Integrating Experimental Toxicology and Machine Learning to Model Levonorgestrel-Induced Oxidative Damage in Zebrafish
Highlights
- Environmentally relevant levonorgestrel exposures elicited organ- and time-dependent redox perturbations in zebrafish, with hepatic tissue exhibiting the most pronounced susceptibility.
- Glutathione peroxidase (GPx) emerged as a robust diagnostic indicator, reflecting consistent oxidative stress trajectories across concentration and duration.
- Advanced ensemble algorithms, particularly Gradient Boosted Trees, achieved near-perfect classification of exposure profiles from integrated biomarker datasets.
- Machine learning-augmented toxicology enables high-resolution detection of subtle xenobiotic effects, extending beyond conventional biomarker interpretation.
- The identification of GPx as a sentinel endpoint strengthens predictive ecotoxicological assessment frameworks and informs environmental monitoring of endocrine-active contaminants.
Abstract
1. Introduction
2. Materials and Methods
2.1. Empirical Toxicology Dataset
2.2. Test Chemical
2.3. Maintenance of Zebrafish and Exposure Procedure
2.4. Tissue Sampling and Homogenization Procedure
2.5. Antioxidative/Oxidative Stress Biomarkers
2.6. Statictics
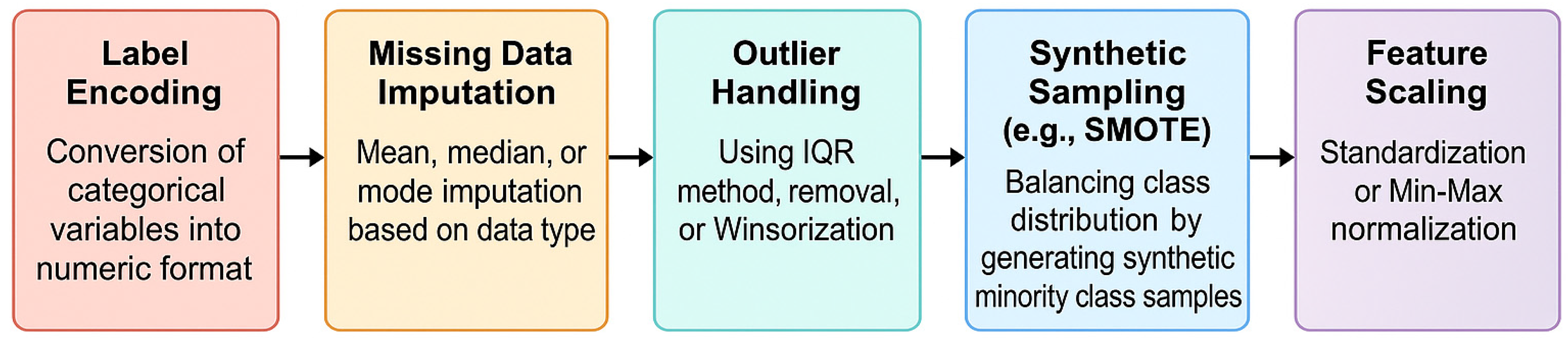
2.7. Data Pre-Processing
2.8. Machine Learning (ML) Models
2.8.1. Logistic Regression (LR)
2.8.2. Multilayer Perceptron (MLP)
2.8.3. Gradient-Boosted Trees (GBT)
2.8.4. Decision Tree (DT)
2.8.5. Random Forest (RF)
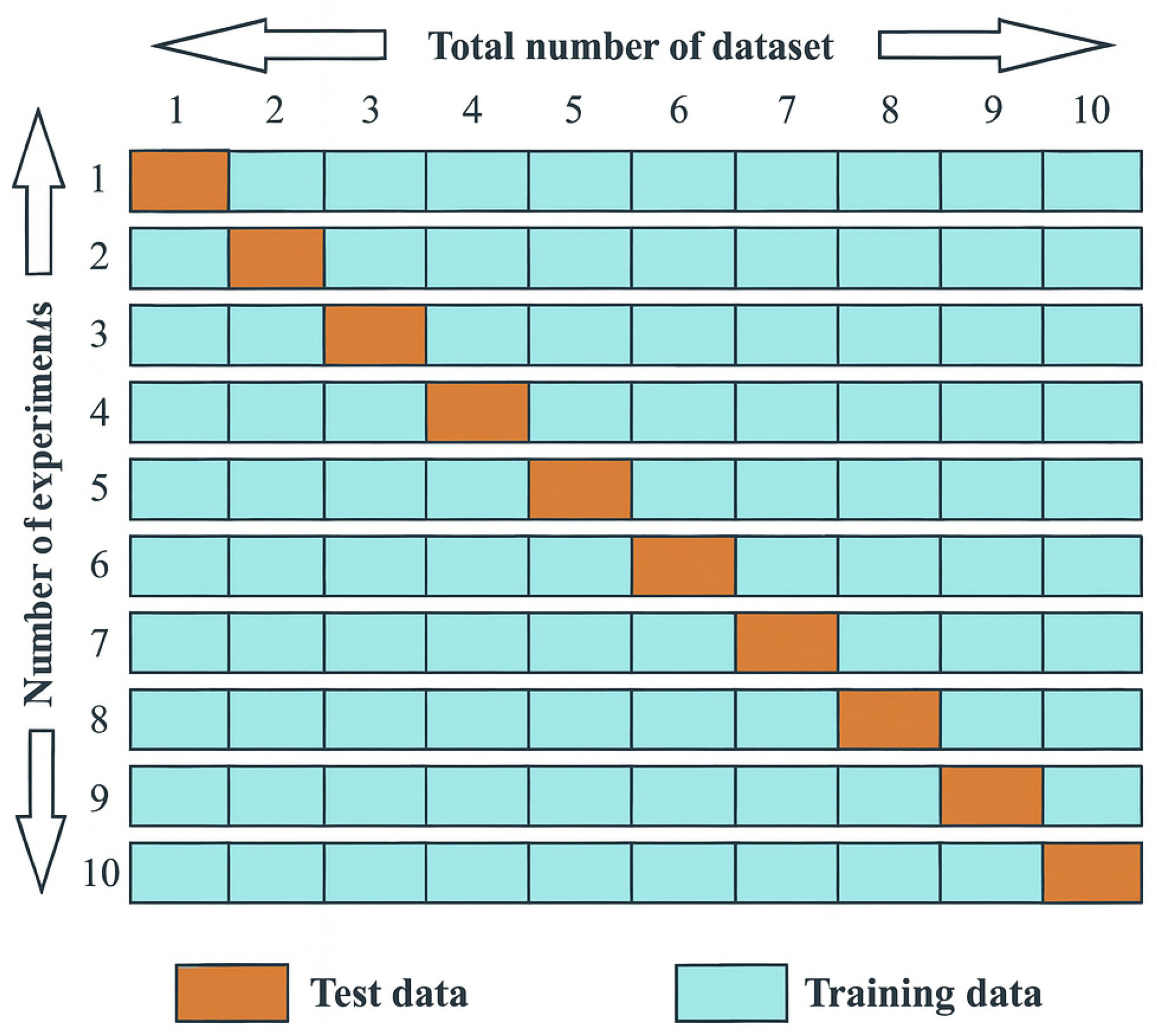
2.9. Model Performance Evaluation
3. Results
3.1. Empirical Outcomes for Antioxidant/Oxidant Biomarkers
3.2. ML Model Outcomes
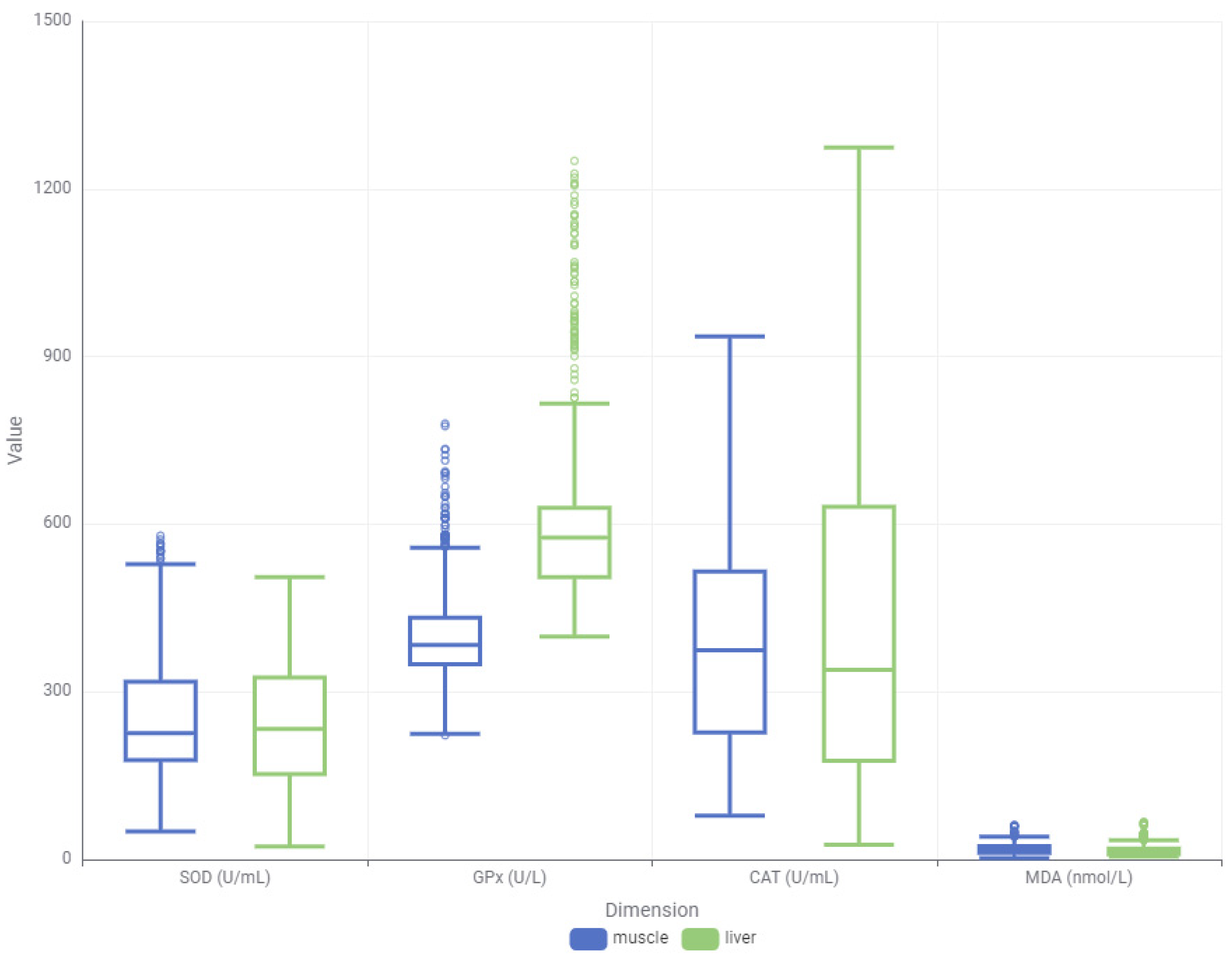
3.3. Distributional Analysis of Oxidative Stress Biomarkers for Tissue and Dose Specifity
4. Discussion
| Study | Focus | Exposure Details | Key Findings | Statistical Significance/Model Accuracy |
|---|---|---|---|---|
| [40] | Morphological abnormalities | Exposure up to 120 hpf * | 8 abnormal phenotypes and 8 organ features classified | mAP > 0.93; Accuracy > 0.86 |
| [72] | Pancreatic toxicity and gene expression | Not specified | Chemical clusters identified affecting pancreatic pathways | RF accuracy 74% |
| [107] | Neuronal development (neuroendogenesis) | LNG: 5 ng; Estradiol: 100 ng; 5 days | ↑ alpha-HUC+ neurons in hypothalamus and related regions | p < 0.001 (hypothalamus), p < 0.01 (preoptic area) |
| [108] | Acute toxicity prediction (QSAR/q-RASAR) | LC50: 0.790 mg/L (exp); 0.763 mg/L (pred) | Phenolphthalein identified as highly toxic | R2 = 0.886, Q2 = 0.814 |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EDCs | Endocrine disrupting chemicals |
| LNG | Levonorgestrel |
| LNG-C | Levonorgestrel-control |
| LNG-H | Levonorgestrel-high concentration |
| LNG-L | Levonorgestrel-low concentration |
| HPT | Hypothalamic–pituitary–thyroid |
| HPG | Hypothalamic–pituitary–gonadal |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GPx | Glutathione peroxidase |
| MDA | Malondialdehyde |
| ML | Machine learning |
| LR | Logistic regression |
| MLP | Multilayer perceptron |
| GBT | Gradient-boosted trees |
| DT | Decision tree |
| RF | Random forest |
| XGBoost | Extreme gradient boosting |
| SMOTE | Synthetic minority over-sampling technique |
| QSAR | Quantitative structure–activity relationship |
| q-RASAR | quantitative read-across structure–activity relationship |
| OECD | Organisation for Economic Co-operation and Development |
| SVM | Support vector machine |
| DO2 | Dissolved oxygen |
| °C | Temperature |
| ORP | Oxidation-reduction potential |
| NH3-N | Ammonia |
| NO3-N | Nitrate |
| NO2-N | Nitrite |
| APHA | American Public Health Association |
| TSE | Türk Standartları Enstitüsü |
| AVMA | American Veterinary Medical Association |
| EU | European Union |
| KCl | Potassium chloride |
| H2O2 | Hydrogen peroxide |
| GSSG | Oxidized glutathione |
| GSH | Reduced glutathione |
| NADPH | Nicotinamide adenine dinucleotide phosphate (reduced form) |
| NADP+ | Nicotinamide adenine dinucleotide phosphate (oxidized form) |
| NBT | Nitroblue tetrazolium |
| IU | International units |
| U | Units |
| TBA | Thiobarbituric acid |
| IQR | Interquartile range |
| ReLU | Rectified linear unit |
| MAE | Mean absolute error |
| RMSE | Root mean square error |
| ROC | Receiver operating characteristic |
| AUROC | Area under the receiver operating characteristic curve |
| PPV | Positive predictive value |
| TPR | True positive rate |
| TNR | True negative rate |
| κ | Cohen’s Kappa |
| FPR | False positive Rate |
| R2 | The coefficient of determination |
| UPGMA | Unweighted pair group method with arithmetic mean |
| AOP | Adverse outcome pathway |
| ERA | Environmental risk assessment |
| EMEA | European medicines agency |
| MECs | Measured environmental concentrations |
| RQs | Risk quotients |
| PNEC | Predicted no-effect concentration |
References
- Damstra, T.; Barlow, S.; Bergman, A.; Kavlock, R.; Van Der Kraak, G.J. Global Assessment of the State of the Science of Endocrine Disruptors; World Health Organization: Geneva, Switzerland, 2002; p. 180.
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Kloas, W.; Urbatzka, R.; Opitz, R.; Würtz, S.; Behrends, T.; Hermelink, B.; Hofmann, F.; Jagnytsch, O.; Kroupova, H.; Lorenz, C.; et al. Endocrine disruption in aquatic vertebrates. Ann. N. Y. Acad. Sci. 2009, 1163, 187–200. [Google Scholar] [CrossRef]
- Frątczak, M.; Kaczmarski, M.; Szkudelska, K.; Tryjanowski, P. Assessing species bias in amphibian research on endocrine disruptors: Beyond Xenopus laevis. Front. Environ. Sci. 2025, 13, 1556788. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Q.; Li, X.; Xu, H.; Ren, C.; Yang, Y.; Xu, S.; Wei, G.; Duan, Y.; Tan, Z.; et al. Norgestrel causes digestive gland injury in the clam Mactra veneriformis: An integrated histological, transcriptomics, and metabolomics study. Sci. Total Environ. 2023, 871, 162110. [Google Scholar] [CrossRef]
- Besse, J.; Garric, J. Progestagens for human use, exposure and hazard assessment for the aquatic environment. Environ. Pollut. 2009, 157, 3485–3494. [Google Scholar] [CrossRef]
- Liu, Z.; Ogejo, J.A.; Pruden, A.; Knowlton, K.F. Occurrence, fate and removal of synthetic oral contraceptives (SOCs) in the natural environment: A review. Sci. Total Environ. 2011, 409, 5149–5161. [Google Scholar] [CrossRef]
- Fent, K. Progestins as endocrine disrupters in aquatic ecosystems: Concentrations, effects and risk assessment. Environ. Int. 2015, 84, 115–130. [Google Scholar] [CrossRef]
- Orlando, E.F.; Ellestad, L.E. Sources, concentrations and exposure effects of environmental gestagens on fish and other aquatic wildlife, with an emphasis on reproduction. Gen. Comp. Endocrinol. 2014, 203, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, F.; Kloas, W. The synthetic progestogen, levonorgestrel, but not natural progesterone, affects male mate calling behavior of Xenopus laevis. Gen. Comp. Endocrinol. 2012, 176, 385–390. [Google Scholar] [CrossRef]
- Runnalls, T.J.; Beresford, N.; Losty, E.; Scott, A.P.; Sumpter, J.P. Several synthetic progestins with different potencies adversely affect reproduction of fish. Environ. Sci. Technol. 2013, 47, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wan, Y.; Wu, S.; Fan, Z.; Hu, J. Occurrence of androgens and progestogens in wastewater treatment plants and receiving river waters: Comparison to estrogens. Water Res. 2011, 45, 732–740. [Google Scholar] [CrossRef]
- Maasz, G.; Zrinyi, Z.; Takacs, P.; Lovas, S.; Fodor, I.; Kiss, T.; Pirger, Z. Complex molecular changes induced by chronic progestogens exposure in roach (Rutilus rutilus). Ecotoxicol. Environ. Saf. 2017, 139, 9–17. [Google Scholar] [CrossRef][Green Version]
- Šauer, P.; Stará, A.; Golovko, O.; Valentová, O.; Bořík, A.; Grabic, R.; Kocour Kroupová, H. Two synthetic progestins and natural progesterone are responsible for most of the progestagenic activities in municipal wastewater treatment plant effluents in the Czech and Slovak republics. Water Res. 2018, 137, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, C.; Contardo-Jara, V.; Trubiroha, A.; Krüger, A.; Viehmann, V.; Wiegand, C.; Pflugmacher, S.; Nützmann, G.; Lutz, I.; Kloas, W. The synthetic gestagen levonorgestrel disrupts sexual development in Xenopus laevis by affecting gene expression of pituitary gonadotropins and gonadal steroidogenic enzymes. Toxicol. Sci. 2011, 124, 311–319. [Google Scholar] [CrossRef]
- Kumar, V.; Johnson, A.C.; Trubiroha, A.; Tumová, J.; Ihara, M.; Grabic, R.; Kloas, W.; Tanaka, H.; Kroupová, H.K. The challenge presented by progestins in ecotoxicological research: A critical review. Environ. Sci. Technol. 2015, 49, 2625–2638. [Google Scholar] [CrossRef] [PubMed]
- Croxatto, H.B. Mechanisms that explain the contraceptive action of progestin implants for women. Contraception 2002, 65, 21–27. [Google Scholar] [CrossRef]
- Rocha, M.J.; Rocha, E. Synthetic progestins in waste and surface waters: Concentrations, impacts and ecological risk. Toxics 2022, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, C.; Opitz, R.; Trubiroha, A.; Lutz, I.; Ziková, A.; Kloas, W. The synthetic gestagen levonorgestrel directly affects gene expression in thyroid and pituitary glands of Xenopus laevis tadpoles. Aquat. Toxicol. 2016, 177, 63–73. [Google Scholar] [CrossRef]
- Svensson, J.; Mustafa, A.; Fick, J.; Schmitz, M.; Brunström, B. Developmental exposure to progestins causes male bias and precocious puberty in zebrafish (Danio rerio). Aquat. Toxicol. 2016, 177, 316–323. [Google Scholar] [CrossRef]
- Kroupová, H.K.; Trubiroha, A.; Lorenz, C.; Contardo-Jara, V.; Lutz, I.; Grabic, R.; Kocour, M.; Kloas, W. The progestin levonorgestrel disrupts gonadotropin expression and sex steroid levels in pubertal roach (Rutilus rutilus). Aquat. Toxicol. 2014, 154, 154–162. [Google Scholar] [CrossRef]
- Zrinyi, Z.; Maasz, G.; Zhang, L.; Vertes, A.; Lovas, S.; Kiss, T.; Elekes, K.; Pirger, Z. Effect of progesterone and its synthetic analogs on reproduction and embryonic development of a freshwater invertebrate model. Aquat. Toxicol. 2017, 190, 94–103. [Google Scholar] [CrossRef]
- DeQuattro, Z.A.; Peissig, E.J.; Antkiewicz, D.S.; Lundgren, E.J.; Hedman, C.J.; Hemming, J.D.C.; Barry, T.P. Effects of progesterone on reproduction and embryonic development in the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2012, 31, 851–856. [Google Scholar] [CrossRef]
- Fuentes, A.K.; Ho-Shing, B.K.; Zamora, E.; Hinojosa, G.; Dearth, R.K. Fetal exposure to the synthetic-progesterone levonorgestrel (LNG) targets the brain resulting in hyperactive behavior using the zebrafish (Danio rerio) as a model. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Teigeler, M.; Schaudien, D.; Böhmer, W.; Länge, R.; Schäfers, C. Effects of the gestagen levonorgestrel in a life cycle test with zebrafish (Danio rerio). Environ. Toxicol. Chem. 2022, 41, 580–591. [Google Scholar] [CrossRef]
- Ziková, A.; Lorenz, C.; Hoffmann, F.; Kleiner, W.; Lutz, I.; Stöck, M.; Kloas, W. Endocrine disruption by environmental gestagens in amphibians—A short review supported by new in vitro data using gonads of Xenopus laevis. Chemosphere 2017, 181, 74–82. [Google Scholar] [CrossRef]
- Liang, Y.-Q.; Huang, G.-Y.; Ying, G.-G.; Liu, S.-S.; Jiang, Y.-X.; Liu, S.; Peng, F.-J. A time-course transcriptional kinetics of the hypothalamic-pituitary-gonadal and hypothalamic-pituitary-adrenal axes in zebrafish eleutheroembryos after exposure to norgestrel. Environ. Toxicol. Chem. 2015, 34, 112–119. [Google Scholar] [CrossRef]
- Hua, J.; Han, J.; Guo, Y.; Zhou, B. The progestin levonorgestrel affects sex differentiation in zebrafish at environmentally relevant concentrations. Aquat. Toxicol. 2015, 166, 19–27. [Google Scholar] [CrossRef]
- Cardoso, P.G.; Rodrigues, D.; Madureira, T.V.; Oliveira, N.; Rocha, M.J.; Rocha, E. Warming modulates the effects of the endocrine disruptor progestin levonorgestrel on the zebrafish fitness, ovary maturation kinetics and reproduction success. Environ. Pollut. 2017, 229, 300–311. [Google Scholar] [CrossRef]
- Chen, S.; Lin, C.; Tan, J.; Wang, Y.; Wang, A.; Wang, X.; Wang, X.; Liu, L.; Li, J.; Hou, L.; et al. Reproductive potential of mosquitofish is reduced by the masculinizing effect of a synthetic progesterone, gestodene: Evidence from morphology, courtship behaviour, ovary histology, sex hormones and gene expressions. Sci. Total Environ. 2021, 769, 144570. [Google Scholar] [CrossRef]
- Frankel, T.E.; Meyer, M.T.; Orlando, E.F. Aqueous exposure to the progestin, levonorgestrel, alters anal fin development and reproductive behavior in the eastern mosquitofish (Gambusia holbrooki). Gen. Comp. Endocrinol. 2016, 234, 161–169. [Google Scholar] [CrossRef]
- Steinbach, C.; Císař, P.; Šauer, P.; Klicnarová, J.; Schmidt-Posthaus, H.; Golovko, O.; Kroupová, H.K. Synthetic progestin etonogestrel negatively affects mating behavior and reproduction in Endler’s guppies (Poecilia wingei). Sci. Total Environ. 2019, 663, 206–215. [Google Scholar] [CrossRef]
- Cano-Nicolau, J.; Garoche, C.; Hinfray, N.; Pellegrini, E.; Boujrad, N.; Pakdel, F.; Kah, O.; Brion, F. Several synthetic progestins disrupt the glial cell specific-brain aromatase expression in developing zebra fish. Toxicol. Appl. Pharmacol. 2016, 305, 12–21. [Google Scholar] [CrossRef]
- Liang, Y.; Huang, G.; Lin, Z.; Li, J.; Yang, J.; Zhong, L.; Ying, G. Reproductive effects of synthetic progestin norgestrel in zebrafish (Danio rerio). Chemosphere 2018, 190, 17–24. [Google Scholar] [CrossRef]
- Paulos, P.; Runnalls, T.J.; Nallani, G.; La Point, T.; Scott, A.P.; Sumpter, J.P.; Huggett, D.B. Reproductive responses in fathead minnow and Japanese medaka following exposure to a synthetic progestin, norethindrone. Aquat. Toxicol. 2010, 99, 256–262. [Google Scholar] [CrossRef]
- Hinfray, N.; Tebby, C.; Garoche, C.; Piccini, B.; Bourgine, G.; Aït-Aïssa, S.; Kah, O.; Pakdel, F.; Brion, F. Additive effects of levonorgestrel and ethinylestradiol on brain aromatase (cyp19a1b) in zebrafish-specific in vitro and in vivo bioassays. Toxicol. Appl. Pharmacol. 2016, 307, 108–114. [Google Scholar] [CrossRef]
- Liang, Y.-Q.; Huang, G.-Y.; Zhao, J.-L.; Shi, W.-J.; Hu, L.-X.; Tian, F.; Liu, S.-S.; Jiang, Y.-X.; Ying, G.-G. Transcriptional alterations induced by binary mixtures of ethinylestradiol and norgestrel during the early development of zebrafish (Danio rerio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 195, 60–67. [Google Scholar] [CrossRef]
- Länge, R.; Hutchinson, T.H.; Croudace, C.P.; Siegmund, F.; Schweinfurth, H.; Hampe, P.; Panter, G.H.; Sumpter, J.P. Effects of the synthetic estrogen 17α-ethinylestradiol on the life-cycle of the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2001, 20, 1216–1227. [Google Scholar] [CrossRef]
- Thomson, P.; Pineda, M.; Yargeau, V.; Langlois, V.S. Chronic exposure to two gestagens differentially alters morphology and gene expression in Silurana tropicalis. Arch. Environ. Contam. Toxicol. 2021, 80, 745–759. [Google Scholar] [CrossRef]
- Dong, Z.; Li, X.; Chen, Y.; Zhang, N.; Wang, Z.; Liang, Y.-Q.; Guo, Y. Short-term exposure to norethisterone affected swimming behavior and antioxidant enzyme activity of medaka larvae, and led to masculinization in the adult population. Chemosphere 2023, 310, 136844. [Google Scholar] [CrossRef]
- Mannai, A.; Hmida, L.; Bouraoui, Z.; Guerbej, H.; Gharred, T.; Jebali, J. Does thermal stress modulate the biochemical and physiological responses of Ruditapes decussatus exposed to the progestin levonorgestrel? Environ. Sci. Pollut. Res. 2022, 29, 85211–85228. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Malhotra, J.D.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress: A vicious cycle or a double-edged sword. Antioxid. Redox Signal. 2007, 9, 2277–2293. [Google Scholar] [CrossRef]
- Wang, X.; Tan, Z.; Chen, S.; Gui, L.; Li, X.; Ke, D.; Hou, L.; Leung, J.Y.S. Norethindrone causes cellular and hepatic injury in zebrafish by compromising the metabolic processes associated with antioxidant defence: Insights from metabolomics. Chemosphere 2021, 275, 130049. [Google Scholar] [CrossRef]
- Fortuna, M.; Varella, A.C.C.; Siqueira, L.; Soares, S.M.; Freddo, N.; Nardi, J.; Barletto, Í.P.; Bertuol, M.Z.; Barcellos, L.J.G. Transgenerational Effects of the Levonorgestrel-Based Birth Control Pill in Zebrafish Offspring. Environ. Toxicol. Pharmacol. 2024, 110, 104540. [Google Scholar] [CrossRef]
- Brander, S.M.; White, J.W.; DeCourten, B.M.; Major, K.; Hutton, S.J.; Connon, R.E.; Mehinto, A.C. Accounting for Transgenerational Effects of Toxicant Exposure in Population Models Alters the Predicted Long-Term Population Status. Environ. Epigenetics 2022, 8, dvac023. [Google Scholar] [CrossRef]
- Oropesa, A.L.; Guimarães, L. Occurrence of Levonorgestrel in Water Systems and Its Effects on Aquatic Organisms: A Review. Rev. Environ. Contam. Toxicol. 2021, 254, 57–84, Erratum in Environ. Contam. Toxicol. 2021, 254, 217. https://doi.org/10.1007/398_2020_52. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, S.; Castiglioni, S.; Fent, K. Progestins and Antiprogestins Affect Gene Expression in Early Development in Zebrafish (Danio rerio) at Environmental Concentrations. Environ. Sci. Technol. 2012, 46, 5183–5192. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, K.; Fent, K. Regulation of Zebrafish (Danio rerio) Locomotor Behavior and Circadian Rhythm Network by Environmental Steroid Hormones. Environ. Pollut. 2018, 232, 422–429. [Google Scholar] [CrossRef]
- Sinhorin, V.D.G.; Sinhorin, A.P.; Teixeira, J.M.S.; Lazarotto Miléski, K.M.; Hansen, P.C.; Moreira, P.S.A.; Kawashita, N.H.; Baviera, A.M.; Loro, V.L. Effects of the acute exposition to glyphosate-based herbicide on oxidative stress parameters and antioxidant responses in a hybrid Amazon fish surubim (Pseudoplatystoma sp.). Ecotoxicol. Environ. Saf. 2014, 106, 181–187. [Google Scholar] [CrossRef]
- Santos, T.G.; Martinez, C.B.R. Atrazine promotes biochemical changes and DNA damage in a Neotropical fish species. Chemosphere 2012, 89, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Mukherjee, S.; Chattopadhyay, A.; Bhattacharya, S. Low dose of arsenic trioxide triggers oxidative stress in zebrafish brain: Expression of antioxidant genes. Ecotoxicol. Environ. Saf. 2014, 107, 1–8. [Google Scholar] [CrossRef]
- Ibrahim, Z.A.; Oniye, S.J.; Luka, S.A.; Mathias, C.A. Bioaccumulation and impact of levonorgestrel on the growth, photosynthetic pigments, and oxidative stress response of Chlorogonium elongatum. SSRN 2024. [Google Scholar] [CrossRef]
- Russell, S.; Norvig, P. Artificial Intelligence: A Modern Approach, 4th ed.; Pearson: Hoboken, NJ, USA, 2020. [Google Scholar]
- Davidovic, L.M.; Laketic, D.; Cumic, J.; Jordanova, E.; Pantic, I. Application of artificial intelligence for detection of chemico-biological interactions associated with oxidative stress and DNA damage. Chem. Biol. Interact. 2021, 345, 109533. [Google Scholar] [CrossRef]
- van de Waterbeemd, H.; Gifford, E. ADMET in silico modelling: Towards prediction paradise? Nat. Rev. Drug Discov. 2003, 2, 192–204. [Google Scholar] [CrossRef]
- Butler, K.T.; Davies, D.W.; Cartwright, H.; Isayev, O.; Walsh, A. Machine learning for molecular and materials science. Nature 2018, 559, 547–555. [Google Scholar] [CrossRef]
- Wainberg, M.; Merico, D.; DeLong, A.; Frey, B.J. Deep learning in biomedicine. Nat. Biotechnol. 2018, 36, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Prezioso, E.; Giampaolo, F.; Mazzocca, C.; Bujari, A.; Mele, V.; Amato, F. Machine learning insights for behavioral data analysis supporting the autonomous vehicles scenario. IEEE Internet Things J. 2023, 10, 3107–3117. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, G. Machine learning-based toxicity prediction: From chemical structural description to transcriptome analysis. Int. J. Mol. Sci. 2018, 19, 2358. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sun, L.; Li, W.; Liu, G.; Tang, Y. In silico prediction of chemical toxicity for drug design using machine learning methods and structural alerts. Front. Chem. 2018, 6, 30. [Google Scholar] [CrossRef]
- Dávila-Santiago, E.; Shi, C.; Mahadwar, G.; Medeghini, B.; Insinga, L.; Hutchinson, R.; Good, S.; Jones, G.D. Machine learning applications for chemical fingerprinting and environmental source tracking using non-target chemical data. Environ. Sci. Technol. 2022, 56, 4080–4090. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lu, D.; Zhang, A.; Liu, Q.; Jiang, G. Data-driven machine learning in environmental pollution: Gains and problems. Environ. Sci. Technol. 2022, 56, 2124–2133. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Varma, M.; Laux, P.; Choudhary, S.; Datusalia, A.K.; Gupta, N.; Luch, A.; Gandhi, A.; Kulkarni, P.; Nath, B.; et al. Artificial intelligence and machine learning disciplines with the potential to improve the nanotoxicology and nanomedicine fields: A comprehensive review. Arch. Toxicol. 2023, 97, 963–979. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, Q.; Mu, L.; Hu, X. Machine learning in the identification, prediction and exploration of environmental toxicology: Challenges and perspectives. J. Hazard. Mater. 2022, 438, 129487. [Google Scholar] [CrossRef]
- Wang, R.; Wang, B.; Chen, A. Application of machine learning in the study of development, behavior, nerve, and genotoxicity of zebrafish. Environ. Pollut. 2024, 328, 124473. [Google Scholar] [CrossRef]
- Doğankaya, L.; Gültekin, T.; Coşkun, T.; Alptekin, E. Binaural beat stimulation—A non-invasive method for inducing zebrafish growth. Iran. J. Fish. Sci. 2020, 19, 2308–2321. [Google Scholar]
- MacRae, C.; Peterson, R. Zebrafish as a mainstream model for in vivo systems pharmacology and toxicology. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 43–64. [Google Scholar] [CrossRef]
- Tal, T.; Yaghoobi, B.; Lein, P. Translational toxicology in zebrafish. Curr. Opin. Toxicol. 2020, 23–24, 13–20. [Google Scholar] [CrossRef]
- Lin, Z.; Chou, W.C. Machine learning and artificial intelligence in toxicological sciences. Toxicol. Sci. 2022, 189, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.V.; Sant, K.; George, U.Z. Integrating network analysis and machine learning to elucidate chemical-induced pancreatic toxicity in zebrafish embryos. Toxicol. Sci. 2025, 206, 330–353. [Google Scholar] [CrossRef]
- Contardo-Jara, V.; Lorenz, C.; Pflugmacher, S.; Nützmann, G.; Kloas, W.; Wiegand, C. Molecular effects and bioaccumulation of levonorgestrel in the non-target organism Dreissena polymorpha. Environ. Pollut. 2011, 159, 38–44. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Keramati, V.; Jamili, S.; Ramin, M. Effect of diazinon on catalase antioxidant enzyme activity in liver tissue of Rutilus rutilus. J. Fish. Aquat. Sci. 2010, 5, 368–376. [Google Scholar] [CrossRef][Green Version]
- TS 5676; Su Kirliliği Kontrolü—Zehirlilik Deneyleri—Kısım 1 [Water Pollution Control—Toxicity Tests—Part 1]. Turkish Standards Institution (TSE): Ankara, Turkey, 1988. (In Turkish)
- TS 8264; Endüstriyel Sıvı Atıklar ve Atıksular—Akut Zehirlilik Deneyleri—Canlılık Deney Metodları [Industrial Liquid Wastes and Wastewaters—Acute Toxicity Tests—Survival Test Methods]. Turkish Standards Institution (TSE): Ankara, Turkey, 1990. (In Turkish)
- OECD. Test No. 203: Fish, Acute Toxicity Test; OECD Publishing: Paris, France, 1992. [Google Scholar]
- Durak, I.; Canbolat, O.; Kavutcu, M.; Öztürk, H.S.; Yurtaslani, Z. Activities of total, cytoplasmic and mitochondrial superoxide dismutase enzymes in sera and pleural fluids from patients with lung cancer. J. Clin. Lab. Anal. 1996, 10, 17–20. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; pp. 673–677. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Dahle, L.K.; Hill, E.G.; Holman, R.T. The thiobarbituric acid reaction and the autooxidants of polyunsaturated fatty acid methyl esters. Arch. Biochem. Biophys. 1962, 98, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Cox, D.R. The regression analysis of binary sequences. J. R. Stat. Soc. Ser. B-Methodol. 1958, 20, 215–242. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression, 3rd ed.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Rumelhart, D.E.; Hinton, G.E.; Williams, R.J. Learning representations by back-propagating errors. Nature 1986, 323, 533–536. [Google Scholar] [CrossRef]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016; ISBN 978-0-262-03561-3. [Google Scholar]
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Schapire, R.E. The strength of weak learnability. Mach. Learn. 1990, 5, 197–227. [Google Scholar] [CrossRef]
- Polikar, R. Ensemble learning. In Ensemble Machine Learning: Methods and Applications; Zhang, C., Ma, Y., Eds.; Springer: New York, NY, USA, 2012; pp. 1–34. [Google Scholar] [CrossRef]
- Gupte, A.; Joshi, S.; Gadgul, P.; Kadam, A. Comparative study of classification algorithms used in sentiment analysis. Int. J. Comput. Sci. Inf. Technol. 2014, 5, 6261–6264. [Google Scholar]
- Quinlan, J.R. Induction of decision trees. Mach. Learn. 1986, 1, 81–106. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Mitchell, T.M. Machine learning and data mining. Commun. ACM 1999, 42, 30–36. [Google Scholar] [CrossRef]
- Cardoso, P.G.; Resende-de-Oliveira, R.; Rocha, E. Combined effects of increased temperature and levonorgestrel exposure on zebrafish female liver, using stereology and immunohistochemistry against catalase, CYP1A, HSP90 and vitellogenin. Environ. Pollut. 2019, 252, 1059–1067. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Teng, Y.; Ji, C.; Wu, H. Characterization of oxidative damage induced by nanoparticles via mechanism-driven machine learning approaches. Sci. Total Environ. 2023, 871, 162103. [Google Scholar] [CrossRef]
- Zhang, H.; Lenaghan, S.C.; Connolly, M.H.; Parker, L.E. Zebrafish larva locomotor activity analysis using machine learning techniques. In Proceedings of the 12th IEEE International Conference on Machine Learning and Applications (ICMLA 2013), Miami, FL, USA, 4–7 December 2013; pp. 103–108. [Google Scholar] [CrossRef]
- Sawaki, R.; Sato, D.; Nakayama, H.; Nakagawa, Y.; Shimada, Y. ZF-AutoML: An easy machine-learning-based method to detect anomalies in fluorescent-labelled zebrafish. Inventions 2019, 4, 72. [Google Scholar] [CrossRef]
- Mugoni, V.; Camporeale, A.; Santoro, M.M. Analysis of oxidative stress in zebrafish embryos. J. Vis. Exp. 2014, 100, e51328. [Google Scholar] [CrossRef]
- Fang, L.; Miller, Y.I. Emerging applications for zebrafish as a model organism to study oxidative mechanisms and their roles in inflammation and vascular accumulation of oxidized lipids. Free Radic. Biol. Med. 2012, 53, 1411–1420. [Google Scholar] [CrossRef]
- Gutha, R.; Yarrappagaari, S.; Thopireddy, L.; Reddy, K.; Saddala, R. Effect of abiotic and biotic stress factors analysis using machine learning methods in zebrafish. Comp. Biochem. Physiol. D Genom. Proteom. 2018, 27, 168–175. [Google Scholar] [CrossRef]
- Jones, R.A.; Renshaw, M.; Barry, D.J.; Smith, J.C. Automated staging of zebrafish embryos using machine learning. Wellcome Open Res. 2022, 7, 275. [Google Scholar] [CrossRef]
- Philip, R.C.; Rodriguez, J.J.; Nihori, M.; Francis, R.H.; Mudery, J.A.; Caskey, J.S.; Krupinski, E.A.; Jacob, A. Automated high-throughput damage scoring of zebrafish lateral line hair cells after ototoxin exposure. Zebrafish 2018, 15, 145–155. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saikia, S. Use of zebrafish as a model organism to study oxidative stress: A review. Zebrafish 2022, 19, 470–479. [Google Scholar] [CrossRef]
- Hinojosa, G.; Ruiz, M.; Garcia, K.; Dearth, R.K. The Contraceptive Synthetic-Progesterone Levonorgestrel (LNG) Significantly Accelerates Fetal Hypothalamic Neuronal Development (Neuroendogenesis) in the Zebrafish (Danio rerio). J. Endocr. Soc. 2022, 6 (Suppl. 1), A446. [Google Scholar] [CrossRef]
- Italiya, G.; Subramanian, S. Leveraging new approach methodologies: Ecotoxicological modelling of endocrine disrupting chemicals to Danio rerio through machine learning and toxicity studies. Toxicol. Mech. Methods 2025, 35, 197–213. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on the Environmental Risk Assessment of Medicinal Products for Human Use; Committee for Medicinal Products for Human Use (CHMP); Document No. EMEA/CHMP/SWP/4447/00 corr 2; European Medicines Agency: London, UK, 2006. [Google Scholar]
- Thrupp, T.J.; Runnalls, T.J.; Scholze, M.; Kugathas, S.; Kortenkamp, A.; Sumpter, J.P. The Consequences of Exposure to Mixtures of Chemicals: Something from ‘Nothing’ and ‘A Lot from a Little’ When Fish Are Exposed to Steroid Hormones. Sci. Total Environ. 2018, 619–620, 1482–1492. [Google Scholar] [CrossRef]
- Ankley, G.T.; Bennett, R.S.; Erickson, R.J.; Hoff, D.J.; Hornung, M.W.; Johnson, R.D.; Mount, D.R.; Nichols, J.W.; Russom, C.L.; Schmieder, P.K.; et al. Adverse Outcome Pathways: A Conceptual Framework to Support Ecotoxicology Research and Risk Assessment. Environ. Toxicol. Chem. 2010, 29, 730–741. [Google Scholar] [CrossRef]
- Perkins, E.J.; Ashauer, R.; Burgoon, L.; Conolly, R.; Landesmann, B.; Mackay, C.; Murphy, C.A.; Pollesch, N.; Wheeler, J.R.; Zupanic, A.; et al. Building and Applying Quantitative Adverse Outcome Pathway Models for Chemical Hazard and Risk Assessment. Environ. Toxicol. Chem. 2019, 38, 1850–1865. [Google Scholar] [CrossRef]

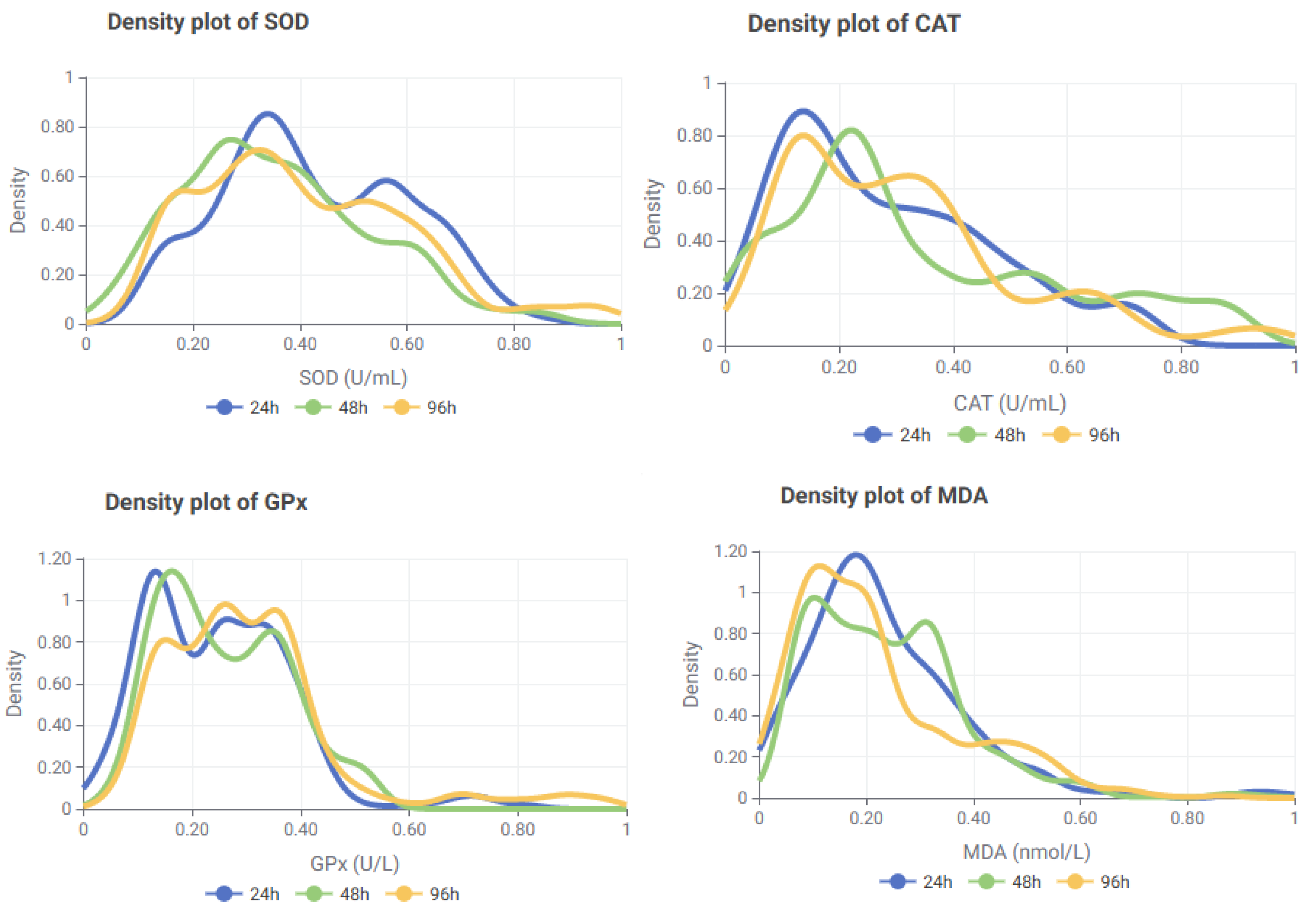

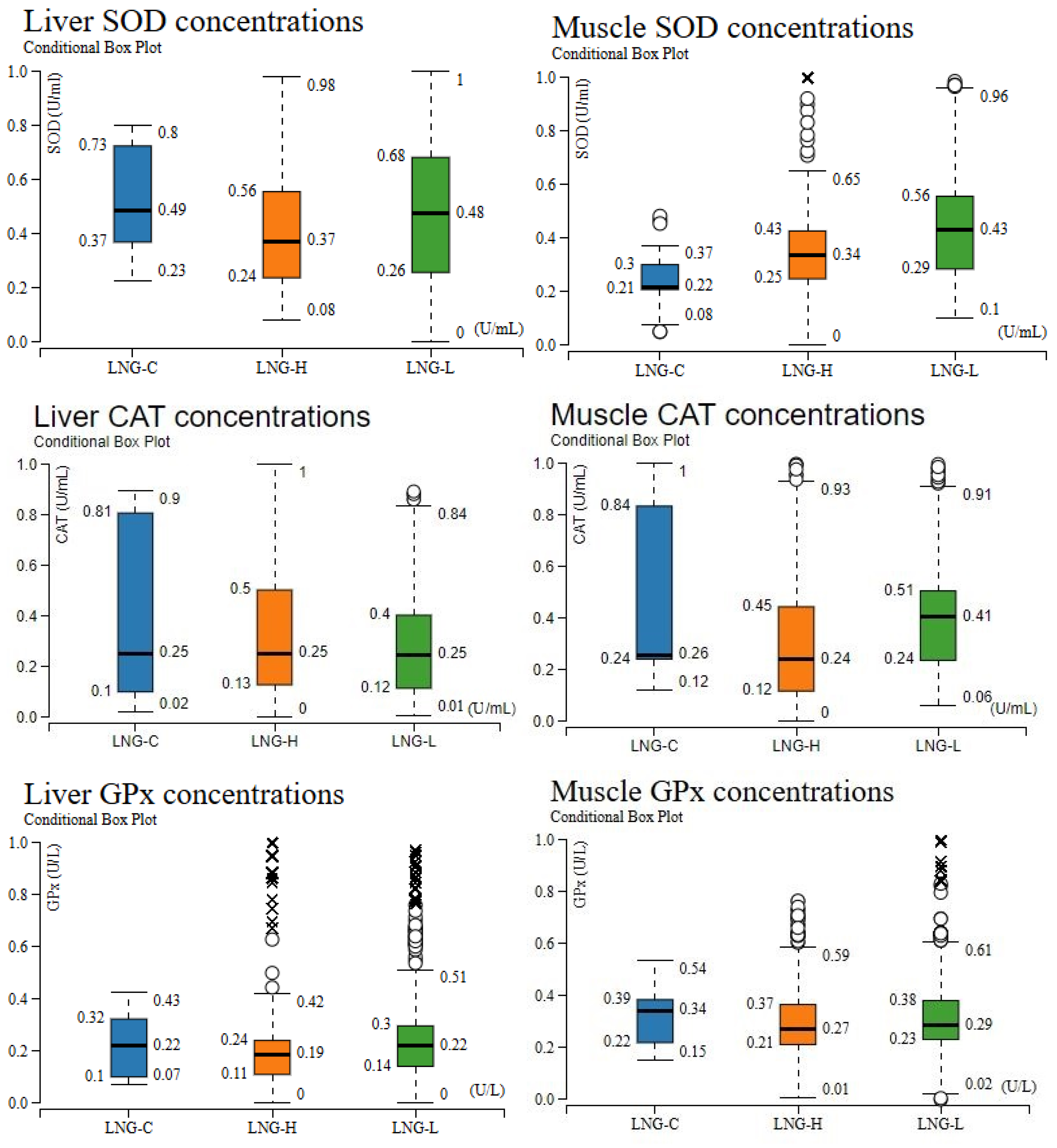
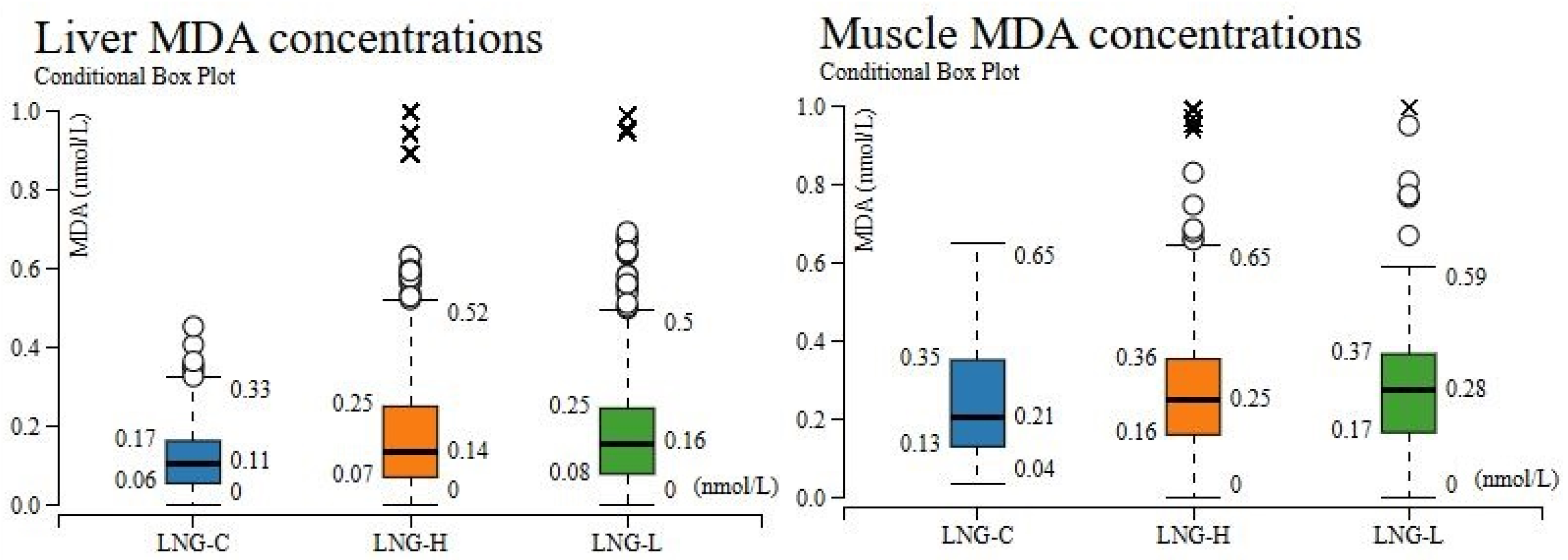
| Treatment * | Time (h) | Parameter | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DO2 (mg/L) | °C | pH | ORP(mV) | NH3-N (mg/L) | NO3-N (mg/L) | NO2-N (mg/L) | Hardness (mg/L) | Alkalinity (mg/L CaCO3) | ||
| LNG-C | 24 | 7.15 ± 0.27 | 24.24 ± 0.54 | 8.37 ± 0.10 | 17.88 ± 2.47 | 0.16 ± 0.02 | 1.22 ± 0.36 | 0.19 ± 0.11 | 11.27 ± 2.46 | 7.00 ± 1.60 |
| 48 | 7.24 ± 0.49 | 24.56 ± 0.51 | 8.32 ± 0.09 | 18.22 ± 2.15 | 0.18 ± 0.00 | 0.99 ± 0.47 | 0.06 ± 0.01 | 18.00 ± 1.70 | 4.00 ± 0.00 | |
| 96 | 7.13 ± 0.17 | 24.17 ± 0.16 | 8.38 ± 0.03 | 17.91 ± 1.88 | 0.15 ± 0.01 | 1.42 ± 0.32 | 0.16 ± 0.24 | 11.67 ± 1.87 | 6.00 ± 0.00 | |
| LNG-L | 24 | 7.31 ± 0.29 | 24.13 ± 0.90 | 8.33 ± 0.04 | 15.68 ± 0.71 | 0.17 ± 0.01 | 1.07 ± 0.13 | 0.06 ± 0.00 | 17.67 ± 1.87 | 4.00 ± 0.00 |
| 48 | 7.18 ± 0.43 | 24.71 ± 0.69 | 8.27 ± 0.05 | 15.92 ± 1.86 | 0.17 ± 0.00 | 1.04 ± 0.19 | 0.06 ± 0.00 | 18.00 ± 1.71 | 4.00 ± 0.00 | |
| 96 | 7.76 ± 0.43 | 24.78 ± 0.08 | 8.19 ± 0.09 | 14.61 ± 1.43 | 0.16 ± 0.03 | 1.46 ± 0.83 | 0.04 ± 0.01 | 9.33 ± 6.68 | 3.47 ± 0.82 | |
| LNG-H | 24 | 8.33 ± 0.25 | 23.98 ± 0.37 | 8.09 ± 0.25 | 16.03 ± 7.49 | 0.09 ± 0.06 | 1.53 ± 0.53 | 0.08 ± 0.10 | 17.67 ± 0.78 | 4.00 ± 0.00 |
| 48 | 8.29 ± 0.59 | 24.99 ± 1.33 | 8.15 ± 0.10 | 16.74 ± 3.57 | 0.12 ± 0.06 | 1.35 ± 0.51 | 0.05 ± 0.00 | 16.00 ± 1.21 | 4.00 ± 0.00 | |
| 96 | 7.87 ± 0.54 | 24.74 ± 0.64 | 8.16 ± 0.04 | 12.64 ± 1.43 | 0.14 ± 0.02 | 1.63 ± 0.43 | 0.03 ± 0.00 | 5.33 ± 1.97 | 3.67 ± 0.78 | |
| Algorithm * | Accuracy (%) | Error (%) | Cohen’s Kappa |
|---|---|---|---|
| GBT | 96.17 | 3.83 | 0.923 |
| RF | 94.97 | 5.03 | 0.899 |
| DT | 93.47 | 6.58 | 0.868 |
| MLP | 85.24 | 14.77 | 0.704 |
| LR | 82.06 | 17.96 | 0.642 |
| Model * | Class | Recall | Precision | Sensitivity | Specificity | F-Measure |
|---|---|---|---|---|---|---|
| GBT | Muscle | 0.958 | 0.964 | 0.958 | 0.965 | 0.961 |
| GBT | Liver | 0.965 | 0.96 | 0.965 | 0.958 | 0.962 |
| RF | Muscle | 0.938 | 0.959 | 0.938 | 0.961 | 0.948 |
| RF | Liver | 0.961 | 0.941 | 0.961 | 0.938 | 0.951 |
| DT | Muscle | 0.937 | 0.928 | 0.937 | 0.931 | 0.932 |
| DT | Liver | 0.931 | 0.94 | 0.931 | 0.937 | 0.936 |
| MLP | Muscle | 0.833 | 0.858 | 0.833 | 0.87 | 0.845 |
| MLP | Liver | 0.847 | 0.847 | 0.87 | 0.833 | 0.859 |
| LR | Muscle | 0.856 | 0.791 | 0.856 | 0.787 | 0.822 |
| LR | Liver | 0.787 | 0.853 | 0.787 | 0.856 | 0.819 |
| Biomarker * | R2 ** | MAE | RMSE |
|---|---|---|---|
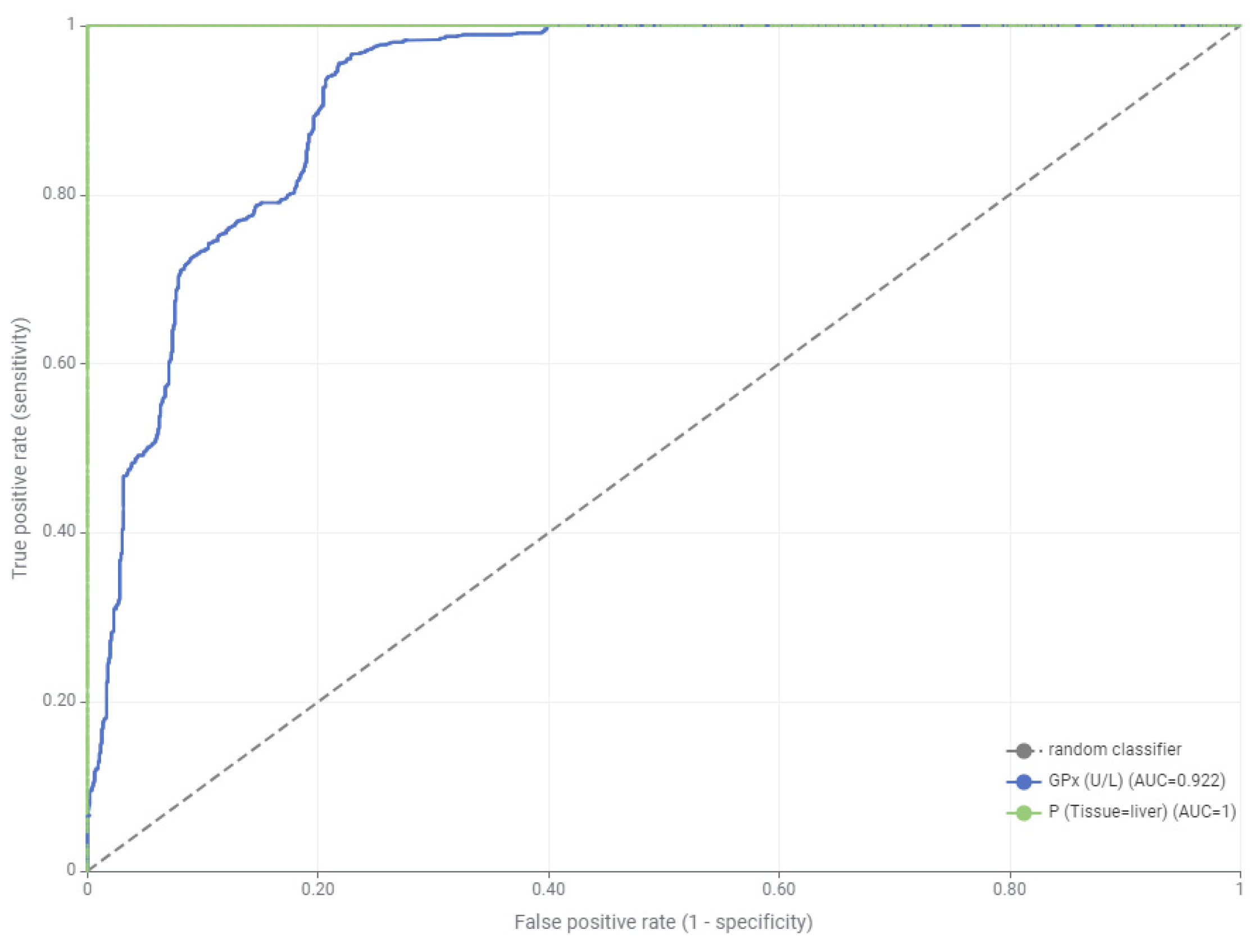
| GPx | 0.922 | 0.019 | 0.041 |
| MDA | 0.849 | 0.113 | 0.273 |
| SOD | 0.81 | 0.123 | 0.307 |
| CAT | 0.78 | 0.159 | 0.330 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meriç Turgut, İ.; Yapıcı, M.; Gerdan Koc, D. Integrating Experimental Toxicology and Machine Learning to Model Levonorgestrel-Induced Oxidative Damage in Zebrafish. Toxics 2025, 13, 764. https://doi.org/10.3390/toxics13090764
Meriç Turgut İ, Yapıcı M, Gerdan Koc D. Integrating Experimental Toxicology and Machine Learning to Model Levonorgestrel-Induced Oxidative Damage in Zebrafish. Toxics. 2025; 13(9):764. https://doi.org/10.3390/toxics13090764
Chicago/Turabian StyleMeriç Turgut, İlknur, Melek Yapıcı, and Dilara Gerdan Koc. 2025. "Integrating Experimental Toxicology and Machine Learning to Model Levonorgestrel-Induced Oxidative Damage in Zebrafish" Toxics 13, no. 9: 764. https://doi.org/10.3390/toxics13090764
APA StyleMeriç Turgut, İ., Yapıcı, M., & Gerdan Koc, D. (2025). Integrating Experimental Toxicology and Machine Learning to Model Levonorgestrel-Induced Oxidative Damage in Zebrafish. Toxics, 13(9), 764. https://doi.org/10.3390/toxics13090764







