Cadmium Nephrotoxicity Is Associated with Altered MicroRNA Expression in the Rat Renal Cortex
Abstract
1. Introduction
2. Material and Methods
2.1. Animal Protocol
2.2. Biomarker Determination
2.3. RNA Isolation
2.4. µParafloTM MicroRNA Microarray Assay
2.5. MicroRNA Real-Time PCR
3. Results
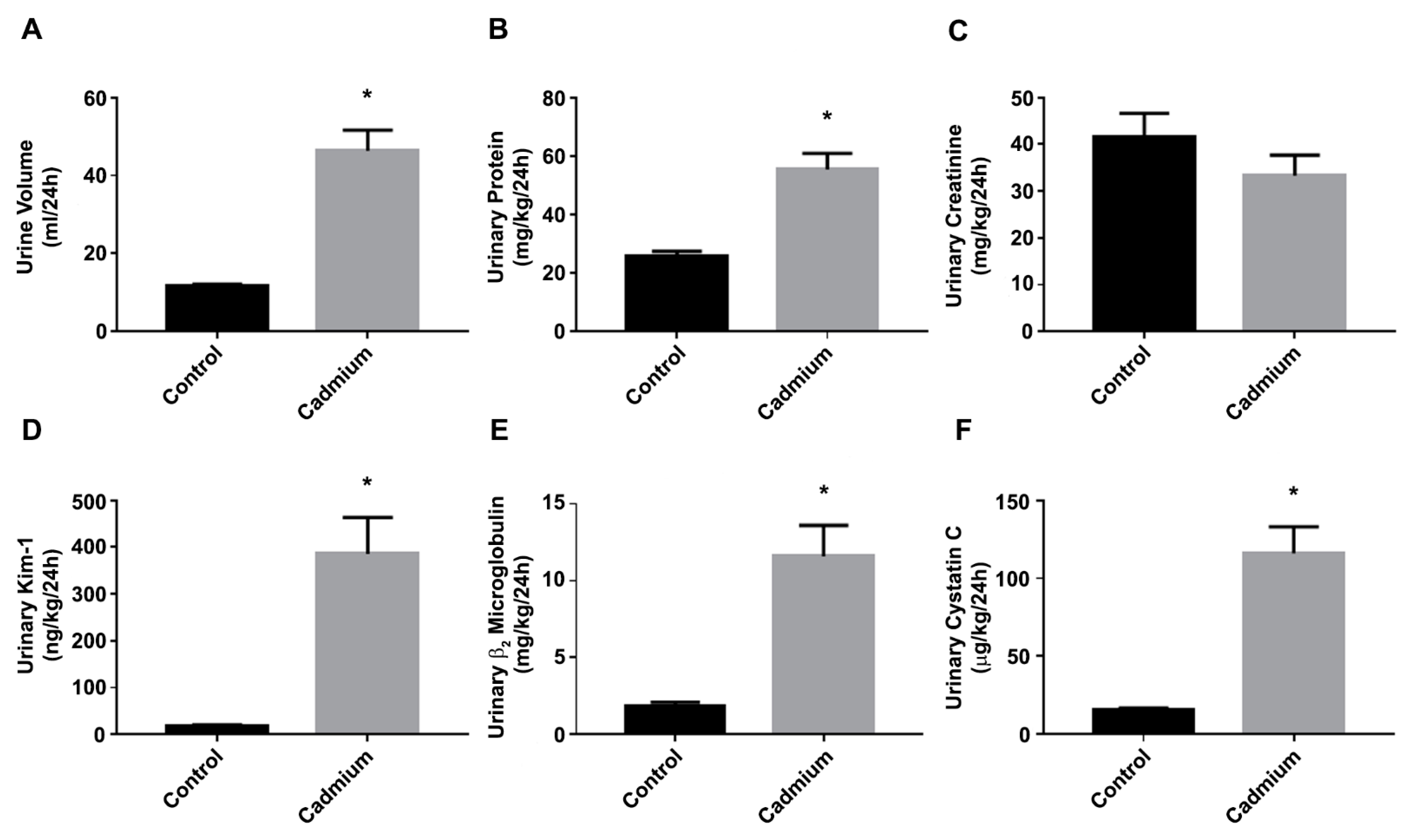
3.1. Cd-Induced Kidney Injury in a Sub-Chronic Rat Model
3.2. µParaflo™ MicroRNA Microarray
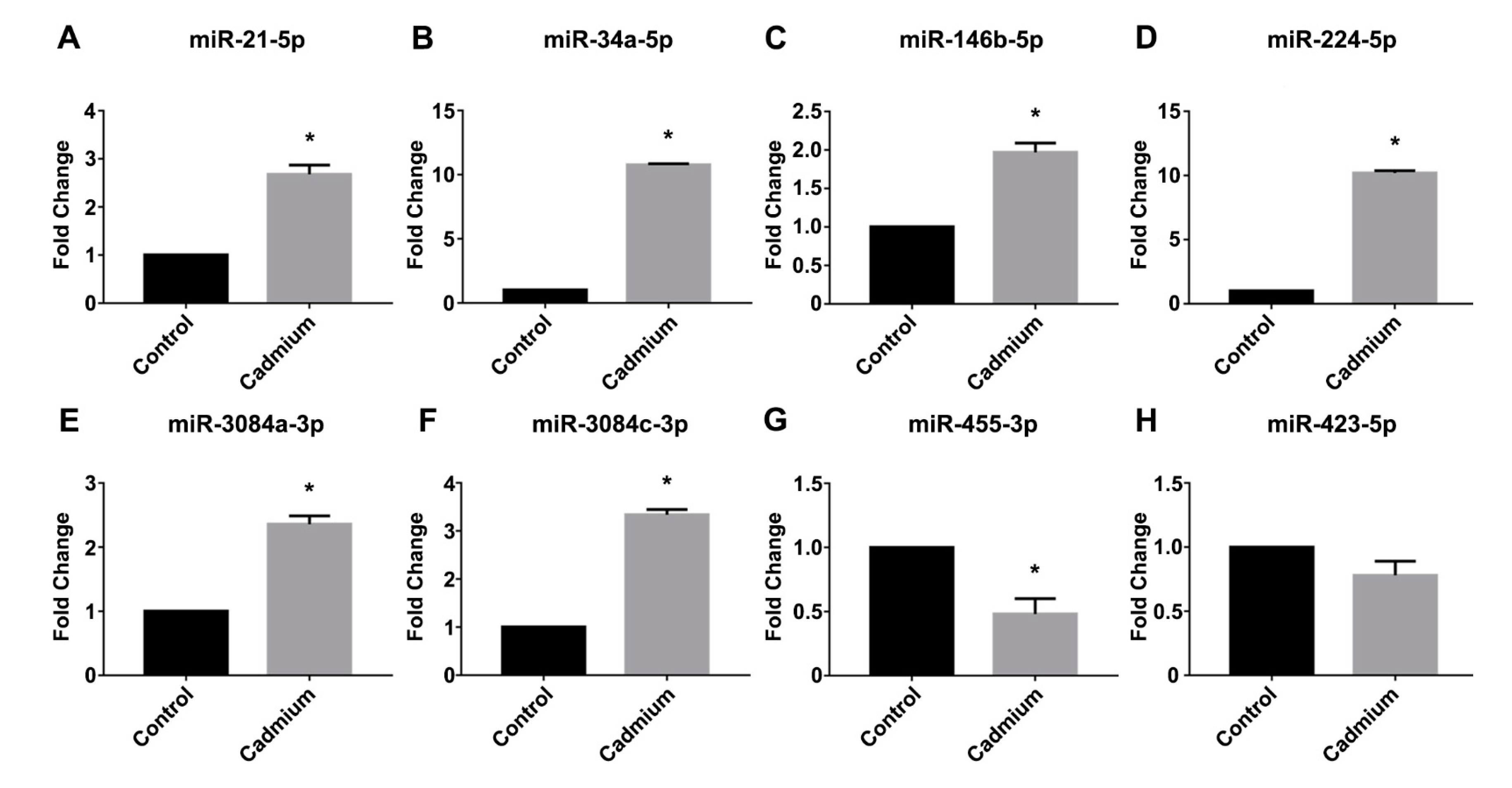
3.3. Real-Time PCR Validation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- ATSDR’s Substance Priority List. Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 30 January 2018).
- Jarup, L.; Akesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Bridges, C.C.; Zalups, R.K. Molecular and ionic mimicry and the transport of toxic metals. Toxicol. Appl. Pharmacol. 2005, 204, 274–308. [Google Scholar] [CrossRef] [PubMed]
- Jarup, L. Cadmium overload and toxicity. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Renal Assoc. 2002, 17, 35–39. [Google Scholar] [CrossRef]
- Kjellstrom, T. Renal effects. In Cadmium and Health: A Toxicoloogical and Epidemiological Appraisal; Friberg, L., Elinder, C.-G., Kjellstrom, T., Nordberg, G.F., Eds.; CRC Press: Boca Raton, FL, USA, 1986; Volume 2, pp. 21–109. [Google Scholar]
- Prozialeck, W.C.; Edwards, J.R. Mechanisms of cadmium-induced proximal tubule injury: New insights with implications for biomonitoring and therapeutic interventions. J. Pharmacol. Exp. Ther. 2012, 343, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Thevenod, F.; Lee, W.K. Cadmium and cellular signaling cascades: Interactions between cell death and survival pathways. Arch. Toxicol. 2013, 87, 1743–1786. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. MicroRNAs: Tiny regulators with great potential. Cell 2001, 107, 823–826. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, K.; Karolina, D.S.; Sepramaniam, S.; Armugam, A.; Wintour, E.M.; Bertram, J.F.; Jeyaseelan, K. Role of microRNAs in kidney homeostasis and disease. Kidney Int. 2012, 81, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Kreidberg, J.A. MicroRNAs in renal development. Pediatr. Nephrol. 2013, 28, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, T.; Belliere, J.; Bascands, J.L.; Neau, E.; Klein, J.; Schanstra, J.P. MiRNAs in urine: A mirror image of kidney disease? Expert Rev. Mol. Diagn. 2015, 15, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Trionfini, P.; Benigni, A.; Remuzzi, G. MicroRNAs in kidney physiology and disease. Nat. Rev. Nephrol. 2015, 11, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Chen, Z.; Zou, Y.; Wan, X. Roles of non-coding RNAs in acute kidney injury. Kidney Blood Press. Res. 2016, 41, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Badal, S.S.; Danesh, F.R. MicroRNAs and their applications in kidney diseases. Pediatr. Nephrol. 2015, 30, 727–740. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fan, P.C.; Chen, C.C.; Chen, Y.C.; Chang, Y.S.; Chu, P.H. MicroRNAs in acute kidney injury. Hum. Genom. 2016, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, C.V.; Vaidya, V.S. MicroRNAs in injury and repair. Arch. Toxicol. 2017, 91, 2781–2797. [Google Scholar] [CrossRef] [PubMed]
- Pavkovic, M.; Vaidya, V.S. MicroRNAs and drug-induced kidney injury. Pharmacol. Ther. 2016, 163, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Vaidya, V.S.; Liu, J.; Waalkes, M.P.; Edwards, J.R.; Lamar, P.C.; Bernard, A.M.; Dumont, X.; Bonventre, J.V. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int. 2007, 72, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Lamar, P.C.; Edwards, J.R. Effects of sub-chronic Cd exposure on levels of copper, selenium, zinc, iron and other essential metals in rat renal cortex. Toxicol. Rep. 2016, 3, 740–746. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prozialeck, W.C.; Edwards, J.R.; Vaidya, V.S.; Bonventre, J.V. Preclinical evaluation of novel urinary biomarkers of cadmium nephrotoxicity. Toxicol. Appl. Pharmacol. 2009, 238, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Edwards, J.R.; Lamar, P.C.; Liu, J.; Vaidya, V.S.; Bonventre, J.V. Expression of kidney injury molecule-1 (Kim-1) in relation to necrosis and apoptosis during the early stages of Cd-induced proximal tubule injury. Toxicol. Appl. Pharmacol. 2009, 238, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Shoucri, R.M.; Pouliot, M. Some observations on the kinetics of the Jaffe reaction for creatinine. Clin. Chem. 1977, 23, 1527–1530. [Google Scholar] [PubMed]
- Gao, X.; Gulari, E.; Zhou, X. In situ synthesis of oligonucleotide microarrays. Biopolymers 2004, 73, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Hong, A.; Sheng, N.; Zhang, X.; Matejko, A.; Jun, K.Y.; Srivannavit, O.; Gulari, E.; Gao, X.; Zhou, X. Microparaflo biochip for nucleic acid and protein analysis. Methods Mol. Biol. 2007, 382, 287–312. [Google Scholar] [PubMed]
- Bolstad, B.M.; Irizarry, R.A.; Astrand, M.; Speed, T.P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003, 19, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Baxter, S.S.; Carlson, L.A.; Mayer, A.M.; Hall, M.L.; Fay, M.J. Granulocytic differentiation of HL-60 promyelocytic leukemia cells is associated with increased expression of Cul5. In Vitro Cell. Dev. Biol. Anim. 2009, 45, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; VanDreel, A.; Ackerman, C.D.; Stock, I.; Papaeliou, A.; Yasmine, C.; Wilson, K.; Lamar, P.C.; Sears, V.L.; Gasiorowski, J.Z.; et al. Evaluation of cystatin C as an early biomarker of cadmium nephrotoxicity in the rat. Biometals Int. J. Role Metal Ions Biol. Biochem. Med. 2016, 29, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Piscator, M. The nephropathy of chronic cadmium poisoning. In Cadmium, Handbook of Experimental Pharmocology; Faulkes, E.C., Ed.; Springer: New York, NY, USA, 1986; Volume 80, pp. 194–197. [Google Scholar]
- Bernard, A. Renal dysfunction induced by cadmium: Biomarkers of critical effects. Biometals Int. J. Role Metal Ions Biol. Biochem. Med. 2004, 17, 519–523. [Google Scholar] [CrossRef]
- Lauwerys, R.R.; Bernard, A.M.; Roels, H.A.; Buchet, J.P. Cadmium: Exposure markers as predictors of nephrotoxic effects. Clin. Chem. 1994, 40, 1391–1394. [Google Scholar] [PubMed]
- Kobayashi, E.; Suwazono, Y.; Uetani, M.; Inaba, T.; Oishi, M.; Kido, T.; Nishijo, M.; Nakagawa, H.; Nogawa, K. Estimation of benchmark dose as the threshold levels of urinary cadmium, based on excretion of total protein, beta2-microglobulin, and N-acetyl-beta-D-glucosaminidase in cadmium nonpolluted regions in Japan. Environ. Res. 2006, 101, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Edwards, J.R. Early biomarkers of cadmium exposure and nephrotoxicity. Biometals Int. J. Role Metal Ions Biol. Biochem. Med. 2010, 23, 793–809. [Google Scholar] [CrossRef] [PubMed]
- Saikumar, J.; Hoffmann, D.; Kim, T.M.; Gonzalez, V.R.; Zhang, Q.; Goering, P.L.; Brown, R.P.; Bijol, V.; Park, P.J.; Waikar, S.S.; et al. Expression, circulation, and excretion profile of microRNA-21, -155, and -18a following acute kidney injury. Toxicol. Sci. Off. J. Soc. Toxicol. 2012, 129, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, X.; Zhang, S.; Lin, Y.; Yang, J.; Zhang, C. MicroRNA-21 protects against the H2O2-induced injury on cardiac myocytes via its target gene PDCD4. J. Mol. Cell. Cardiol. 2009, 47, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Jing, Y.; Hao, J.; Frankfort, N.C.; Zhou, X.; Shen, B.; Liu, X.; Wang, L.; Li, R. MicroRNA-21 in the pathogenesis of acute kidney injury. Protein Cell 2013, 4, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, K.; Zhou, L.; Mi, Q.S.; Huang, S.; She, J.X.; Dong, Z. MicroRNA-34a is induced via p53 during cisplatin nephrotoxicity and contributes to cell survival. Mol. Med. 2010, 16, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Kim, J.G.; Kim, H.J.; Kwon, H.K.; Cho, I.J.; Choi, D.W.; Lee, W.H.; Kim, W.D.; Hwang, S.J.; Choi, S.; et al. Discovery of an integrative network of microRNAs and transcriptomics changes for acute kidney injury. Kidney Int. 2014, 86, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Pavkovic, M.; Riefke, B.; Ellinger-Ziegelbauer, H. Urinary microRNA profiling for identification of biomarkers after cisplatin-induced kidney injury. Toxicology 2014, 324, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Hong, Q.; Wang, Z.; Yu, Y.Y.; Zou, X.; Xu, L.H. MicroRNA-34a suppresses autophagy in tubular epithelial cells in acute kidney injury. Am. J. Nephrol. 2015, 42, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, K.L.; Gerlach, C.V.; Craciun, F.L.; Ramachandran, K.; Bijol, V.; Kissick, H.T.; Vaidya, V.S. Application of small RNA sequencing to identify microRNAs in acute kidney injury and fibrosis. Toxicol. Appl. Pharmacol. 2016, 312, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.H.; Lee, H.; Kim, H.R.; Choi, K.H.; Joung, J.G.; Kim, H.H. Regulation of PLK1 through competition between hnRNPK, miR-149–3p and miR-193b-5p. Cell Death Differ. 2017, 24, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Du, G.; Xu, A.; Xi, X.; Li, D. Expression of miR-149–3p inhibits proliferation, migration, and invasion of bladder cancer by targeting S100A4. Am. J. Cancer Res. 2017, 7, 2209–2219. [Google Scholar] [PubMed]
- Yu, D.; Wu, L.; Gill, P.; Tolleson, W.H.; Chen, S.; Sun, J.; Knox, B.; Jin, Y.; Xiao, W.; Hong, H.; et al. Multiple microRNAs function as self-protective modules in acetaminophen-induced hepatotoxicity in humans. Arch. Toxicol. 2018, 92, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Singh, R.S.; Kumari, M.; Garg, D.; Upadhyay, A.; Ecelbarger, C.M.; Tripathy, S.; Tiwari, S. Urinary exosomal microRNA-451–5p is a potential early biomarker of diabetic nephropathy in rats. PLoS ONE 2016, 11, e0154055. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Gu, T.; Shi, E.; Wang, Y.; Fang, Q.; Wang, C. Dysregulation of renal microRNA expression after deep hypothermic circulatory arrest in rats. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2016, 49, 1725–1731. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.; Molne, J.; Forssell-Aronsson, E.; Konijnenberg, M.; Bernhardt, P. Nephrotoxicity profiles and threshold dose values for [177Lu]-DOTATATE in nude mice. Nucl. Med. Biol. 2012, 39, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Schuler, E.; Parris, T.Z.; Helou, K.; Forssell-Aronsson, E. Distinct microRNA expression profiles in mouse renal cortical tissue after 177Lu-octreotate administration. PLoS ONE 2014, 9, e112645. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, I.S.; Chang, K.C.; Tsai, Y.T.; Ke, J.Y.; Lu, P.J.; Lee, K.H.; Yeh, S.D.; Hong, T.M.; Chen, Y.L. MicroRNA-320 suppresses the stem cell-like characteristics of prostate cancer cells by downregulating the Wnt/beta-catenin signaling pathway. Carcinogenesis 2013, 34, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Niewenhuis, R.J. Cadmium (Cd2+) disrupts intercellular junctions and actin filaments in LLC-PK1 cells. Toxicol. Appl. Pharmacol. 1991, 107, 81–97. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Niewenhuis, R.J. Cadmium (Cd2+) disrupts Ca(2+)-dependent cell-cell junctions and alters the pattern of E-cadherin immunofluorescence in LLC-PK1 cells. Biochem. Biophys. Res. Commun. 1991, 181, 1118–1124. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Lamar, P.C. Cadmium (Cd2+) disrupts E-cadherin-dependent cell-cell junctions in MDCK cells. In Vitro Cell. Dev. Biol. Anim. 1997, 33, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Lamar, P.C.; Lynch, S.M. Cadmium alters the localization of N-cadherin, E-cadherin, and beta-catenin in the proximal tubule epithelium. Toxicol. Appl. Pharmacol. 2003, 189, 180–195. [Google Scholar] [CrossRef]
- Edwards, J.R.; Kolman, K.; Lamar, P.C.; Chandar, N.; Fay, M.J.; Prozialeck, W.C. Effects of cadmium on the sub-cellular localization of beta-catenin and beta-catenin-regulated gene expression in NRK-52E cells. Biometals Int. J. Role Metal Ions Biol. Biochem. Med. 2013, 26, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Urani, C.; Sacco, M.G.; Procaccianti, C.; Gribaldo, L. Whole genome analysis and microRNAs regulation in HepG2 cells exposed to cadmium. Altex 2012, 29, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Boyerinas, B.; Park, S.M.; Hau, A.; Murmann, A.E.; Peter, M.E. The role of let-7 in cell differentiation and cancer. Endocr.-Relat. Cancer 2010, 17, F19–F36. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Deng, J.; Sun, Z.M.; Pan, A.P.; Xiang, X.J.; Zhang, L.; Yu, F.; Chen, J.; Sun, Z.; Feng, M.; et al. Interference with the beta-catenin gene in gastric cancer induces changes to the miRNA expression profile. Tumour Biol. J. Int. Soci. Oncodev. Biol. Med. 2015, 36, 6973–6983. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, Y.; Chen, X.; Wei, Q.; Cao, B.; Shang, H. Downregulation of microRNA-193b-3p promotes autophagy and cell survival by targeting TSC1/mTOR signaling in NSC-34 cells. Front. Mol. Neurosci. 2017, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Denaro, M.; Ugolini, C.; Poma, A.M.; Borrelli, N.; Materazzi, G.; Piaggi, P.; Chiarugi, M.; Miccoli, P.; Vitti, P.; Basolo, F. Differences in miRNA expression profiles between wild-type and mutated NIFTPs. Endocr.-Relat. Cancer 2017, 24, 543–553. [Google Scholar] [CrossRef] [PubMed]

| MicroRNA | p-Value | Control Mean (RFS *) | Cadmium Mean (RFS *) | Log 2 (Cadmium/Control) |
|---|---|---|---|---|
| miR-3084a-3p | 1.05 × 10−6 | 1019 | 3016 | 1.57 |
| miR-34a-5p | 4.57 × 10−6 | 99 | 612 | 2.62 |
| miR-1949 | 1.10 × 10−5 | 41 | 326 | 2.98 |
| miR-224-5p | 3.75 × 10−5 | 12 | 390 | 5.06 |
| miR-222-3p | 3.00 × 10−4 | 622 | 1127 | 0.86 |
| miR-221-3p | 3.95 × 10−4 | 968 | 1643 | 0.76 |
| miR-146b-5p | 8.79 × 10−4 | 200 | 558 | 1.48 |
| miR-210-5p | 1.81 × 10−3 | 1140 | 1740 | 0.61 |
| miR-20a-5p | 1.87 × 10−3 | 1179 | 1756 | 0.58 |
| miR-146a-5p | 2.89 × 10−3 | 3840 | 5884 | 0.62 |
| miR-3084c-3p | 4.34 × 10−3 | 1174 | 3419 | 1.54 |
| miR-92a-3p | 6.52 × 10−3 | 1083 | 1926 | 0.83 |
| miR-21-5p | 6.98 × 10−3 | 10,943 | 22,388 | 1.03 |
| miR-466b-2-3p | 7.25 × 10−3 | 2101 | 3143 | 0.58 |
| miR-320-3p | 1.18 × 10−2 | 1377 | 1882 | 0.45 |
| miR-15b-5p | 1.29 × 10−2 | 1032 | 1647 | 0.67 |
| miR-466c-3p | 1.29 × 10−2 | 3427 | 5220 | 0.61 |
| miR-214-3p | 1.64 × 10−2 | 1582 | 2094 | 0.40 |
| miR-483-5p | 1.74 × 10−2 | 711 | 1184 | 0.74 |
| miR-149-3p | 1.78 × 10−2 | 1573 | 3796 | 1.27 |
| let-7i-5p | 2.67 × 10−2 | 3498 | 4619 | 0.40 |
| miR-762 | 2.84 × 10−2 | 915 | 1702 | 0.90 |
| miR-466d | 3.47 × 10−2 | 370 | 675 | 0.87 |
| miR-346 | 3.57 × 10−2 | 315 | 440 | 0.48 |
| miR-17-5p | 3.60 × 10−2 | 877 | 1269 | 0.53 |
| miR-451-5p | 3.63 × 10−2 | 552 | 1177 | 1.09 |
| miR-92b-3p | 3.81 × 10−2 | 471 | 759 | 0.69 |
| miR-466c-5p | 3.83 × 10−2 | 229 | 389 | 0.76 |
| miR-32-3p | 4.07 × 10−2 | 622 | 1144 | 0.88 |
| Statistically significant transcripts with low signals (signal < 500) | ||||
| miR-138-5p | 4.00 × 10−4 | 43 | 140 | 1.71 |
| miR-130b-3p | 7.36 × 10−4 | 12 | 59 | 2.25 |
| miR-187-3p | 3.82 × 10−3 | 84 | 242 | 1.53 |
| miR-155-5p | 6.57 × 10−3 | 33 | 197 | 2.57 |
| miR-1839-3p | 7.09 × 10−3 | 293 | 417 | 0.51 |
| miR-187-5p | 8.23 × 10−3 | 66 | 114 | 0.79 |
| miR-132-3p | 9.57 × 10−3 | 59 | 189 | 1.66 |
| miR-34a-3p | 1.08 × 10−2 | 7 | 24 | 1.86 |
| miR-452-3p | 1.71 × 10−2 | 5 | 27 | 2.36 |
| miR-511-5p | 1.99 × 10−2 | 36 | 90 | 1.32 |
| miR-758-5p | 2.08 × 10−2 | 203 | 281 | 0.47 |
| miR-487b-5p | 2.13 × 10−2 | 29 | 69 | 1.22 |
| miR-327 | 2.19 × 10−2 | 28 | 51 | 0.84 |
| miR-504 | 4.10 × 10−2 | 98 | 136 | 0.47 |
| miR-6332 | 4.30 × 10−2 | 16 | 28 | 0.82 |
| MicroRNA | p-Value | Control Mean (RFS *) | Cadmium Mean (RFS *) | Log 2 (Cadmium/Control) |
|---|---|---|---|---|
| miR-193b-3p | 2.29 × 10−5 | 445 | 137 | −1.70 |
| miR-185-5p | 2.81 × 10−5 | 1150 | 628 | −0.87 |
| miR-455-3p | 2.06 × 10−4 | 764 | 258 | −1.57 |
| miR-195-5p | 4.76 × 10−4 | 4374 | 3035 | −0.53 |
| miR-200a-3p | 2.31 × 10−3 | 5998 | 3725 | −0.69 |
| miR-101b-3p | 2.56 × 10−3 | 465 | 285 | −0.71 |
| miR-194-5p | 2.72 × 10−3 | 13,390 | 7697 | −0.80 |
| miR-99a-5p | 2.79 × 10−3 | 5468 | 3596 | −0.60 |
| miR-505-3p | 3.59 × 10−3 | 539 | 371 | −0.54 |
| miR-342-3p | 4.25 × 10−3 | 1871 | 845 | −1.15 |
| miR-203a-3p | 5.21 × 10−3 | 1327 | 730 | −0.86 |
| miR-378a-3p | 6.43 × 10−3 | 2576 | 1616 | −0.67 |
| miR-378a-5p | 6.67 × 10−3 | 416 | 233 | −0.83 |
| miR-140-5p | 7.56 × 10−3 | 403 | 228 | −0.82 |
| miR-378b | 9.43 × 10−3 | 1985 | 1298 | −0.61 |
| miR-103-3p | 1.73 × 10−2 | 2717 | 2000 | −0.44 |
| miR-107-3p | 1.74 × 10−2 | 2781 | 2052 | −0.44 |
| miR-192-5p | 2.31 × 10−2 | 13,962 | 11,183 | −0.32 |
| miR-152-3p | 2.98 × 10−2 | 971 | 683 | −0.51 |
| miR-100-5p | 3.39 × 10−2 | 2133 | 1318 | −0.70 |
| miR-30a-3p | 3.73 × 10−2 | 837 | 552 | −0.60 |
| miR-30a-5p | 3.81 × 10−2 | 15,805 | 12,197 | −0.37 |
| miR-22-5p | 3.84 × 10−2 | 939 | 812 | −0.21 |
| miR-30b-5p | 3.93 × 10−2 | 14,704 | 11,704 | −0.33 |
| miR-196b-5p | 4.03 × 10−2 | 464 | 318 | −0.54 |
| miR-489-3p | 4.21 × 10−2 | 485 | 311 | −0.64 |
| miR-30e-5p | 4.68 × 10−2 | 10,074 | 6429 | −0.65 |
| Statistically significant transcripts with low signals (signal < 500) | ||||
| miR-203b-3p | 6.03 × 10−5 | 146 | 31 | −2.25 |
| miR-192-3p | 7.66 × 10−5 | 299 | 105 | −1.50 |
| miR-193a-3p | 2.05 × 10−4 | 328 | 104 | −1.65 |
| miR-455-5p | 3.55 × 10−4 | 70 | 17 | −2.05 |
| miR-184 | 6.06 × 10−4 | 27 | 5 | −2.52 |
| miR-375-3p | 7.44 × 10−4 | 39 | 11 | −1.86 |
| miR-345-5p | 1.04 × 10−3 | 183 | 103 | −0.82 |
| miR-29b-5p | 2.03 × 10−3 | 148 | 78 | −0.92 |
| miR-301a-3p | 3.09 × 10−3 | 122 | 60 | −1.03 |
| miR-3559-5p | 5.48 × 10−3 | 298 | 161 | −0.89 |
| miR-582-5p | 9.16 × 10−3 | 165 | 99 | −0.73 |
| miR-345-3p | 9.25 × 10−3 | 58 | 36 | −0.70 |
| miR-24-1-5p | 1.07 × 10−2 | 98 | 53 | −0.88 |
| miR-29c-5p | 1.07 × 10−2 | 274 | 161 | −0.77 |
| miR-24-2-5p | 1.12 × 10−2 | 276 | 181 | −0.61 |
| miR-10b-3p | 1.54 × 10−2 | 200 | 121 | −0.72 |
| miR-3068-5p | 1.86 × 10−2 | 162 | 113 | −0.52 |
| miR-200a-5p | 1.87 × 10−2 | 133 | 73 | −0.86 |
| miR-201-5p | 2.26 × 10−2 | 67 | 33 | −1.00 |
| miR-141-3p | 2.41 × 10−2 | 171 | 83 | −1.05 |
| miR-194-3p | 2.63 × 10−2 | 83 | 44 | −0.92 |
| miR-324-5p | 2.73 × 10−2 | 243 | 180 | −0.43 |
| miR-26b-3p | 3.38 × 10−2 | 27 | 10 | −1.47 |
| miR-193a-5p | 3.45 × 10−2 | 20 | 5 | −2.12 |
| miR-3585-5p | 3.50 × 10−2 | 67 | 34 | −0.98 |
| let-7e-3p | 4.06 × 10−2 | 47 | 23 | −1.00 |
| miR-103-1-5p | 4.71 × 10−2 | 32 | 22 | −0.52 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fay, M.J.; Alt, L.A.C.; Ryba, D.; Salamah, R.; Peach, R.; Papaeliou, A.; Zawadzka, S.; Weiss, A.; Patel, N.; Rahman, A.; et al. Cadmium Nephrotoxicity Is Associated with Altered MicroRNA Expression in the Rat Renal Cortex. Toxics 2018, 6, 16. https://doi.org/10.3390/toxics6010016
Fay MJ, Alt LAC, Ryba D, Salamah R, Peach R, Papaeliou A, Zawadzka S, Weiss A, Patel N, Rahman A, et al. Cadmium Nephrotoxicity Is Associated with Altered MicroRNA Expression in the Rat Renal Cortex. Toxics. 2018; 6(1):16. https://doi.org/10.3390/toxics6010016
Chicago/Turabian StyleFay, Michael J., Lauren A. C. Alt, Dominika Ryba, Ribhi Salamah, Ryan Peach, Alexander Papaeliou, Sabina Zawadzka, Andrew Weiss, Nil Patel, Asad Rahman, and et al. 2018. "Cadmium Nephrotoxicity Is Associated with Altered MicroRNA Expression in the Rat Renal Cortex" Toxics 6, no. 1: 16. https://doi.org/10.3390/toxics6010016
APA StyleFay, M. J., Alt, L. A. C., Ryba, D., Salamah, R., Peach, R., Papaeliou, A., Zawadzka, S., Weiss, A., Patel, N., Rahman, A., Stubbs-Russell, Z., Lamar, P. C., Edwards, J. R., & Prozialeck, W. C. (2018). Cadmium Nephrotoxicity Is Associated with Altered MicroRNA Expression in the Rat Renal Cortex. Toxics, 6(1), 16. https://doi.org/10.3390/toxics6010016






