Effects of ZnFe2O4 Nanoparticles on Development and Rhythmic Behavior of Drosophila melanogaster
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnFe2O4-NPs
2.2. Characterization of ZnFe2O4-NPs
2.3. Exposure to ZnFe2O4-NPs and Experimental Conditions
2.4. TEM Analysis of Guts of D. melanogaster
2.5. Developmental Detection Experiments
2.6. Monitoring of Rhythmic Behavior in Drosophila
2.7. Oxidative Stress Analysis and Neurotransmitter Levels Detection
2.8. QRT-PCR Detection
2.9. Statistical Analysis
3. Results
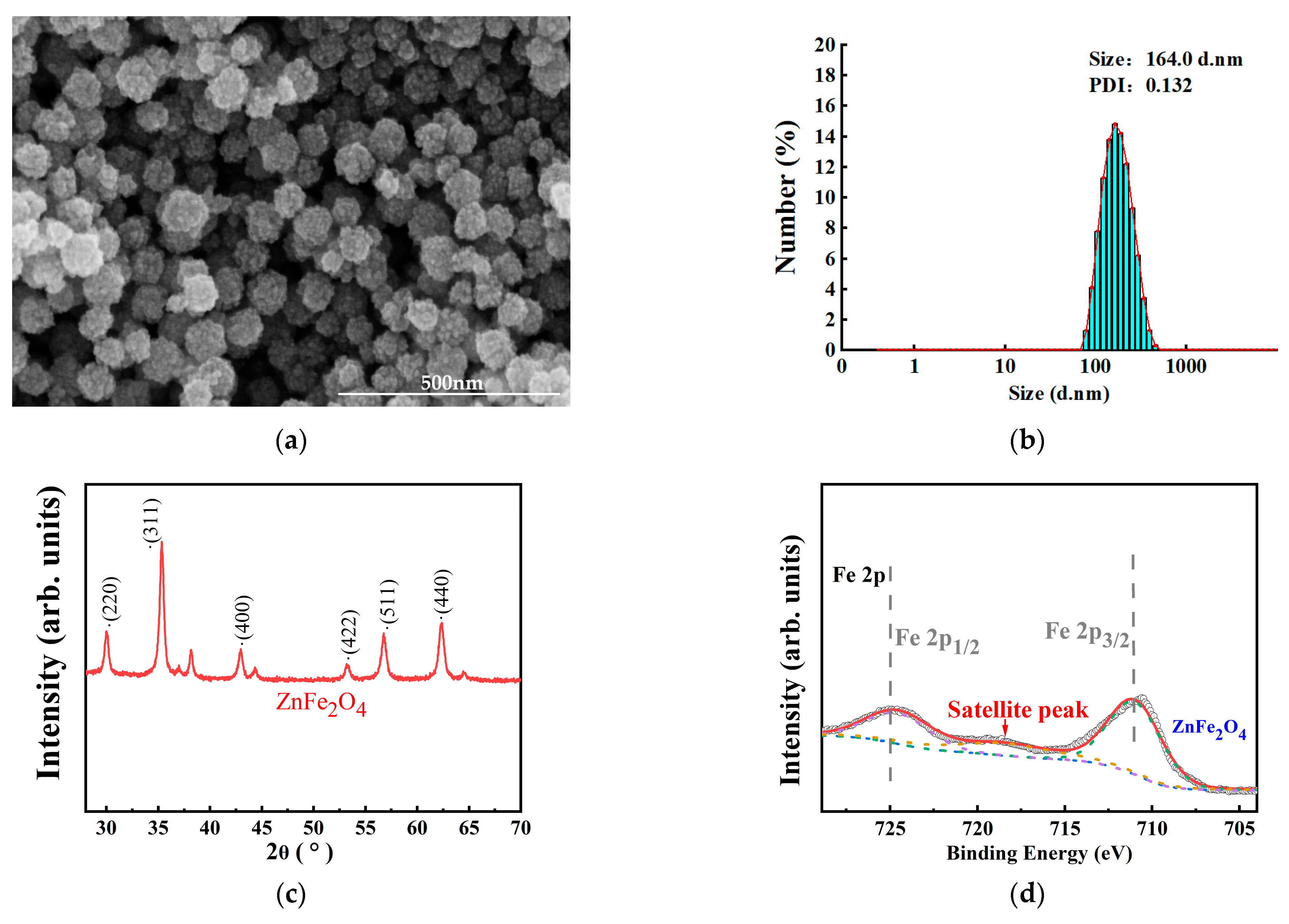
3.1. SEM Analysis of Nano ZnFe2O4 and the TEM Analysis of Intestines of Drosophila melanogaster
3.2. Effects of ZnFe2O4-NPs on Development
3.3. Effects of ZnFe2O4-NPs Exposure on Rhythmic Behaviors
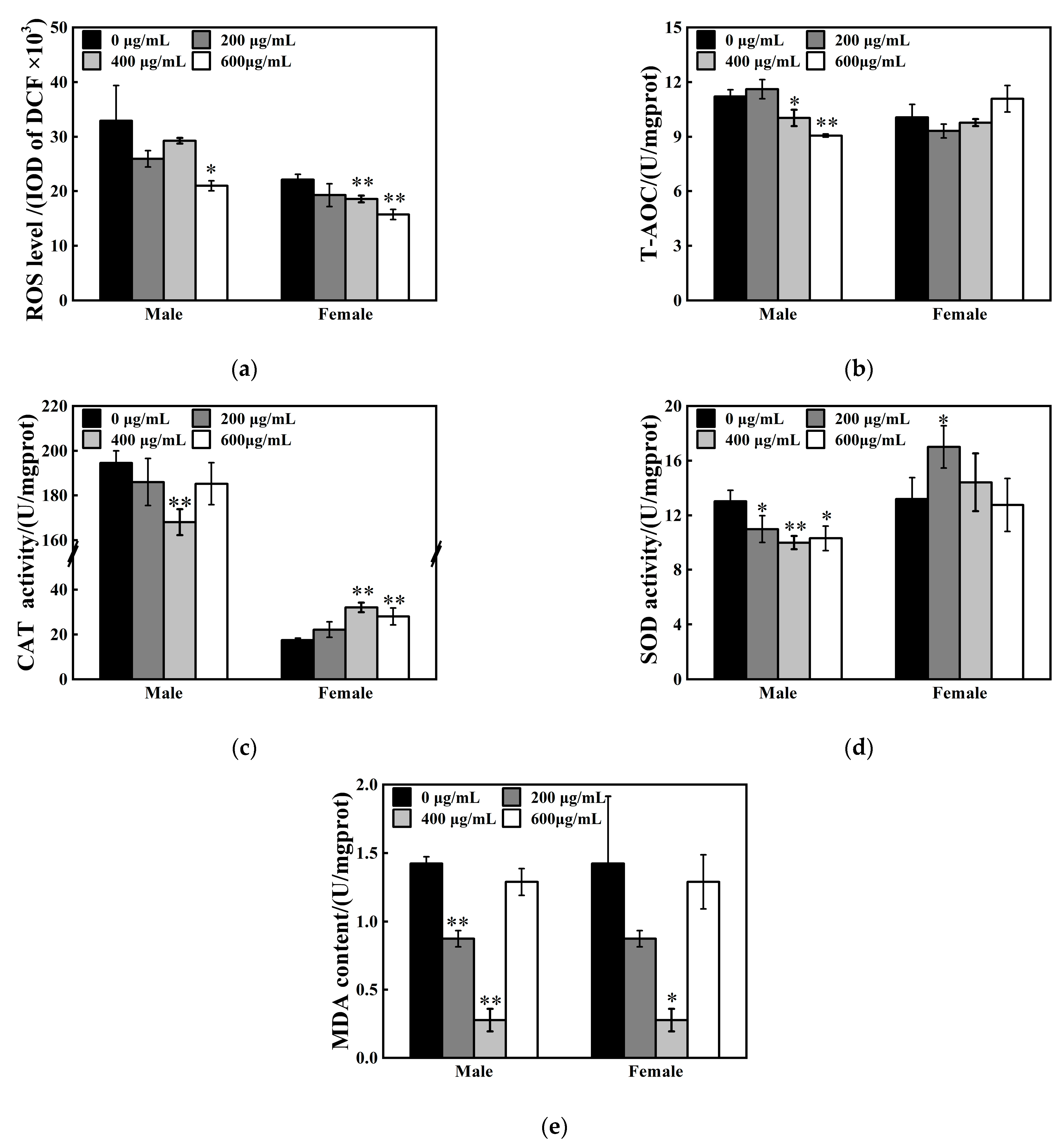
3.4. Oxidative Stress Responses Under ZnFe2O4-NPs Exposure
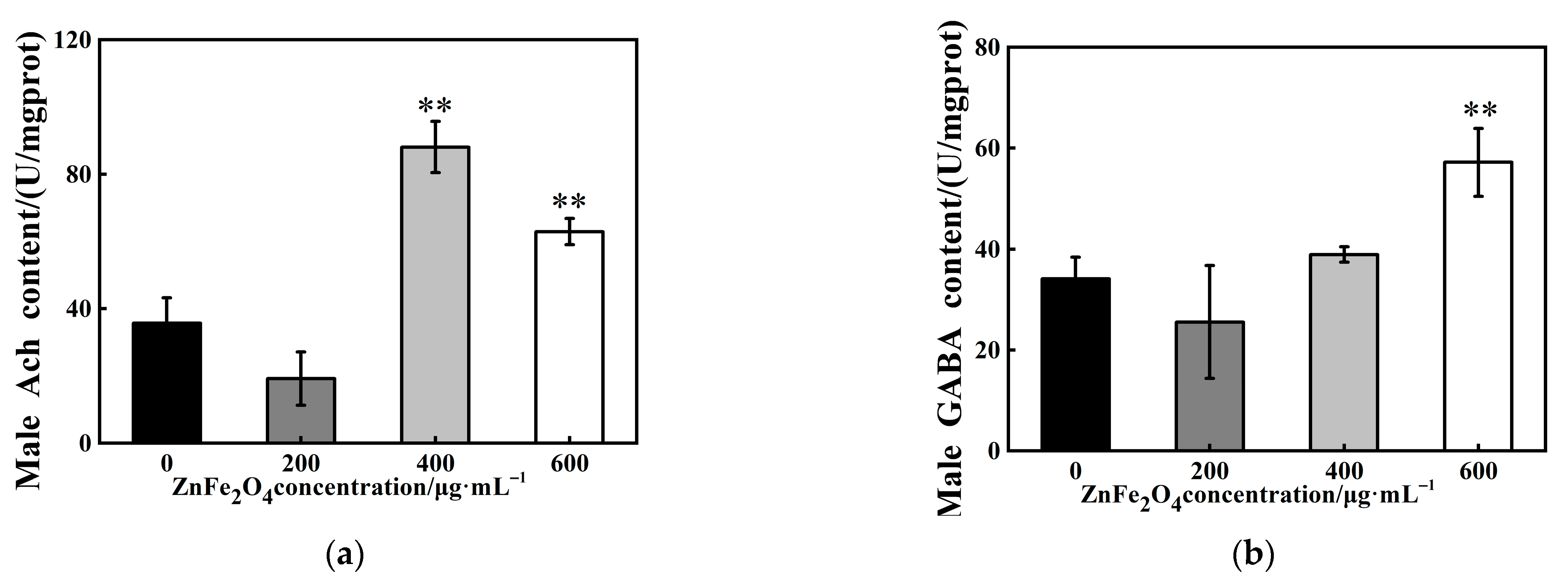
3.5. Effects of ZnFe2O4-NPs on Neurotransmitters Levels
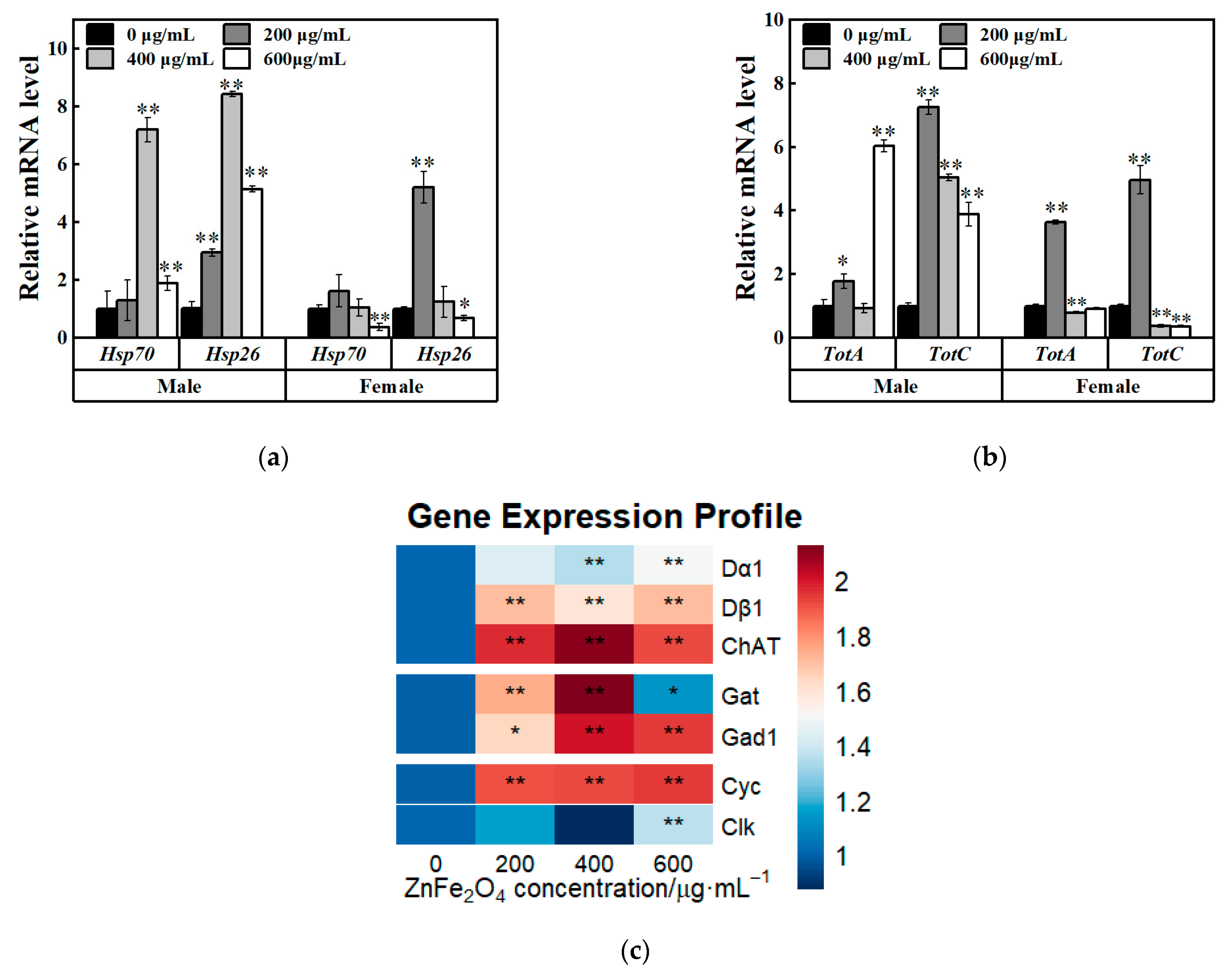
3.6. Relative Expression Levels of Target Genes After ZnFe2O4-NPs Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahim, A.A.; Saed, M.; Al Abdullah, S.; Dellinger, K.; Obare, S.O.; Pathiraja, G. Magnetic zinc ferrite nanostructures: Recent advancements for environmental and biomedical applications. J. Alloys Compd. 2024, 4, 100038. [Google Scholar] [CrossRef]
- Zimina, T.M.; Sitkov, N.O.; Gareev, K.G.; Fedorov, V.; Grouzdev, D.; Koziaeva, V.; Gao, H.; Combs, S.E.; Shevtsov, M. Biosensors and Drug Delivery in Oncotheranostics Using Inorganic Synthetic and Biogenic Magnetic Nanoparticles. Biosensors 2022, 12, 789. [Google Scholar] [CrossRef]
- Rezaei, B.; Yari, P.; Sanders, S.M.; Wang, H.; Chugh, V.K.; Liang, S.; Mostufa, S.; Xu, K.; Wang, J.P.; Gómez-Pastora, J.; et al. Magnetic Nanoparticles: A Review on Synthesis, Characterization, Functionalization, and Biomedical Applications. Small 2024, 20, e2304848. [Google Scholar] [CrossRef]
- Liu, W.; Dong, Z.; Liu, J.; Li, Z.; Wang, Y.; Cao, X.; Zhang, Z.; Liu, Y. Hollow S-Doped ZnFe2O4 Microcubes with Magnetic Separability for Photocatalytic Removal of Uranium(VI) under Different Light Intensity. Inorg. Chem. 2024, 63, 11369–11380. [Google Scholar] [CrossRef]
- Omelyanchik, A.; Levada, K.; Pshenichnikov, S.; Abdolrahim, M.; Baricic, M.; Kapitunova, A.; Galieva, A.; Sukhikh, S.; Astakhova, L.; Antipov, S.; et al. Green Synthesis of Co-Zn Spinel Ferrite Nanoparticles: Magnetic and Intrinsic Antimicrobial Properties. Materials 2020, 13, 5014. [Google Scholar] [CrossRef]
- Rabbani, A.; Haghniaz, R.; Khan, T.; Khan, R.; Khalid, A.; Naz, S.S.; Ul-Islam, M.; Vajhadin, F.; Wahid, F. Development of bactericidal spinel ferrite nanoparticles with effective biocompatibility for potential wound healing applications. RSC Adv. 2021, 11, 1773–1782. [Google Scholar] [CrossRef]
- Qin, H.; He, Y.; Xu, P.; Huang, D.; Wang, Z.; Wang, H.; Wang, Z.; Zhao, Y.; Tian, Q.; Wang, C. Spinel ferrites (MFe2O4): Synthesis, improvement and catalytic application in environment and energy field. Adv. Colloid Interface Sci. 2021, 294, 102486. [Google Scholar] [CrossRef] [PubMed]
- Tabesh, F.; Mallakpour, S.; Hussain, C.M. Recent advances in magnetic semiconductor ZnFe2O4 nanoceramics: History, properties, synthesis, characterization, and applications. J. Solid State Chem. 2023, 322, 123940. [Google Scholar] [CrossRef]
- Ferrite Market—Forecast (2025–2031). Available online: https://www.industryarc.com/Report/15854/ferrite-market.html (accessed on 14 August 2025).
- Nowack, B. Evaluation of Environmental Exposure Models for Engineered Nanomaterials in a Regulatory Context. NanoImpact 2017, 8, 38–47. [Google Scholar] [CrossRef]
- Thakur, D.; Ta, Q.T.H.; Noh, J.S. Photosensitizer-Conjugated Hollow ZnFe2O4 Nanoparticles for Antibacterial Near-Infrared Photodynamic Therapy. ACS Appl. Nano Mater. 2022, 5, 1533–1541. [Google Scholar] [CrossRef]
- Nguyen, V.V.H.; Chu, M.H.; Nguyen, D.H.; Nguyen, V.D.; Park, I.; Nguyen, V.H. Excellent detection of H2S gas at ppb concentrations using ZnFe2O4 nanofibers loaded with reduced graphene oxide. Sens. Actuators B Chem. 2019, 282, 876–884. [Google Scholar] [CrossRef]
- Xu, Z.; Long, X.; Jia, Y.; Zhao, D.; Pan, X. Occurrence, transport, and toxicity of nanomaterials in soil ecosystems: A review. Environ. Chem. Lett. 2022, 20, 3943–3969. [Google Scholar] [CrossRef]
- Tao, Z.; Zhou, Q.; Zheng, T.; Mo, F.; Ouyang, S. Iron oxide nanoparticles in the soil environment: Adsorption, transformation, and environmental risk. J. Hazard. Mater. 2023, 459, 132107. [Google Scholar] [CrossRef]
- Surendra, D.M.; Chamaraja, N.A.; Godipurge, S.S.; Yallappa, S. Synthesis and functionalization of silver ferrite (AgFe2O3) nanoparticles with l-methionine: In vivo toxicity studies against Drosophila melanogaster (Diptera: Drosophilidae). Results Chem. 2022, 4, 100565. [Google Scholar] [CrossRef]
- Pilaquinga, F.; Cárdenas, S.; Vela, D.; Jara, E.; Morey, J.; Gutiérrez-Coronado, J.L.; Debut, A.; Piña, M.L.N. Fertility and Iron Bioaccumulation in Drosophila melanogaster Fed with Magnetite Nanoparticles Using a Validated Method. Molecules 2021, 26, 2808. [Google Scholar] [CrossRef] [PubMed]
- Güneş, M.; Aktaş, K.; Yalçın, B.; Burgazlı, A.Y.; Asilturk, M.; Ünşar, A.E.; Kaya, B. In vivo assessment of the toxic impact of exposure to magnetic iron oxide nanoparticles (IONPs) using Drosophila melanogaster. Environ Toxicol. Pharmacol. 2024, 107, 104412. [Google Scholar] [CrossRef] [PubMed]
- Bag, J.; Mukherjee, S.; Ghosh, S.K.; Das, A.; Mukherjee, A.; Sahoo, J.K.; Tung, K.S.; Sahoo, H.; Mishra, M. Fe3O4 coated guargum nanoparticles as non-genotoxic materials for biological application. Int. J. Biol. Macromol. 2020, 165, 333–345. [Google Scholar] [CrossRef]
- Malhotra, N.; Chen, J.R.; Sarasamma, S.; Audira, G.; Siregar, P.; Liang, S.T.; Lai, Y.H.; Lin, G.M.; Ger, T.R.; Hsiao, C.D. Ecotoxicity Assessment of Fe3O4 Magnetic Nanoparticle Exposure in Adult Zebrafish at an Environmental Pertinent Concentration by Behavioral and Biochemical Testing. Nanomaterials 2019, 9, 873. [Google Scholar] [CrossRef]
- Tawfik, M.M.; Mohamed, M.H.; Sadak, M.S.; Thalooth, A.T. Iron oxide nanoparticles effect on growth, physiological traits and nutritional contents of Moringa oleifera grown in saline environment. Bull. Natl. Res. Cent. 2021, 45, 177. [Google Scholar] [CrossRef]
- Chauhan, S.; Naik, S.; Kumar, R.; Ruokolainen, J.; Kesari, K.K.; Mishra, M.; Gupta, P.K. In Vivo Toxicological Analysis of the ZnFe2O4@poly(tBGE-alt-PA) Nanocomposite: A Study on Fruit Fly. ACS Omega 2024, 9, 6549–6555. [Google Scholar] [CrossRef]
- Ong, C.; Yung, L.Y.; Cai, Y.; Bay, B.H.; Baeg, G.H. Drosophila melanogaster as a model organism to study nanotoxicity. Nanotoxicology 2015, 9, 396–403. [Google Scholar] [CrossRef]
- Parashar, S.; Raj, S.; Srivastava, P.; Singh, A.K. Comparative toxicity assessment of selected nanoparticles using different experimental model organisms. J. Pharmacol. Toxicol. Methods 2024, 130, 107563. [Google Scholar] [CrossRef]
- Ng, C.T.; Yu, L.E.; Ong, C.N.; Bay, B.H.; Baeg, G.H. The use of Drosophila melanogaster as a model organism to study immune-nanotoxicity. Nanotoxicology 2019, 13, 429–446. [Google Scholar] [CrossRef]
- Raj, A.; Shah, P.; Agrawal, N. Sedentary behavior and altered metabolic activity by AgNPs ingestion in Drosophila melanogaster. Sci. Rep. 2017, 7, 15617. [Google Scholar] [CrossRef]
- Gautam, A.; Gautam, C.; Mishra, M.; Mishra, V.K.; Hussain, A.; Sahu, S.; Nanda, R.; Kisan, B.; Biradar, S.; Gautam, R.K. Enhanced mechanical properties of hBN-ZrO2 composites and their biological activities on Drosophila melanogaster: Synthesis and characterization. RSC Adv. 2019, 9, 40977–40996. [Google Scholar] [CrossRef]
- Parmalee, N.L.; Aschner, M. Metals and Circadian Rhythms. Adv. Neurotoxicol. 2017, 1, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, W.; Ashburner, M.; Hawley, R.S. Drosophila Protocols; Science Press: Bejing, China, 2004; pp. 589–590. [Google Scholar]
- Chen, H.; Wang, B.; Feng, W.; Du, W.; Ouyang, H.; Chai, Z.; Bi, X. Oral magnetite nanoparticles disturb the development of Drosophila melanogaster from oogenesis to adult emergence. Nanotoxicology 2015, 9, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Rocabert, A.; Martín-Pérez, J.; Pareras, L.; Egea, R.; Alaraby, M.; Cabrera-Gumbau, J.M.; Sarmiento, I.; Martínez-Urtaza, J.; Rubio, L.; Barguilla, I.; et al. Nanoplastic exposure affects the intestinal microbiota of adult Drosophila flies. Sci. Total Environ. 2025, 980, 179545. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Zhang, Z.; Sun, B.; Tang, C.; Zhang, L.; Jiang, Z.; Ding, B.; Liao, Y.; Cai, P. Simulated mobile communication frequencies (3.5 GHz) emitted by a signal generator affects the sleep of Drosophila melanogaster. Environ. Pollut. 2021, 283, 117087. [Google Scholar] [CrossRef]
- Donelson, N.C.; Kim, E.Z.; Slawson, J.B.; Vecsey, C.G.; Huber, R.; Griffith, L.C. High-resolution positional tracking for long-term analysis of Drosophila sleep and locomotion using the “tracker” program. PLoS ONE 2012, 7, e37250. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rommelaere, S.; Carboni, A.; Bada Juarez, J.F.; Boquete, J.P.; Abriata, L.A.; Teixeira Pinto Meireles, F.; Rukes, V.; Vincent, C.; Kondo, S.; Dionne, M.S.; et al. A humoral stress response protects Drosophila tissues from antimicrobial peptides. Curr. Biol. 2024, 34, 1426–1437.e6. [Google Scholar] [CrossRef]
- Amstrup, A.B.; Bæk, I.; Loeschcke, V.; Givskov Sørensen, J. A functional study of the role of Turandot genes in Drosophila melanogaster: An emerging candidate mechanism for inducible heat tolerance. J. Insect Physiol. 2022, 143, 104456. [Google Scholar] [CrossRef]
- Bushra, R.; Ahmad, M.; Alam, K.; Seidi, F.; Qurtulen; Shakeel, S.; Song, J.; Jin, Y.; Xiao, H. Recent advances in magnetic nanoparticles: Key applications, environmental insights, and future strategies. Sustain. Mater. Technol. 2024, 40, e00985. [Google Scholar] [CrossRef]
- Soares, G.A.; Faria, J.V.C.; Pinto, L.A.; Prospero, A.G.; Pereira, G.M.; Stoppa, E.G.; Buranello, L.P.; Bakuzis, A.F.; Baffa, O.; Miranda, J.R.A. Long-Term Clearance and Biodistribution of Magnetic Nanoparticles Assessed by AC Biosusceptometry. Materials 2022, 15, 2121. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Qi, Y.; Liu, G.; Song, Y.; Jiang, X.; Du, B. Size-Dependent In Vivo Transport of Nanoparticles: Implications for Delivery, Targeting, and Clearance. ACS Nano 2023, 17, 20825–20849. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharmacother. 2019, 111, 666–675. [Google Scholar] [CrossRef]
- Manzanares, D.; Ceña, V. Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell. Pharmaceutics 2020, 12, 371. [Google Scholar] [CrossRef]
- Alaraby, M.; Annangi, B.; Hernández, A.; Creus, A.; Marcos, R. A comprehensive study of the harmful effects of ZnO nanoparticles using Drosophila melanogaster as an in vivo model. J. Hazard. Mater. 2015, 296, 166–174. [Google Scholar] [CrossRef]
- Bacchetta, R.; Moschini, E.; Santo, N.; Fascio, U.; Del Giacco, L.; Freddi, S.; Camatini, M.; Mantecca, P. Evidence and uptake routes for Zinc oxide nanoparticles through the gastrointestinal barrier in Xenopus laevis. Nanotoxicology 2013, 8, 728–744. [Google Scholar] [CrossRef] [PubMed]
- Iacovita, C.; Stiufiuc, R.; Radu, T.; Florea, A.; Stiufiuc, G.; Dutu, A.; Mican, S.; Tetean, R.; Lucaciu, C.M. Polyethylene Glycol-Mediated Synthesis of Cubic Iron Oxide Nanoparticles with High Heating Power. Nanoscale Res. Lett. 2015, 10, 391. [Google Scholar] [CrossRef]
- Vela, D.; Rondal, J.; Cárdenas, S.; Gutiérrez-Coronado, J.; Jara, E.; Debut, A.; Pilaquinga, F. Assessment of the Toxic Effects of Chitosan-Coated Magnetite Nanoparticles on Drosophila melanogaster. Am. J. Appl. Sci. 2020, 17, 204–213. [Google Scholar] [CrossRef]
- Faria, F.S.; Areal, M.; Bitner-Mathé, B.C. Thermal Stress and Adult Fitness in a Drosophila suzukii Neotropical Propagule. Neotrop. Entomol. 2023, 52, 993–1004. [Google Scholar] [CrossRef]
- Matthews, S.; Xu, E.G.; Roubeau Dumont, E.; Meola, V.; Pikuda, O.; Cheong, R.; Guo, M.; Tahara, R.; Larsson, H.; Tufenkji, N. Polystyrene micro- and nanoplastics affect locomotion and daily activity of Drosophila melanogaster. Environ. Sci. Nano 2021, 8, 110–121. [Google Scholar] [CrossRef]
- Adamczyk-Grochala, J.; Wnuk, M.; Oklejewicz, B.; Klimczak, K.; Błoniarz, D.; Deręgowska, A.; Rzeszutek, I.; Stec, P.; Ciuraszkiewicz, A.; Kądziołka-Gaweł, M.; et al. Evaluation of anticancer activity of urotropine surface modified iron oxide nanoparticles using a panel of forty breast cancer cell lines. Nanotoxicology 2025, 19, 50–68. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, L.; Xu, H.F.; Dai, Z.H.; Hao, W.; Cao, J.M. Effect of Fe2O3 Magnetic Nanoparticles on Circadian Rhythm in Mice. In Proceedings of the 23rd National Congress of the Chinese Association for Physiological Sciences, Xi’an, China, 18–21 October 2010; Department of Physiology and Pathophysiology, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences & Peking Union Medical College, Eds.; Science Press: Beijing, China, 2010; pp. 230–231. [Google Scholar]
- Ma, P.; Luo, Q.; Chen, J.; Gan, Y.; Du, J.; Ding, S.; Xi, Z.; Yang, X. Intraperitoneal injection of magnetic Fe3O4-nanoparticle induces hepatic and renal tissue injury via oxidative stress in mice. Int. J. Nanomed. 2012, 7, 4809–4818. [Google Scholar] [CrossRef]
- Yu, S.; Rui, Q.; Cai, T.; Wu, Q.; Li, Y.; Wang, D. Close association of intestinal autofluorescence with the formation of severe oxidative damage in intestine of nematodes chronically exposed to Al2O3-nanoparticle. Environ. Toxicol. Pharmacol. 2011, 32, 233–241. [Google Scholar] [CrossRef]
- Musachio, E.A.S.; Poetini, M.R.; Janner, D.E.; Meichtry, L.B.; Poleto, K.H.; Fernandes, E.J.; Guerra, G.P.; Prigol, M. Sex-specific changes in oxidative stress parameters and longevity produced by Bisphenol F and S compared to Bisphenol A in Drosophila melanogaster. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 257, 109329. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Slobodian, M.R.; Petahtegoose, J.D.; Wallis, A.L.; Levesque, D.C.; Merritt, T.J.S. The Effects of Essential and Non-Essential Metal Toxicity in the Drosophila melanogaster Insect Model: A Review. Toxics 2021, 9, 269. [Google Scholar] [CrossRef]
- Qiang, W.; Huang, Y.; Wan, Z.; Zhou, B. Metal-metal interaction mediates the iron induction of Drosophila MtnB. Biochem. Biophys. Res. Commun. 2017, 487, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Rivero, M.; Marín-Barba, M.; Gutiérrez, L.; Lozano-Velasco, E.; Wheeler, G.N.; Sánchez-Marcos, J.; Muñoz-Bonilla, A.; Morris, C.J.; Ruiz, A. Toxicity and biodegradation of zinc ferrite nanoparticles in Xenopus laevis. J. Nanopart. Res. 2019, 21, 181. [Google Scholar] [CrossRef]
- Alaraby, M.; Abass, D.; Gutiérrez, J.; Velázquez, A.; Hernández, A.; Marcos, R. Reproductive Toxicity of Nanomaterials Using Silver Nanoparticles and Drosophila as Models. Molecules 2024, 29, 5802. [Google Scholar] [CrossRef]
- Yang, P.; Yang, X.; Sun, L.; Han, X.; Xu, L.; Gu, W.; Zhang, M. Effects of cadmium on oxidative stress and cell apoptosis in Drosophila melanogaster larvae. Sci. Rep. 2022, 12, 4762. [Google Scholar] [CrossRef]
- Pickering, A.M.; Vojtovich, L.; Tower, J.; Davies, K.J.A. Oxidative stress adaptation with acute, chronic, and repeated stress. Free Radic. Biol. Med. 2013, 55, 109–118. [Google Scholar] [CrossRef]
- Medina-Leendertz, S.; Mora, M.; Vielma, J.R.; Bravo, Y.; Atencio-Bracho, L.; Leal-Yépez, A.; Arcaya, J.L.; Bonilla, E. Melatonin decreases oxidative stress in Drosophila melanogaster exposed to manganese. Investig. Clin. 2018, 59, 230–241. [Google Scholar] [CrossRef]
- Bélteky, P.; Rónavári, A.; Igaz, N.; Szerencsés, B.; Tóth, I.Y.; Pfeiffer, I.; Kiricsi, M.; Kónya, Z. Silver nanoparticles: Aggregation behavior in biorelevant conditions and its impact on biological activity. Int. J. Nanomed. 2019, 14, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, L.; Wang, X. Molecular toxicity and defense mechanisms induced by silver nanoparticles in Drosophila melanogaster. J. Environ. Sci. 2023, 125, 616–629. [Google Scholar] [CrossRef]
- Ahamed, M.; Posgai, R.; Gorey, T.J.; Nielsen, M.; Hussain, S.M.; Rowe, J.J. Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster. Toxicol. Appl. Pharmacol. 2010, 242, 263–269. [Google Scholar] [CrossRef]
- Park, E.J.; Park, K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol. Lett. 2009, 184, 18–25. [Google Scholar] [CrossRef]
- Ekengren, S.; Hultmark, D. A family of Turandot-related genes in the humoral stress response of Drosophila. Biochem. Biophys. Res. Commun. 2001, 284, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Nayak, N.; Mohapatra, S.; Sahoo, J.K.; Sahoo, H.; Mishra, M. Strontium ferrite as a nontoxic nanomaterial to improve metabolism in a diabetic model of Drosophila melanogaster. Mater. Chem. Phys. 2022, 281, 125906. [Google Scholar] [CrossRef]
- Ekengren, S.; Tryselius, Y.; Dushay, M.S.; Liu, G.; Steiner, H.; Hultmark, D. A humoral stress response in Drosophila. Curr. Biol. 2001, 11, 1479. [Google Scholar] [CrossRef]
- Tataroglu, O.; Emery, P. Studying circadian rhythms in Drosophila melanogaster. Methods 2014, 68, 140–150. [Google Scholar] [CrossRef]
- Parasram, K.; Bachetti, D.; Carmona-Alcocer, V.; Karpowicz, P. Fluorescent Reporters for Studying Circadian Rhythms in Drosophila melanogaster. Methods Mol. Biol. 2022, 2482, 353–371. [Google Scholar] [CrossRef]
- Masuda, K.; Sakurai, T.; Hirano, A. A coupled model between circadian, cell-cycle, and redox rhythms reveals their regulation of oxidative stress. Sci. Rep. 2024, 14, 15479. [Google Scholar] [CrossRef]
- Xiao, N.; Xu, S.; Li, Z.K.; Tang, M.; Mao, R.; Yang, T.; Ma, S.X.; Wang, P.H.; Li, M.T.; Sunilkumar, A.; et al. A single photoreceptor splits perception and entrainment by cotransmission. Nature 2023, 623, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Roach, J.P.; Ben-Jacob, E.; Sander, L.M.; Zochowski, M.R. Modeling the formation and dynamics of cortical waves induced by cholinergic modulation. BMC Neurosci. 2015, 16 (Suppl. 1), P304. [Google Scholar] [CrossRef]
- Jones, B.E. Arousal and sleep circuits. Neuropsychopharmacology 2020, 45, 6–20. [Google Scholar] [CrossRef]
- Tang, J.; Liu, A.; Chen, K.; Shi, Y.; Qiu, X. Exposure to amitriptyline disturbs behaviors in adult zebrafish and their offspring via altering neurotransmitter levels. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2025, 288, 110079. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.; Emery, P. Fly into tranquility: GABA’s role in Drosophila sleep. Curr. Opin. Insect Sci. 2024, 64, 101219. [Google Scholar] [CrossRef] [PubMed]
- Ossowska, K. Zona incerta as a therapeutic target in Parkinson’s disease. J. Neurol. 2020, 267, 591–606. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, W.; Guo, Y.; Li, P.; Zhang, Z.; Yang, J.; Sun, Y. Effects of ZnFe2O4 Nanoparticles on Development and Rhythmic Behavior of Drosophila melanogaster. Toxics 2025, 13, 779. https://doi.org/10.3390/toxics13090779
Yan W, Guo Y, Li P, Zhang Z, Yang J, Sun Y. Effects of ZnFe2O4 Nanoparticles on Development and Rhythmic Behavior of Drosophila melanogaster. Toxics. 2025; 13(9):779. https://doi.org/10.3390/toxics13090779
Chicago/Turabian StyleYan, Wenhao, Yunfan Guo, Penghui Li, Ziyan Zhang, Jinjun Yang, and Yongyan Sun. 2025. "Effects of ZnFe2O4 Nanoparticles on Development and Rhythmic Behavior of Drosophila melanogaster" Toxics 13, no. 9: 779. https://doi.org/10.3390/toxics13090779
APA StyleYan, W., Guo, Y., Li, P., Zhang, Z., Yang, J., & Sun, Y. (2025). Effects of ZnFe2O4 Nanoparticles on Development and Rhythmic Behavior of Drosophila melanogaster. Toxics, 13(9), 779. https://doi.org/10.3390/toxics13090779








