Synergy of Arsenic and Graphene Oxide in Utero and Lactation Exacerbates Reproductive Disorders in Female Rat Offspring Undergoing Puberty and Maturity
Highlights
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.1.1. Sodium Arsenite
2.1.2. Graphene Oxide
Synthesis of Graphene Oxide
Characterization Analysis of Graphene Oxide
2.2. Sodium Arsenite Dose
2.3. Graphene Oxide Dose
2.4. Ethics Statement
2.5. Animals Fetch
2.6. Mating and Pregnancy Layout
2.7. Experimental Assignment and Design
2.8. Experimental Milestones
Maternal Toxicity Monitoring
2.9. Female Offspring
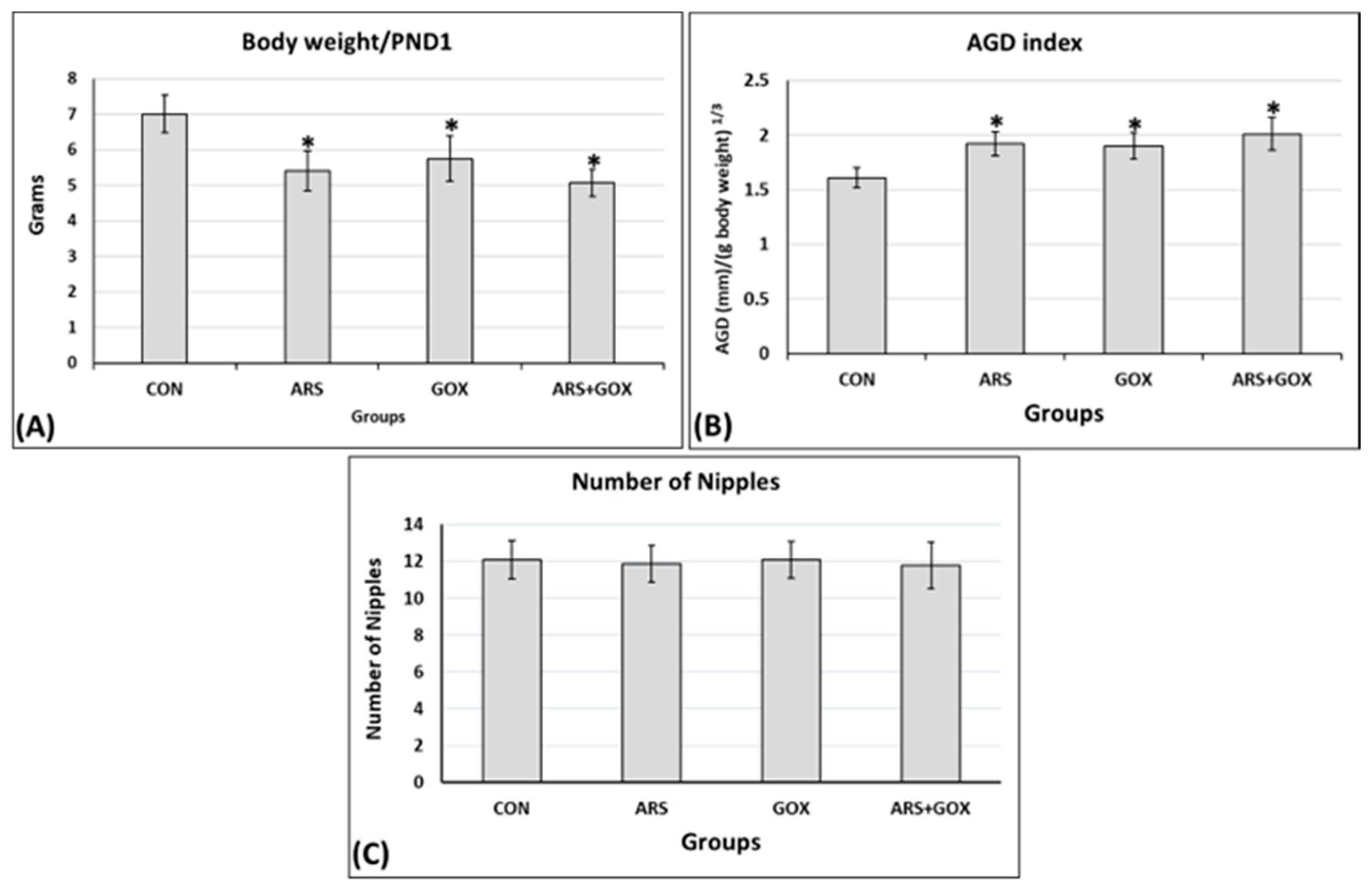
2.9.1. Measurement of Body Weight and Anogenital Distance
2.9.2. Determination of Location and Count of Nipples and Areolae
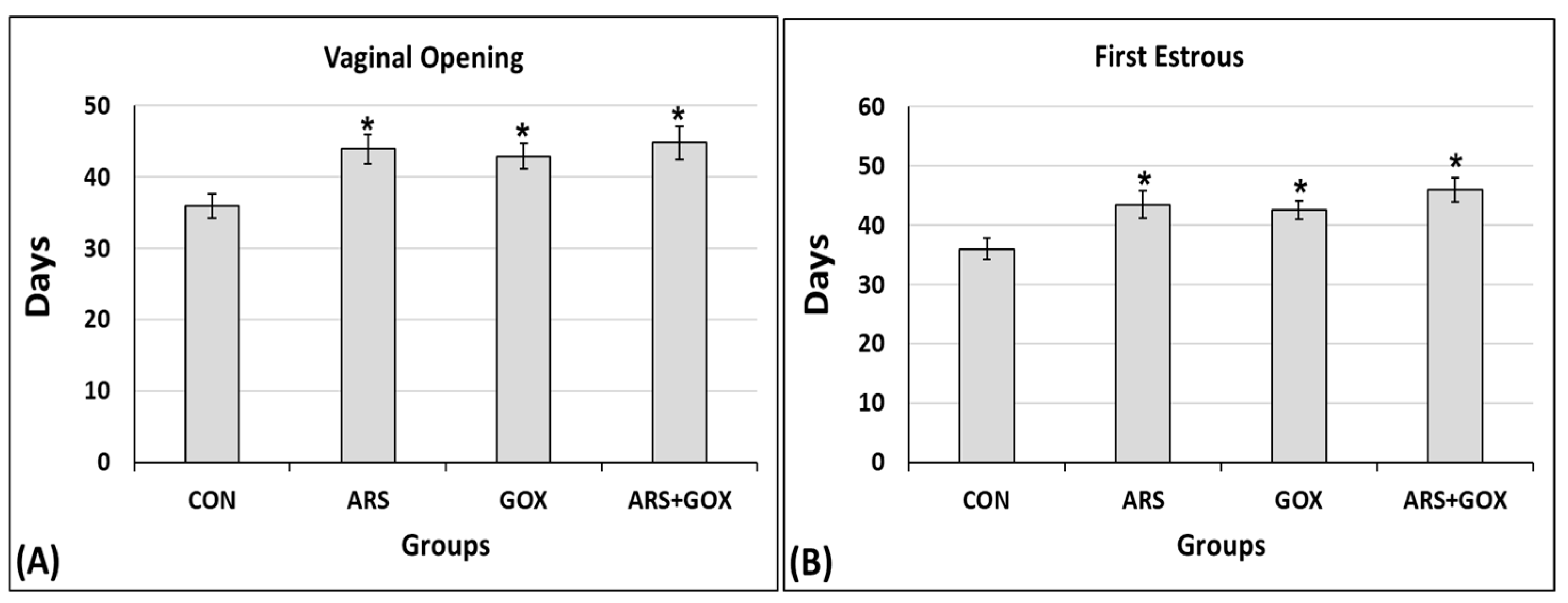
2.9.3. Estimation of Onset of Puberty
Vaginal Opening, First Estrus, and Ovulation
2.9.4. Scrutiny of Reproductive Cycle (Estrous Cycle) (Maturation Phase)
2.9.5. Assessment of Lordosis and Sexual Receptivity
2.9.6. Test of Natural Mating for Fertility and Reproductive Performance
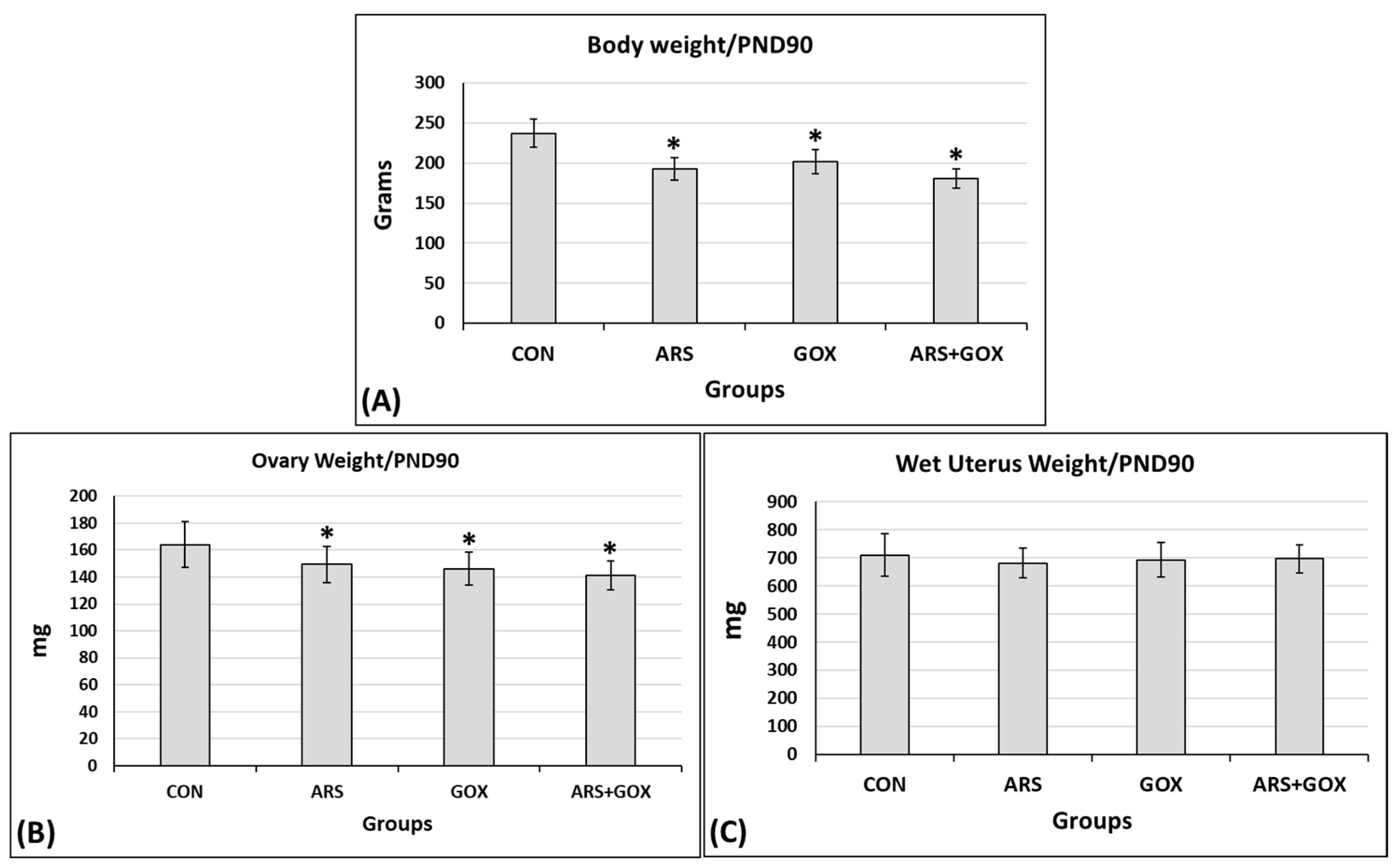
2.9.7. Assessment of Body, Ovary, and Uterus Weights at PND 90
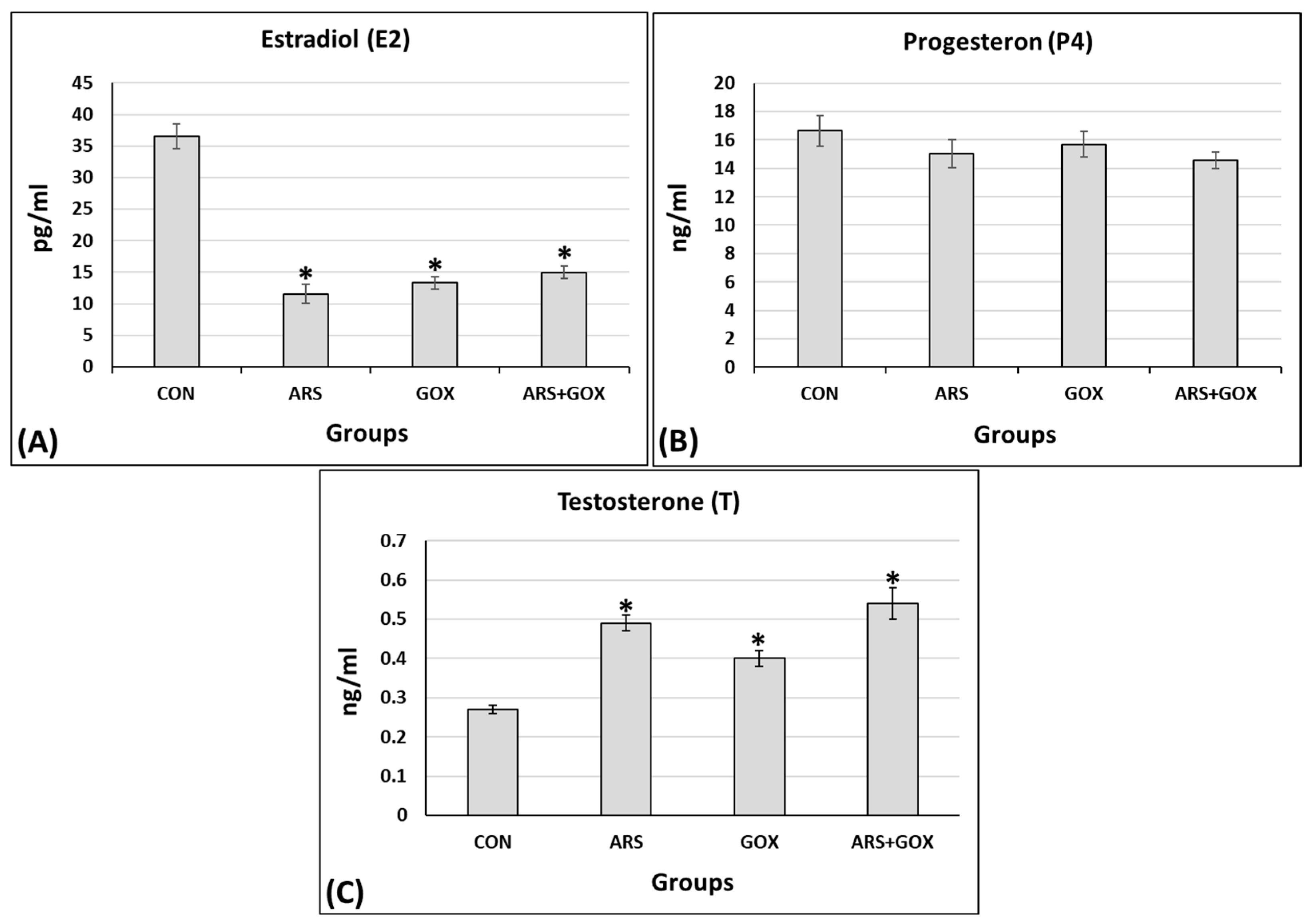
2.9.8. Reproductive Hormone Assays
2.10. Statistical Analysis
3. Results
3.1. Characterization Panel of Graphene Oxide
3.2. Maternal Toxicity
3.3. Offspring Toxicity from Birth to Puberty (PND 1–PND 90)
3.3.1. Body Weight, Anogenital Distance, and Number of Nipples/Areolae
3.3.2. Signs of Puberty Onset (Vaginal Opening and First Estrus)
3.3.3. Estrous Cycle
3.3.4. Sexual Behaviour and Receptivity of Female Offspring
3.3.5. Fertility and Reproductive Performance
3.3.6. Biometric Parameters of Female Offspring at PND 90
3.3.7. Status of Hormone Levels
4. Discussion
5. Conclusions
Limitations of the Study
- High Dose Levels: The doses of arsenic and graphene oxide administered in this study were relatively high compared to typical environmental or occupational exposure levels. While this approach is valuable for identifying potential toxic effects and mechanisms, the findings may not directly reflect the risks associated with lower, chronic, real-life exposures.
- Single-Species Model: The use of female rats as an experimental model provides important information, but extrapolation to humans should be carried out cautiously. Differences in physiology, metabolism, and reproductive biology between rodents and humans may limit the generalizability of the results.
- Exposure Duration and Route: The exposure duration and route (oral, intraperitoneal, etc.) may not fully mimic human exposure scenarios, where chronic low-dose exposure through water, food, or inhalation is more common. This difference may influence the distribution, bioaccumulation, and toxicokinetics of arsenic and graphene oxide.
- Combined Exposure Assessment: While the study investigated co-exposure to arsenic and graphene oxide, the design may not capture the full complexity of interactive effects at lower, environmentally relevant concentrations. The observed toxicity could be amplified by the supraphysiological doses used.
- Limited Mechanistic Exploration: Although the reproductive endpoints of offspring puberty and maturity were assessed, mechanistic insights at the molecular and cellular levels (e.g., oxidative stress pathways or epigenetic modifications) were not comprehensively investigated. Such analyses would be valuable for clarifying the underlying basis of the observed effects.
- Long-Term Offspring Outcomes: The assessment of offspring was limited to early developmental and reproductive parameters. Long-term effects across the lifespan, including transgenerational impacts, were not evaluated and remain to be determined. In addition, the present study aimed to investigate the prepubertal, pubertal, and maturity endpoints of female offspring, which was conducted only in early developmental stages (short-term offspring outcomes).
- Daily intake water consumption (mg/kg/day): Noting the absence of direct estimated intake data, we recommend that future studies incorporate the daily monitoring of water consumption to allow more accurate dose–response assessment.
- Lack of Molecular and Histological Endpoints: A further limitation of the present study is the absence of direct molecular and histological assessments to support the proposed mechanistic pathways. While the reproductive and developmental outcomes observed suggest possible involvement of oxidative stress, endocrine disruption, and neuroendocrine signalling alterations associated with puberty and maturity, these mechanisms remain hypothetical without corroborating molecular markers or tissue-level evidence. Future studies incorporating gene expression profiling, protein analyses, and histopathological examinations of reproductive and neural tissues are warranted to validate and expand upon the mechanistic interpretations suggested here.
- Analysis of Gonadotropins (LH, FSH): The inclusion of gonadotropins (LH and FSH) would have provided a more comprehensive assessment of hypothalamic–pituitary–gonadal (HPG) axis disruption. We also highlight that future work should include gonadotropin measurements alongside sex steroid hormones to better characterize the endocrine mechanisms underlying arsenic and graphene oxide toxicity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, N.; Xiong, G.; Liu, Z. Toxicity of metal-based nanoparticles: Challenges in the nano era. Front. Bioeng. Biotechnol. 2022, 10, 1001572. [Google Scholar] [CrossRef]
- Qu, J.; Wang, Q.; Sun, X.; Li, Y. The environment and female reproduction: Potential mechanism of cadmium poisoning to the growth and development of ovarian follicle. Ecotoxicol. Environ. Saf. 2022, 244, 114029. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-K.; Lim, J.; Jeong, Y.-J.; Hwang, S.; Lee, J.-Y.; Choi, B.-Y. Recent pollution and source identification of metal(loid)s in a sediment core from Gunsan Reservoir, South Korea. J. Haz. Mat. 2021, 416, 126204. [Google Scholar] [CrossRef] [PubMed]
- Ommati, M.M.; Manthari, R.K.; Tikka, C.; Niu, R.; Sun, Z.; Sabouri, S.; Zamiri, M.J.; Ahmadi, H.N.; Ghaffari, H.; Heidari, R.; et al. Arsenic-induced Autophagic Alterations and Mitochondrial Impairments in HPG-S Axis of Mature Male Mice Offspring (F1-generation): A persistent toxicity study. Toxicol. Lett. 2020, 326, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Nunzio, A.D.; Giarra, A.; Toscanesi, M.; Amoresano, A.; Piscopo, M.; Ceretti, E.; Zani, C.; Lorenzetti, S.; Trifuoggi, M.; Montano, L. Comparison between Macro and Trace Element Concentrations in Human. Semen. 636 and Blood Serum in Highly Polluted Areas in Italy. Int. J. Environ. Res. Public Health 2022, 19, 11635. [Google Scholar] [CrossRef]
- Liu, T.; Xu, Y.; Yi, C.X.; Tong, Q.; Cai, D. The hypothalamus for whole-body physiology: From metabolism to aging. Protein Cell 2022, 13, 394–421. [Google Scholar] [CrossRef]
- Marciniak, W.; Matoušek, T.; Domchek, S.; Paradiso, A.; Patruno, M.; Irmejs, A.; Roderte, I.; Derkacz, R.; Baszuk, P.; Kuświk, M.; et al. Blood Arsenic Levels as a Marker of Breast Cancer Risk among BRCA1 Carriers. Cancers 2021, 13, 3345. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, Z.; Niu, R.; Manthari, R.K.; Yuan, M.; Yang, K.; Cheng, M.; Gong, Z.; Wang, J. Effect of arsenic and/or fluoride gestational exposure on renal autophagy in offspring mice. Chemosphere 2020, 241, 124861. [Google Scholar] [CrossRef]
- Bourguignon, N.S.; Bonaventura, M.M.; Rodríguez, D.; Bizzozzero, M.; Ventura, C.; Nuñez, M.; Lux-Lantos, V.A.; Libertun, C. Evaluation of sodium arsenite exposure on reproductive competence in pregnant and postlactational dams and their offspring. Reprod. Toxicol. 2017, 69, 1–12. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, H.; Wang, X.; Wu, Y.; Zhang, Y.; Chen, S.; Zhang, W.; Sun, X.; Zheng, T.; Xia, W.; et al. Prenatal arsenic exposure, arsenic metabolism and neurocognitive development of 2-year-old children in low-arsenic areas. Environ. Int. 2023, 174, 107918. [Google Scholar] [CrossRef]
- Couto-Santos, F.; Souza, A.C.F.; Bastos, D.S.S.; Ervilha, L.O.G.; Dias, F.C.R.; de Sales Araújo, L.; Guimarães, S.E.F.; de Oliveira, L.L.; Machado-Neves, M. Prepubertal exposure to arsenic alters male reproductive parameters in pubertal and adult rats. Toxicol. Appl. Pharmacol. 2020, 409, 115304. [Google Scholar] [CrossRef]
- Chen, P.; Luo, Q.; Lin, Y.; Jin, J.; Hu, K.L.; Wang, F.; Sun, J.; Chen, R.; Wei, J.; Chen, G.; et al. Arsenic exposure during juvenile and puberty significantly affected reproductive system development of female SD rats. Ecotoxicol. Environ. Saf. 2022, 242, 113857. [Google Scholar] [CrossRef]
- Rodriguez, K.F.; Ungewitter, E.K.; Crespo-Mejias, Y.; Liu, C.; Nicol, B.; Kissling, G.E.; Yao, H.H. Effects of in utero exposure to arsenic during the second half of gestation on reproductive end points and metabolic parameters in female CD-1 mice. Environ. Health Perspect. 2016, 124, 336–343. [Google Scholar] [CrossRef]
- Souza, A.C.F.; Ervilha, L.O.G.; Coimbra, J.L.P.; Bastos, D.S.S.; Guimarães, S.E.F.; Machado-Neves, M. Reproductive disorders in female rats after prenatal exposure to sodium arsenite. J. Appl. Toxicol. 2020, 40, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Buerki-Thurnherr, T.; Kaur, J.; Wick, P.; Pelin, M.; Tubaro, A.; Carniel, F.C.; Tretiach, M.; Flahaut, E.; Iglesias, D.; et al. Environmental and Health Impacts of Graphene and Other Two-Dimensional Materials: A Graphene Flagship Perspective. ACS Nano 2024, 18, 6038–6094. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, J.; Fang, H.; Wang, M.; Wang, Q.; Zhou, B. Coexposure to environmental concentrations of cis-bifenthrin and graphene oxide: Adverse effects on the nervous system during metamorphic development of Xenopus laevis. J. Hazard. Mater. 2020, 381, 120995. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Wang, B.; Feng, Q.; Wang, R. Potential toxicity of carbonaceous nanomaterials on aquatic organisms and their alleviation strategies: A review. Ecotoxicol. Environ. Saf. 2024, 285, 117019. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, C.; Khan, I.A.; Tang, Y.; Liu, S.; Yang, M. Toxic effects of different-sized graphene oxide particles on zebrafish embryonic development. Ecotoxicol. Environ. Saf. 2020, 197, 110608. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Baharudin, F.; Zhang, Y.; Tan, K.; Zhang, Y.; Li, M.; Liang, Z.; Wu, M.; Zhang, M.; Zhang, D. Exposure to nanoscale graphene oxide deteriorates the quality of porcine oocytes via induction of oxidative stress and the apoptosis. J. Assist. Reprod. Genet. 2025, 2, 1–15. [Google Scholar] [CrossRef]
- Domenech, J.; Rodríguez-Garraus, A.; López de Cerain, A.; Azqueta, A.; Catalán, J. Genotoxicity of Graphene-Based Materials. Nanomaterials 2022, 12, 1795. [Google Scholar] [CrossRef] [PubMed]
- Holmannova, D.; Borsky, P.; Svadlakova, T.; Borska, L.; Fiala, Z. Reproductive and Developmental Nanotoxicity of Carbon Nanoparticles. Nanomaterials 2022, 12, 1716. [Google Scholar] [CrossRef]
- Jiang, Y.; Raliya, R.; Fortner, J.D.; Biswas, P. Graphene Oxides in Water: Correlating Morphology and Surface Chemistry with Aggregation Behavior. Environ. Sci. Technol. 2016, 50, 6964–6973. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Aquino, A.M.; Salata, G.C.; Pinho, C.F.; de Freitas, A.T.A.G.; Périco, L.L.; de Lion Siervo, G.E.M.; Mendes, L.O.; De Medeiros, P.D.; Justulin, L.A.; Fernandes, G.S.A.; et al. Arsenic exposure during prepuberty alters prostate maturation in pubescent rats. Reprod. Toxicol. 2019, 89, 136–144. [Google Scholar]
- WHO. Guidelines for drinking-water quality. In Health Criteria and Other Supporting Information: Addendum; World Health Organization: Geneva, Switzerland, 1998; Volume 2. [Google Scholar]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Mukherjee, A.B.; Bhattacharya, P.; Jacks, G.; Banerjee, D.M.; Ramanathan, A.L.; Mahanta, C.; Chandrashekharan, D.; Chatterjee, D.; Naidu, R. Ground water Arsenic Contamination in India: Extent and Severity. In Managing Arsenic in the Environment: From Soil to Human Health; Naidu, R., Smith, E., Owens, G., Bhattacharya, P., Nadebaum, P., Eds.; CSIRO Publishing: Melbourne, Australia, 2006; pp. 533–594. [Google Scholar]
- Fu, C.; Liu, T.; Li, L.; Liu, H.; Liang, Q.; Meng, X. Effects of graphene oxide on the development of offspring mice in lactation period. Biomaterials 2015, 40, 23–31. [Google Scholar] [CrossRef] [PubMed]
- OECD. Principles of Good Laboratory Practice (GLP), May, Doc C (81) 30 (Final) Annex 2; Organization for Economic Cooperation and Development: Paris, France, 1981. [Google Scholar]
- NRC (National Research Council). Recognition and Alleviation of Distress in Laboratory Animals; The National Academies Press: Washington, DC, USA, 2008. [Google Scholar]
- ElMazoudy, R.H.; Attia, A.A.; AbdElGawad, H.S. Evaluation of developmental toxicity induced by anticholinesterase insecticide, diazinon in female rats. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2011, 92, 534–542. [Google Scholar] [CrossRef]
- Borges, C.D.; Dias, A.F.; Silva, P.V.; Rosa, J.L.; Guerra, M.T.; Silva, R.F.; Kiguti, L.R.; Pupo, A.S.; Kempinas, W.G. Long-term adverse effects on reproductive function in male rats exposed prenatally to the glucocorticoid betamethasone. Toxicology 2017, 376, 15. [Google Scholar] [CrossRef]
- Gallavan, R.H., Jr.; Holson, J.F.; Stump, D.G.; Knapp, J.F.; Reynolds, V.L. Interpreting the toxicologic significance of alterations in anogenital distance: Potential for confounding effects of progeny body weights. Reprod. Toxicol. 1999, 13, 383–390. [Google Scholar] [CrossRef]
- Mylchreest, E.; Wallace, D.G.; Cattley, R.C.; Foster, P.M. Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to Di(n-butyl) phthalate during late gestation. Toxicol. Sci. 2000, 55, 143–151. [Google Scholar] [CrossRef]
- Ajayi, A.F.; Akhigbe, R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: An update. Fertil. Res. Pract. 2020, 6, 5. [Google Scholar] [CrossRef]
- ElMazoudy, R.; Attia, A. Ginger causes subfertility and abortifacient in mice by targeting both estrous cycle and blastocyst implantation without teratogenesis. Phytomedicine 2018, 50, 300–308. [Google Scholar] [CrossRef] [PubMed]
- ElMazoudy, R.; Attia, A. Efficacy of Ginkgo biloba on Vaginal Estrous and Ovarian Histological Alterations for Evaluating An-ti-Implantation and Abortifacient Potentials in Albino Female Mice. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2012, 95, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.S.B.; Decoster, L.; Trova, S.; Mimouni, N.E.H.; Delli, V.; Chachlaki, K.; Yu, Q.; Boehm, U.; Prevot, V.; Giacobini, P. Female sexual behavior is disrupted in a preclinical mouse model of PCOS via an attenuated hypothalamic nitric oxide pathway. Proc. Natl. Acad. Sci. USA 2022, 119, e2203503119. [Google Scholar] [CrossRef]
- USEPA. Guidelines for Ecological Risk Assessment. Report No. EPA/630/R-95/002F; USEPA: Washington, DC, USA, 1998.
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Higginbotham, A.L.; Kosynkin, D.V.; Sinitskii, A.; Sun, Z.; Tour, J.M. Lower-defect graphene oxide nanoribbons from multiwalled carbon nanotubes. ACS Nano 2010, 4, 2059–2069. [Google Scholar] [CrossRef]
- Thomas, J.; Kumar, V.; Sharma, N.; John, N.; Umesh, M.; Kumar Dasarahally Huligowda, L.; Kaur, K.; Utreja, D. Recent approaches in nanotoxicity assessment for drug delivery applications: Challenges and prospects. Med. Drug Discov. 2025, 25, 100204. [Google Scholar] [CrossRef]
- Havelikar, U.; Ghorpade, K.B.; Kumar, A.; Patel, A.; Singh, M.; Banjare, N.; Gupta, P.N. Comprehensive insights into mechanism of nanotoxicity, assessment methods and regulatory challenges of nanomedicines. Discover Nano 2024, 19, 165. [Google Scholar] [CrossRef]
- Ghulam, A.N.; Dos Santos, O.A.L.; Hazeem, L.; Pizzorno Backx, B.; Bououdina, M.; Bellucci, S. Graphene Oxide (GO) Materials-Applications and Toxicity on Living Organisms and Environment. J. Funct. Biomater. 2022, 13, 77. [Google Scholar] [CrossRef]
- Zhou, D.; Cai, Y.; Yang, Z.; Wan, H. Interplay of compound pollutants with microplastics transported in saturated porous media: Effect of co-existing graphene oxide and tetracycline. J. Contam. Hydro. 2023, 259, 104255. [Google Scholar] [CrossRef]
- Mikhael, S.; Punjala-Patel, A.; Gavrilova-Jordan, L. Hypothalamic-Pituitary-Ovarian Axis Disorders Impacting Female Fertility. Biomedicines 2019, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, C.R.; Borowicz, P.P.; Ward, A.K.; Caton, J.S.; Czernik, M.; Palazzese, L.; Loi, P.; Reynolds, L.P. Programming of Embryonic Development. Int. J. Mol. Sci. 2021, 22, 11668. [Google Scholar] [CrossRef]
- Sokou, R.; Lianou, A.; Lampridou, M.; Panagiotounakou, P.; Kafalidis, G.; Paliatsiou, S.; Volaki, P.; Tsantes, A.G.; Boutsikou, T.; Iliodromiti, Z.; et al. Neonates at Risk: Understanding the Impact of High-Risk Pregnancies on Neonatal Health. Medicina 2025, 61, 1077. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, X.; Sun, J.; Zhou, Q. Specific nanotoxicity of graphene oxide during zebrafish embryogenesis. Nanotoxicology 2016, 10, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Song, B.; Wu, J.; Zhang, Y.; Chen, A.; Shao, L. Potential adverse effects of nanoparticles on the reproductive system. Int. J. Nanomed. 2018, 13, 8487–8506. [Google Scholar] [CrossRef]
- Jocsak, G.; Ioja, E.; Kiss, D.S.; Toth, I.; Barany, Z.; Bartha, T.; Frenyo, L.V.; Zsarnovszky, A. Endocrine Disruptors Induced Distinct Expression of Thyroid and Estrogen Receptors in Rat versus Mouse Primary Cerebellar Cell Cultures. Brain Sci. 2019, 9, 359. [Google Scholar] [CrossRef]
- Howard, S.R. Interpretation of reproductive hormones before, during and after the pubertal transition—Identifying health and disordered puberty. Clin. Endocrinol. 2021, 95, 702–715. [Google Scholar] [CrossRef]
- Shan, D.; Wen, X.; Guan, X.; Fang, H.; Liu, Y.; Qin, M.; Wang, H.; Xu, J.; Lv, J.; Zhao, J.; et al. Pubertal lead exposure affects ovary development, folliculogenesis and steroidogenesis by activation of IRE1α-JNK signaling pathway in rat. Ecotoxicol. Environ. Saf. 2023, 257, 114919. [Google Scholar] [CrossRef]
- Biswas, S.; Maitra, S. Altered redox homeostasis in steroid-depleted follicles attenuates hCG regulation of follicular events: Cross-talk between endocrine and IGF axis in maturing oocytes. Free Radic. Biol. Med. 2021, 172, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.C.; Wu, K.Y.; Lin, I.W.; Yang, Z.J.; Chang, A.A.; Hu, M.C. Bisphenol A disrupts steroidogenesis and induces a sex hormone imbalance through c-Jun phosphorylation in Leydig cells. Chemosphere 2017, 185, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Parodi, D.A.; Greenfield, M.; Evans, C.; Chichura, A.; Alpaugh, A.; Williams, J.; Martin, M.B. Alteration of mammary gland development and gene expression by in utero exposure to arsenic. Reprod. Toxicol. 2015, 54, 66–75. [Google Scholar] [CrossRef]
- Reilly, M.P.; Saca, J.C.; Hamilton, A.; Solano, R.F.; Rivera, J.R.; Whitehouse-Innis, W.; Parsons, J.G.; Dearth, R.K. Prepubertal exposure to arsenic (III) suppresses circulating insulin-like growth factor-1 (IGF-1) delaying sexual maturation in female rats. Reprod. Toxicol. 2014, 44, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Kang, Y.; Zhang, C.; Xie, Y.; Wang, C.; Liu, J.; Yu, C.; Zhao, H.; Huang, D. The Role of Kisspeptin in the Control of the Hypothalamic-Pituitary-Gonadal Axis and Reproduction. Front. Endocrinol. 2022, 13, 925206. [Google Scholar] [CrossRef]
- Koysombat, K.; Tsoutsouki, J.; Patel, A.H.; Comninos, A.N.; Dhillo, W.S.; Abbara, A. Kisspeptin and neurokinin B: Roles in reproductive health. Physiol. Rev. 2025, 105, 707–764. [Google Scholar] [CrossRef]
- Graceli, J.B.; Dettogni, R.S.; Merlo, E.; Niño, O.; da Costa, C.S.; Zanol, J.F.; Ríos Morris, E.A.; Miranda-Alves, L.; Denicol, A.C. The impact of endocrine-disrupting chemical exposure in the mammalian hypothalamic-pituitary axis. Mol. Cell. Endocrinol. 2020, 518, 110997. [Google Scholar] [CrossRef]
- Marlatt, V.L.; Bayen, S.; Castaneda-Cortès, D.; Delbès, G.; Grigorova, P.; Langlois, V.S.; Martyniuk, C.J.; Metcalfe, C.D.; Parent, L.; Rwigemera, A.; et al. Impacts of endocrine disrupting chemicals on reproduction in wildlife and humans. Environ. Res. 2022, 208, 112584. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, J.; Kim, P.; Choi, K.; Kim, S.; Shon, W.; Park, K. A transfer of silver nanoparticles from pregnant rat to offspring. Toxicol. Res. 2012, 28, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, K.Y.; Salim, E.I.; Abdel-Latif, A.S.; Abu-Risha, S.E. Tissue distribution, placental transfer and excretion of silver nanoparticles in pregnant rats after a single oral dose. Environ. Anal. Health Toxicol. 2023, 38, e2023023. [Google Scholar] [CrossRef]
- Ortega, I.; Cress, A.B.; Wong, D.H.; Villanueva, J.A.; Sokalska, A.; Moeller, B.C.; Stanley, S.D.; Duleba, A.J. Simvastatin reduces steroidogenesis by inhibiting Cyp17a1 gene expression in rat ovarian theca-interstitial cells. Biol. Reprod. 2012, 86, 1–9. [Google Scholar] [CrossRef]
- Kakuta, H.; Iguchi, T.; Sato, T. The Involvement of Granulosa Cells in the Regulation by Gonadotropins of Cyp17a1 in Theca Cells. In Vivo 2018, 32, 1387–1401. [Google Scholar] [CrossRef]
- Liu, C.; Peng, J.; Matzuk, M.M.; Yao, H.H. Lineage specification of ovarian theca cells requires multicellular interactions via oocyte and granulosa cells. Nat. Commun. 2015, 6, 6934. [Google Scholar] [CrossRef]
- Kugathas, I.; Johansson, H.K.L.; Chan Sock Peng, E.; Toupin, M.; Evrard, B.; Darde, T.A.; Boberg, J.; Draskau, M.K.; Rolland, A.D.; Mazaud-Guittot, S.; et al. Transcriptional profiling of the developing rat ovary following intrauterine exposure to the endocrine disruptors diethylstilbestrol and ketoconazole. Arch. Toxicol. 2023, 97, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, H.; Ye, C.; Wang, X.; Ji, D.; Zhang, Z.; Cao, Y.; Zou, W. Graphene Oxide Nanosheets Induce Mitochondrial Toxicity in Human Ovarian Granulosa Cells: Implications for Female Reproductive Health. Int. J. Nanomed. 2025, 20, 4461–4479. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.K.L.; Christiansen, S.; Draskau, M.K.; Svingen, T.; Boberg, J. Classical toxicity endpoints in female rats are insensitive to the human endocrine disruptors diethylstilbestrol and ketoconazole. Reprod. Toxicol. 2021, 101, 9–17. [Google Scholar] [CrossRef]
- Kim, J.-M.; Song, K.-S.; Xu, B.; Wang, T. Role of potassium channels in female reproductive system. Obstet. Gynecol. Sci. 2020, 63, 565–576. [Google Scholar] [CrossRef]
- Nakanishi, T.; Tanaka, R.; Tonai, S.; Lee, J.Y.; Yamaoka, M.; Kawai, T.; Okamoto, A.; Shimada, M.; Yamashita, Y. LH Induces De Novo Cholesterol Biosynthesis via SREBP Activation in Granulosa Cells During Ovulation in Female Mice. Endocrinology 2021, 162, bqab166. [Google Scholar] [CrossRef]
- Guo, R.; Mao, J.; Yan, L.T. Computer simulation of cell entry of graphene nanosheet. Biomaterials 2013, 34, 4296–4301. [Google Scholar] [CrossRef]
- Yadav, S.; Singh Raman, A.P.; Meena, H.; Goswami, A.G.; Bhawna Kumar, V.; Jain, P.; Kumar, G.; Sagar, M.; Rana, D.K.; Bahadur, I.; et al. An Update on Graphene Oxide: Applications and Toxicity. ACS Omega 2022, 7, 35387–35445. [Google Scholar] [CrossRef]
- Cao, X.; Ma, C.; Zhao, J.; Musante, C.; White, J.C.; Wang, Z.; Xing, B. Interaction of graphene oxide with co-existing arsenite and arsenate: Adsorption, transformation and combined toxicity. Environ. Int. 2019, 131, 104992. [Google Scholar] [CrossRef] [PubMed]
- Dasmahapatra, A.K.; Dasari, T.P.S.; Tchounwou, P.B. Graphene-Based Nanomaterials Toxicity in Fish. Rev. Environ. Contam. Toxicol. 2019, 247, 1–58. [Google Scholar]
- Karimipour, M.; Zirak Javanmard, M.; Ahmadi, A.; Jafari, A. Oral administration of titanium dioxide nanoparticle through ovarian tissue alterations impairs mice embryonic development. Int. J. Reprod. Biomed. 2018, 16, 397–404. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, X.; Zhou, Y.; Yu, D.; Deng, Y.; Ouyang, J.; Yang, B.; Luo, D.; Zhang, D.; Kuang, H. Gestational exposure to titanium dioxide nanoparticles impairs the placentation through dysregulation of vascularization, proliferation and apoptosis in mice. Int. J. Nanomed. 2018, 13, 777–789. [Google Scholar] [CrossRef]
- Dziewięcka, M.; Witas, P.; Karpeta-Kaczmarek, J.; Kwaśniewska, J.; Flasz, B.; Balin, K.; Augustyniak, M. Reduced fecundity and cellular changes in Acheta domesticus after multigenerational exposure to graphene oxide nanoparticles in food. Sci. Total Environ. 2018, 635, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Fasolino, A.; Los, J.H.; Katsnelson, M.I. Intrinsic Ripples in Graphene. Nat. Mater. 2007, 6, 858–861. [Google Scholar] [CrossRef]
- Hou, W.-C.; Chowdhury, I.; Goodwin, D.G.; Henderson, W.M.; Fairbrother, D.H.; Bouchard, D.; Zepp, R.G. Photochemical Transformation of Graphene Oxide in Sunlight. Environ. Sci. Technol. 2015, 49, 3435–3443. [Google Scholar] [CrossRef] [PubMed]
- Salas, E.C.; Sun, Z.; Luttge, A.; Tour, J.M. Reduction of Graphene Oxide via Bacterial Respiration. ACS Nano 2010, 4, 4852–4856. [Google Scholar] [CrossRef]
- Mypati, S.; Sellathurai, A.; Kontopoulou, M.; Docoslis, A.; Barz, D.P. High concentration graphene nanoplatelet dispersions in water stabilized by graphene oxide. Carbon 2021, 174, 581–593. [Google Scholar] [CrossRef]



| Experimental Groups | ||||
|---|---|---|---|---|
| CON | ARS | GOX | ARS + GOX | |
| (%) Females with regular estrous cycles | 100 | 33 | 15 | 25 |
| Number of days in estrous cycle | 5.13± 0.83 | 4.63 ± 0.71 * | 4.56 ± 0.29 * | 4.53 ± 0.21 * |
| Number of estrous cycles | 2.94 ± 0.11 | 3.54 ± 0.12 * | 3.51 ± 0.32 * | 3.61 ± 0.12 * |
| Proestrus/cycle | 1.11 ± 0.013 | 1.00 ± 0.013 | 1.00± 0.033 | 1.09 ± 0.011 |
| Estrus/cycle | 1.25 ± 0.042 | 1.11 ± 0.012 | 1.09 ± 0.012 | 1.07 ± 0.21 |
| Metestrus/cycle | 1.30± 0.032 | 0.52 ± 0.02 * | 0.45 ± 0.022 * | 0.34 ± 0.10 * |
| Diestrus/cycle | 1.47 ± 0.015 | 2.00 ± 0.75 * | 2.02 ± 0.55 * | 2.03 ± 0.21 * |
| Parameters | Experimental Groups | |||
|---|---|---|---|---|
| CON | ARS | GOX | ARS + GOX | |
| % Gestation rate | 100 | 79 * | 77 * | 58 * |
| Fertility potential (efficiency of implantation) | 97.47 ± 4.36 | 74.12 ± 3.11 * | 72.79 ± 3.88 * | 66.37 ± 4.66 * |
| Corpora lutea number/uterus | 13.43 ± 1.42 | 11.67 ± 2.32 * | 11.27 ± 1.83 * | 10.02 ± 1.56 * |
| Implantation number/uterus | 13.09 ± 1.66 | 8.65 ± 1.57 * | 9.33 ± 0.68 * | 6.65 ± 0.48 * |
| Live litter size (viable number) | 12.78 ± 2.64 | 6.10 ± 0.52 * | 7.02 ± 0.68 * | 4.05 ± 0.68 * |
| Stillborn number | 0.0 | 1.10 ± 0.01 * | 1.14 ± 0.05 * | 1.13 ± 0.73 * |
| Resorption number | 0.31 ± 0.01 | 1.45 ± 0.02 * | 1.17 ± 0.02 * | 1.47 ± 0.49 * |
| Pre-implantation loss (%) | 2.53 ± 0.62 | 25.88 ± 0.60 * | 17.214 ± 0.92 * | 33.63 ± 2.61 * |
| Post-implantation loss (%) | 2.37 ± 0.01 | 29.47 ± 2.02 * | 24.76 ± 2.11 * | 39.09 ± 1.32 * |
| Fetus weight (g) | 3.78 ± 0.21 | 3.59 ± 0.21 | 3.63 ± 0.21 | 3.62 ± 0.21 |
| Crown rump length (CRL) (mm) | 41.01 ± 2.12 | 40.01 ± 2.45 | 38.02 ± 1.82 | 38.41 ± 1.23 |
| Placenta weight (mg) | 450 ± 70.13 | 430 ± 69.54 | 440 ± 71.34 | 400 ± 66.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ElMazoudy, R.H.; Attia, A.A.; Saleh, T.A. Synergy of Arsenic and Graphene Oxide in Utero and Lactation Exacerbates Reproductive Disorders in Female Rat Offspring Undergoing Puberty and Maturity. Toxics 2025, 13, 787. https://doi.org/10.3390/toxics13090787
ElMazoudy RH, Attia AA, Saleh TA. Synergy of Arsenic and Graphene Oxide in Utero and Lactation Exacerbates Reproductive Disorders in Female Rat Offspring Undergoing Puberty and Maturity. Toxics. 2025; 13(9):787. https://doi.org/10.3390/toxics13090787
Chicago/Turabian StyleElMazoudy, Reda H., Azza A. Attia, and Tawfik A. Saleh. 2025. "Synergy of Arsenic and Graphene Oxide in Utero and Lactation Exacerbates Reproductive Disorders in Female Rat Offspring Undergoing Puberty and Maturity" Toxics 13, no. 9: 787. https://doi.org/10.3390/toxics13090787
APA StyleElMazoudy, R. H., Attia, A. A., & Saleh, T. A. (2025). Synergy of Arsenic and Graphene Oxide in Utero and Lactation Exacerbates Reproductive Disorders in Female Rat Offspring Undergoing Puberty and Maturity. Toxics, 13(9), 787. https://doi.org/10.3390/toxics13090787








