine (O2-etT) | Unknown ethylating agents | Leukocytes, saliva | LC-NSI-MS/MS | [83,86] |
| O4-ethylthymidine (O4-etT) | Unknown ethylating agents | Leukocytes, saliva | LC-NSI-MS/MS | [83,86] |
| N3-ethylthymidine (N3-etT) | Unknown ethylating agents | Leukocytes, saliva | LC-NSI-MS/MS | [83,84,86,88] |
| 7-(2, 3, 4-trihydroxybut-1-yl) guanine (7-THBG) | 1,3-Butadiene | Leukocytes | Capillary LC-HRMS/MS | [90] |
| 1,N2-propanodeoxyguanosine (Acr-dG) | Acrolein | Buccal cells, sputum, lung, saliva, bladder, liver | Immunochemical, 32P-postlabelling, LC-MS/MS, LC-NSI-HRMS/MS | [53,68,94,95,96] |
| N6-hydroxymethyldeoxyadenosine (HOMe-dA) | Formaldehyde | Saliva | LC-ESI-MS/MS | [95] |
| N2-hydroxymethyldeoxyguanosine (HOMe-dG) | Formaldehyde | Saliva | LC-ESI-MS/MS | [95] |
| N2-ethylidene-deoxyguanosine (Ethylidene-dG) | Acetaldehyde | Saliva, leukocytes, oral cells | LC-ESI-MS/MS | [95,101,102,103] |
| 3-(2′-Deoxyribos-1′-yl)-5,6,7,8-tetrahydro-8-hydroxy-6-methylpyrimido[1,2-a]purine-10(3H)one (Cro-dG) | Crotonaldehyde, acetaldehyde | Buccal cells, sputum, lung, urine | Immunochemical, LC-ESI-MS/MS | [53,100] |
| 7-(2-carbamoyl-2-hydroxyethyl) guanine (7-GAG) | Acrylamide | Urine | LC-ESI-MS/MS | [104,105] |
| 3-(2-deoxy-β-d-erythro-pentafuranosyl)pyrimido[1,2-α]purin-10(3H)-one deoxyguanosine (M1dG) | Malondialdehyde | Leukocytes, nasal epithelium | 32P-postlabelling, LC-NSI-HRMS/MS | [33,48,108,109,110,111,112,113] |
| 8-Oxo-dG | ROS | Semen, retina, leukocytes, urine | Immunochemical, LC-NSI-MS/MS, electrochemical | [114,115,116,117,118,119,120,121] |
| 1,N6-etheno-2′-deoxyadenosine (εdA) | 4-hydroxy-2-nonenal | Urine | LC-ESI-MS/MS | [122] |
| 3,N4-etheno-2′-deoxycytidine (εdC) | 4-hydroxy-2-nonenal | Urine | LC-ESI-MS/MS | [122] |
| B1-B2 | Number of Possible Isomers | Total | |||
|---|---|---|---|---|---|
 |  | 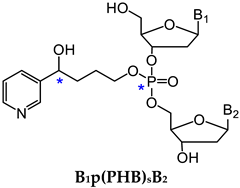 | 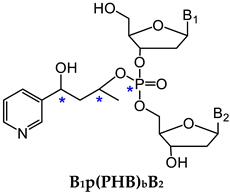 | ||
| A-A | 2 | 2 | 4 | 8 | 16 |
| C-C | 2 | 2 | 4 | 8 | 16 |
| G-G | 2 | 2 | 4 | 8 | 16 |
| T-T | 2 | 2 | 4 | 8 | 16 |
| A-C | 4 | 4 | 8 | 16 | 32 |
| A-G | 4 | 4 | 8 | 16 | 32 |
| A-T | 4 | 4 | 8 | 16 | 32 |
| C-G | 4 | 4 | 8 | 16 | 32 |
| C-T | 4 | 4 | 8 | 16 | 32 |
| G-T | 4 | 4 | 8 | 16 | 32 |
| Total | 32 | 32 | 64 | 128 | 256 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, B.; Stepanov, I.; Hecht, S.S. Recent Studies on DNA Adducts Resulting from Human Exposure to Tobacco Smoke. Toxics 2019, 7, 16. https://doi.org/10.3390/toxics7010016
Ma B, Stepanov I, Hecht SS. Recent Studies on DNA Adducts Resulting from Human Exposure to Tobacco Smoke. Toxics. 2019; 7(1):16. https://doi.org/10.3390/toxics7010016
Chicago/Turabian StyleMa, Bin, Irina Stepanov, and Stephen S. Hecht. 2019. "Recent Studies on DNA Adducts Resulting from Human Exposure to Tobacco Smoke" Toxics 7, no. 1: 16. https://doi.org/10.3390/toxics7010016
APA StyleMa, B., Stepanov, I., & Hecht, S. S. (2019). Recent Studies on DNA Adducts Resulting from Human Exposure to Tobacco Smoke. Toxics, 7(1), 16. https://doi.org/10.3390/toxics7010016





