Effects of Gossypol Exposure on Ovarian Reserve Function: Comprehensive Risk Assessment Based on TRAEC Strategy
Highlights
- TRAEC assessment classifies gossypol as moderate ovarian toxicant (score 4.68).
- Gossypol depletes follicles and impairs oocyte maturation, especially in young mice.
- Gossypol-induced ovarian damage shows limited recovery after 30-day withdrawal.
- Study integrates in vivo, in vitro and omics evidence for robust risk evaluation.
- These findings demonstrate that gossypol exerts moderate but partially irreversible reproductive toxicity, underscoring potential risks for ovarian health.
- The results highlight the necessity for cautious clinical application of gossypol and the importance of further investigations into safer therapeutic alternatives.
Abstract
1. Introduction
2. Materials and Methods
2.1. Risk Assessment of Gossypol-Induced Ovarian Reserve Decline Based on the TRAEC Strategy
2.2. Literature Retrieval and Screening
2.3. Experimental Animals
2.4. Experimental Design
2.5. Follicle Counting
2.6. ELISA for Hormone Quantification
2.7. Oocyte Culture and Maturation
2.8. Immunofluorescence of Oocytes
2.9. KGN Cell Proliferation Assay (EdU)
2.10. KGN Cell Apoptosis Assay (TUNEL)
2.11. Proteomic Analysis of Ovarian Tissue
2.12. Statistical Analysis
3. Results
3.1. Identification of the Scientific Question: Does Gossypol Exposure Affect Ovarian Reserve Function?
3.2. Evidence Collection and Integration
3.2.1. Literature Search and Screening
3.2.2. Overview of Included Studies
3.2.3. Summary of In Vivo Studies
3.2.4. Summary of In Vitro Studies
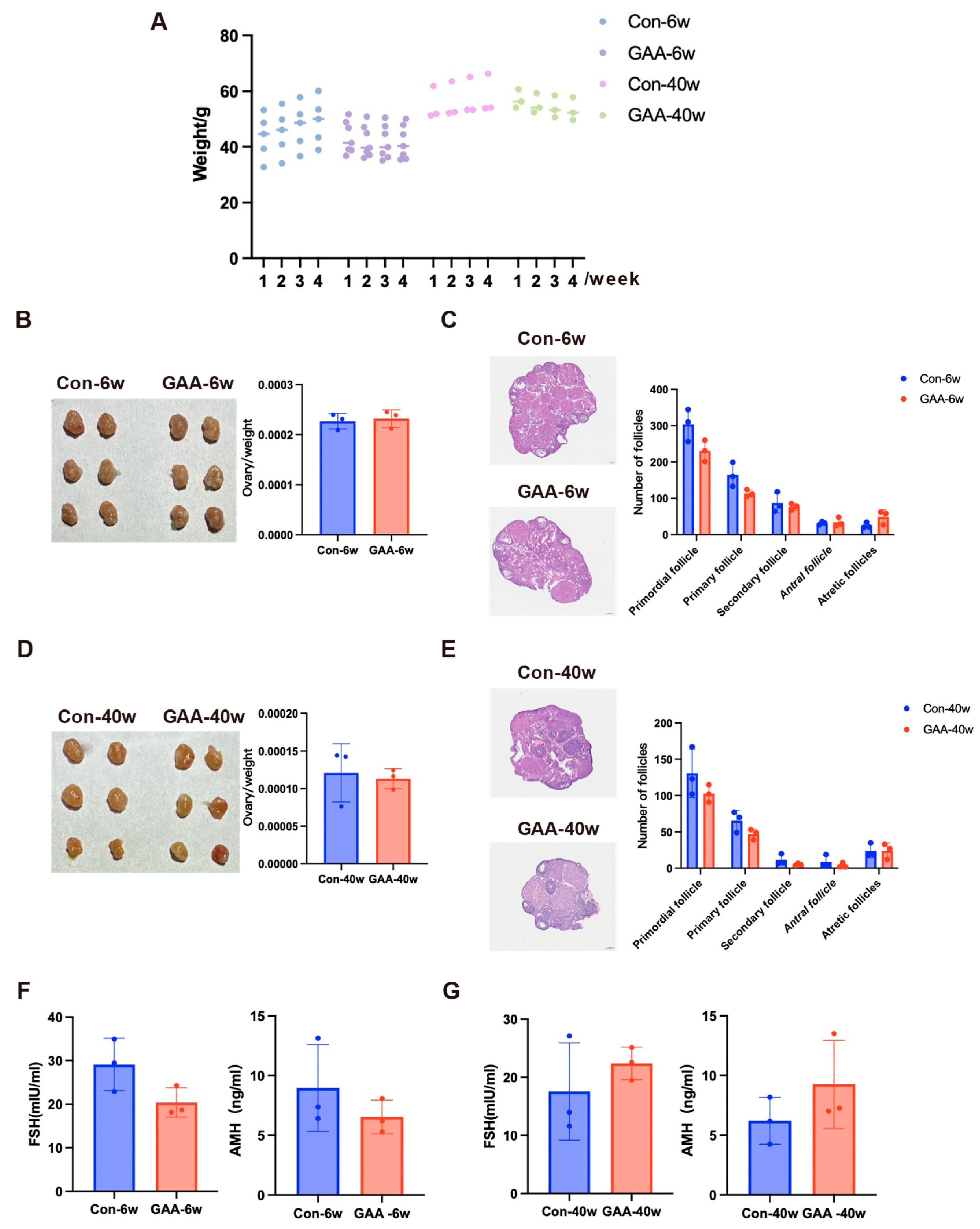
3.3. Gossypol Exposure Induces Decline in Ovarian Reserve Function
3.4. Effects of Gossypol on Mouse Oocytes and KGN Cells
3.4.1. Impairment of Oocyte Maturation
3.4.2. Disruption of Spindle Assembly and Mitochondrial Function
3.4.3. Inhibition of KGN Cell Proliferation and Induction of Apoptosis
3.5. Integrated Risk Assessment of Gossypol Exposure on Ovarian Reserve Function Based on the TRAEC Strategy
3.6. Proteomic Analysis of Ovarian Tissue Following Gossypol Exposure
3.7. Assessment of Reversibility of Gossypol-Induced Ovarian Reserve Decline
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pal, D.; Sahu, P.; Sethi, G.; Wallace, C.E.; Bishayee, A. Gossypol and Its Natural Derivatives: Multitargeted Phytochemicals as Potential Drug Candidates for Oncologic Diseases. Pharmaceutics 2022, 14, 2624. [Google Scholar] [CrossRef]
- Paunovic, D.; Rajkovic, J.; Novakovic, R.; Grujic-Milanovic, J.; Mekky, R.H.; Popa, D.; Calina, D.; Sharifi-Rad, J. The potential roles of gossypol as anticancer agent: Advances and future directions. Chin. Med. 2023, 18, 163. [Google Scholar] [CrossRef] [PubMed]
- Hu, B. Risk Assessment of Free Gossypol Residues in Mutton Sheep. Master’s Thesis, Xinjiang Agricultural University, Urumqi, China, 2016. [Google Scholar]
- Lü, Y.; Wang, X.; Zhao, Q.; Zhang, J. Research progress on safe limits of gossypol in feed and its residues in livestock products. Chin. Agric. Bull. 2010, 26, 1–5. [Google Scholar]
- Zhou, W.; Zheng, W.; Gao, W.; Zuo, X.; Mao, Q.; Hu, B.; Shi, S. Research progress on risk assessment of free gossypol in livestock and poultry products. Herbivores 2014, 5, 12–15. [Google Scholar] [CrossRef]
- Randel, R.D.; Chase, C.C., Jr.; Wyse, S.J. Effects of gossypol and cottonseed products on reproduction of mammals. J. Anim. Sci. 1992, 70, 1628–1638. [Google Scholar] [CrossRef]
- Gadelha, I.C.; Fonseca, N.B.; Oloris, S.C.; Melo, M.M.; Soto-Blanco, B. Gossypol toxicity from cottonseed products. Sci. World J. 2014, 2014, 231635. [Google Scholar] [CrossRef]
- Luz, V.B.; Gadelha, I.C.N.; Cordeiro, L.A.V.; Melo, M.M.; Soto-Blanco, B. In vitro study of gossypol’s ovarian toxicity to rodents and goats. Toxicon Off. J. Int. Soc. Toxinol. 2018, 145, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Brocas, C.; Rivera, R.M.; Paula-Lopes, F.F.; McDowell, L.R.; Calhoun, M.C.; Staples, C.R.; Wilkinson, N.S.; Boning, A.J.; Chenoweth, P.J.; Hansen, P.J. Deleterious actions of gossypol on bovine spermatozoa, oocytes, and embryos. Biol. Reprod. 1997, 57, 901–907. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hernández-Cerón, J.; Jousan, F.D.; Soto, P.; Hansen, P.J. Timing of inhibitory actions of gossypol on cultured bovine embryos. J. Dairy Sci. 2005, 88, 922–928. [Google Scholar] [CrossRef]
- Jimenez, C.R.; Moretti, D.B.; Corrêa, P.S.; da Costa, R.L.D.; Mui, T.S.; Machado-Neto, R.; Louvandini, H. Morphological-metric, ultrastructural and immunohistochemical effects of gossypol on cultured granulosa cells and oocytes of ewes using MOEPF. Anim. Reprod. Sci. 2019, 201, 22–31. [Google Scholar] [CrossRef]
- Ding, T.; Yan, W.; Zhou, T.; Shen, W.; Wang, T.; Li, M.; Zhou, S.; Wu, M.; Dai, J.; Huang, K.; et al. Endocrine disrupting chemicals impact on ovarian aging: Evidence from epidemiological and experimental evidence. Environ. Pollut. (Barking Essex 1987) 2022, 305, 119269. [Google Scholar] [CrossRef]
- Wu, C.; Chen, D.; Stout, M.B.; Wu, M.; Wang, S. Hallmarks of ovarian aging. Trends Endocrinol. Metab. TEM 2025, 36, 418–439. [Google Scholar] [CrossRef]
- Coutinho, E.M.; Athayde, C.; Atta, G.; Gu, Z.P.; Chen, Z.W.; Sang, G.W.; Emuveyan, E.; Adekunle, A.O.; Mati, J.; Otubu, J.; et al. Gossypol blood levels and inhibition of spermatogenesis in men taking gossypol as a contraceptive. A multicenter, international, dose-finding study. Contraception 2000, 61, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Liu, Y.; Chang, Q.; Liu, Z.; Wang, Q.; Niu, R.; Gao, B.; Guan, Q.; Xia, Y. Micro/Nanoplastic Exposure on Placental Health and Adverse Pregnancy Risks: Novel Assessment System Based upon Targeted Risk Assessment Environmental Chemicals Strategy. Toxics 2024, 12, 553. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Ding, C.; Xu, H.; Liu, Z.; Guan, Q.; Xia, Y.; Xu, Q. Effect of per- and polyfluoroalkyl substances on neurodevelopment: Evidence-based risk assessment in the TRAEC strategy context. Environ. Int. 2024, 191, 109003. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.S.; Xu, Q.Q.; Su, Y.; Yin, Z.; Guan, Q.; Liu, Z.; Song, L.; Shen, R. Risk assessment of pyridaben exposure on neurodevelopment of offspring by using TRAEC strategy. Sustain. Horiz. 2025, 13, 100135. [Google Scholar] [CrossRef]
- Jimenez, C.R.; Moretti, D.B.; da Silva, T.P.; Corrêa, P.S.; da Costa, R.L.D.; Siu, T.M.; Louvandini, H. Cottonseed (gossypol) intake during gestation and lactation does affect the ovarian population in ewes and lambs? Res. Vet. Sci. 2021, 135, 557–567. [Google Scholar] [CrossRef]
- Câmara, A.C.; Gadelha, I.C.; Borges, P.A.; de Paiva, S.A.; Melo, M.M.; Soto-Blanco, B. Toxicity of Gossypol from Cottonseed Cake to Sheep Ovarian Follicles. PLoS ONE 2015, 10, e0143708. [Google Scholar] [CrossRef]
- Gadelha, I.C.; de Macedo, M.F.; Oloris, S.C.; Melo, M.M.; Soto-Blanco, B. Gossypol promotes degeneration of ovarian follicles in rats. Sci. World J. 2014, 2014, 986184. [Google Scholar] [CrossRef]
- Ruan, D.; Lin, Y.; Zhang, H.; Chen, W.; Wang, S. Effects of dietary cottonseed meal levels on egg production performance, egg quality, plasma biochemical parameters, ovarian morphology, and gossypol residues in laying ducks at peak production. Chin. J. Anim. Nutr. 2014, 26, 353–362. [Google Scholar]
- Ni, M.; Yuan, Z.; Li, R.; Wu, J.; Yuan, L.; Wen, L.; Yuan, H. Effect of gossypol acetate on apoptosis of luteal cells in mice. In Proceedings of the 7th National Conference on Internal Medicine of Livestock 2011; Chinese Association of Animal Science and Veterinary Medicine: Beijing, China, 2011; Volume 1, pp. 390–397, College of Veterinary Medicine, Hunan Agricultural University. [Google Scholar]
- Randel, R.D.; Willard, S.T.; Wyse, S.J.; French, L.N. Effects of diets containing free gossypol on follicular development, embryo recovery and corpus luteum function in Brangus heifers treated with bFSH. Theriogenology 1996, 45, 911–922. [Google Scholar] [CrossRef]
- Bansode, F.W. Genotoxic effects of gossypol acetate on the ovary of Rhinopoma kinneari wroughton (Microchiroptera: Mammalia). Contraception 1994, 49, 601–607. [Google Scholar] [CrossRef]
- Gray, M.L.; Greene, L.W.; Williams, G.L. Effects of dietary gossypol consumption on metabolic homeostasis and reproductive endocrine function in beef heifers and cows. J. Anim. Sci. 1993, 71, 3052–3059. [Google Scholar] [CrossRef] [PubMed]
- Gambill, M.D.; Humphrey, W.D. Effects of diets containing gossypol on ovarian histology, function and fertility in prepubertal beef heifers. Theriogenology 1993, 40, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, R.; Shen, Q. Study on drug-induced aneuploidy of germ cells and early embryos in mice. Reprod. Contracept. 1992, 81, 34–39. [Google Scholar]
- Bender, H.S.; Saunders, G.K.; Misra, H.P. A histopathologic study of the effects of gossypol on the female rat. Contraception 1988, 38, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.C.; Nadakavukaren, M.J.; Jensen, D.R. Effects of ingested gossypol on the ultrastructure of rat ovarian follicles. Cell Tissue Res. 1987, 250, 215–220. [Google Scholar] [CrossRef]
- Gu, Y.; Anderson, N.O. Effects of gossypol on the estrous cycle and ovarian weight in the rat. Contraception 1985, 32, 491–496. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, M.; Li, C.; Yuan, Q. Study on the toxicological effects of gossypol acetate on pregnant rats. Chin. Pharm. J. 1985, 8, 464–467. [Google Scholar]
- Zhou, L.; Lei, H. Effects of gossypol acetate on uterus and ovary. Acta Pharm. Sin. 1984, 3, 220–223. [Google Scholar] [CrossRef]
- Wu, Y.M.; Chappel, S.C.; Flickinger, G.L. Effects of gossypol on pituitary-ovarian endocrine function, ovulation and fertility in female hamsters. Contraception 1981, 24, 259–268. [Google Scholar] [CrossRef]
- Su, X.; He, Y.; Li, H.; Yu, T.; Sun, Q.; Chen, M.; Zhang, B.; Wang, W.; Ju, S.; Li, Q. Melatonin protects porcine oocytes from gossypol-induced meiosis defects via regulation of SIRT1-mediated mitophagy. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2025, 195, 115122. [Google Scholar] [CrossRef]
- Hong, M.W.; Kim, H.; Choi, S.Y.; Sharma, N.; Lee, S.J. Effect of Gossypol on Gene Expression in Swine Granulosa Cells. Toxins 2024, 16, 436. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.M.; Chen, Y.W.; Wang, Y.S.; Ahmad, M.J.; Yang, S.J.; Duan, Z.Q.; Liu, M.; Yang, C.X.; Xiong, J.J.; Liang, A.X.; et al. Gossypol exposure induces mitochondrial dysfunction and oxidative stress during mouse oocyte in vitro maturation. Chem.-Biol. Interact. 2021, 348, 109642. [Google Scholar] [CrossRef]
- Myat, T.S.; Tetsuka, M. Gossypol inhibits LH-induced steroidogenesis in bovine theca cells. Anim. Sci. J. 2017, 88, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Gadelha, I.C.N.; Lima, M.A.; Melo, M.M.; Soto-Blanco, B. Gossypol promotes the degeneration of chicken ovarian follicles in vitro. Rev. Bras. De Ciência Avícola [Braz. J. Poult. Sci.] 2016, 18, 505–510. [Google Scholar] [CrossRef]
- Basini, G.; Bussolati, S.; Baioni, L.; Grasselli, F. Gossypol, a polyphenolic aldehyde from cotton plant, interferes with swine granulosa cell function. Domest. Anim. Endocrinol. 2009, 37, 30–36. [Google Scholar] [CrossRef]
- Long, A.; Wu, J.; Yuan, L.; Yuan, H. Effects of gossypol on proliferation and apoptosis of primary cultured luteal cells in pigs. Chin. J. Vet. Sci. 2009, 29, 1303–1306. [Google Scholar] [CrossRef]
- Kolena, J.; Vrsanská, S.; Nagyová, E.; Jezová, M. Gossypol inhibits follicle-stimulating hormone-and epidermal growth factor-stimulated expansion of oocyte-cumulus complexes from porcine preovulatory follicles. Physiol. Res. 2001, 50, 627–630. [Google Scholar] [CrossRef]
- Vranová, J.; Jezová, M.; Scsuková, S.; Kolena, J. Inhibitory effect of gossypol on basal and luteinization factor-stimulated progesterone synthesis in porcine granulosa cells. Physiol. Res. 1999, 48, 119–128. [Google Scholar]
- Akira, A.; Ohmura, H.; Uzumcu, M.; Araki, T.; Lin, Y.C. Gossypol inhibits aromatase activity in cultured porcine granulosa cells. Theriogenology 1994, 41, 1489–1497. [Google Scholar] [CrossRef]
- Lin, Y.C.; Gu, Y.; Brueggemeier, R.W.; Rikihisa, Y. Binding of 3H-gossypol in organelles of cultured bovine luteal cells. Life Sci. 1992, 50, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, P.K.; Lin, Y.C.; Rikihisa, Y.; Brueggemeier, R.W. Gossypolone suppresses progesterone synthesis in bovine luteal cells. J. Steroid Biochem. Mol. Biol. 1991, 38, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Chang, C.J.; Rikihisa, Y.; Lin, Y.C. Inhibitory effect of gossypol on human chorionic gonadotropin (hCG)-induced progesterone secretion in cultured bovine luteal cells. Life Sci. 1990, 47, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Lin, Y.C.; Rikihisa, Y. Inhibitory effect of gossypol on steroidogenic pathways in cultured bovine luteal cells. Biochem. Biophys. Res. Commun. 1990, 169, 455–461. [Google Scholar] [CrossRef]
- Lin, J.; Zheng, H. In vitro observation of the effects of gossypol acetate on estradiol and progesterone production by granulosa cells of mature rat follicles. Reprod. Contracept. 1988, 4, 59–64. [Google Scholar]
- Wang, N.G.; Guan, M.Z.; Lei, H.P. Effects of gossypol acetic acid on rat luteal cells in vitro. J. Ethnopharmacol. 1987, 20, 45–51. [Google Scholar] [CrossRef]
- Ye, W.S.; Liang, J.C.; Hsu, T.C. Toxicity of a male contraceptive, gossypol, in mammalian cell cultures. In Vitro 1983, 19, 53–57. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Diao, L. Feasibility analysis of compound gossypol acetate tablets combined with levonorgestrel intrauterine system in the treatment of adenomyosis after lesion excision. Chin. J. Ration. Drug Use Explor. 2021, 18, 69–72. [Google Scholar]
- Jiang, R.; Chen, Z.; Ni, M.; Li, X.; Ying, H.; Fen, J.; Wan, D.; Peng, C.; Zhou, W.; Gu, L. A traditional gynecological medicine inhibits ovarian cancer progression and eliminates cancer stem cells via the LRPPRC-OXPHOS axis. J. Transl. Med. 2023, 21, 504. [Google Scholar] [CrossRef]
- Hsieh, Y.S.; Yu, C.H.; Chu, S.C.; Lin, C.Y.; Chen, P.N. Gossypol Inhibits Metastasis of Lung Cell Carcinoma by Reversing Epithelial to Mesenchymal Transition and Suppressing Proteases Activity. Environ. Toxicol. 2024, 39, 5209–5221. [Google Scholar] [CrossRef]
- Xue, Q.; Wang, W.; Liu, J.; Wang, D.; Zhang, T.; Shen, T.; Liu, X.; Wang, X.; Shao, X.; Zhou, W.; et al. LRPPRC confers enhanced oxidative phosphorylation metabolism in triple-negative breast cancer and represents a therapeutic target. J. Transl. Med. 2025, 23, 372. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shi, S.; Tan, J.; He, L.; Liu, Q.; Fang, F.; Fu, H.; Zhong, M.; Mai, Z.; Sun, R.; et al. Highly Efficient Synergistic Chemotherapy and Magnetic Resonance Imaging for Targeted Ovarian Cancer Therapy Using Hyaluronic Acid-Coated Coordination Polymer Nanoparticles. Adv. Sci. 2024, 11, e2309464. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jin, L.; Li, X.; Deng, H.; Chen, Y.; Lian, Q.; Ge, R.; Deng, H. Gossypol induces apoptosis in ovarian cancer cells through oxidative stress. Mol. Biosyst. 2013, 9, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, H.M.; Tomaszewski, C.E.; Chang, A.L.; Moravek, M.B.; Xu, M.; Padmanabhan, V.; Shikanov, A. The ovarian stroma as a new frontier. Reproduction 2020, 160, R25–R39. [Google Scholar] [CrossRef]
- Chojnacka, K.J.; Elancheliyan, P.; Mussulini, B.H.M.; Mohanraj, K.; Callegari, S.; Gosk, A.; Banach, T.; Góral, T.; Szczepanowska, K.; Rehling, P.; et al. Ovarian carcinoma immunoreactive antigen-like protein 2 (OCIAD2) is a novel complex III-specific assembly factor in mitochondria. Mol. Biol. Cell 2022, 33, ar29. [Google Scholar] [CrossRef] [PubMed]
- Poluektov, Y.; George, M.; Daftarian, P.; Delcommenne, M.C. Assessment of SARS-CoV-2 specific CD4(+) and CD8 (+) T cell responses using MHC class I and II tetramers. Vaccine 2021, 39, 2110–2116. [Google Scholar] [CrossRef]
- Sun, X.; Zhan, M.; Sun, X.; Liu, W.; Meng, X. C1GALT1 in health and disease. Oncol. Lett. 2021, 22, 589. [Google Scholar] [CrossRef]
- Qi, L.; Li, Y.; Zhang, L.; Li, S.; Zhang, X.; Li, W.; Qin, J.; Chen, X.; Ji, Y.; Xue, Z.; et al. Immune and oxidative stress disorder in ovulation-dysfunction women revealed by single-cell transcriptome. Front. Immunol. 2023, 14, 1297484. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, D.; Mei, Y.; Liu, S.; Shao, H.; Sun, Q.; Lu, Q.; Hu, J.; Gu, H. Single-cell RNA sequencing of peripheral blood reveals immune cell dysfunction in premature ovarian insufficiency. Front. Endocrinol. 2023, 14, 1129657. [Google Scholar] [CrossRef]
- Abdelraheim, S.R.; Spiller, D.G.; McLennan, A.G. Mouse Nudt13 is a Mitochondrial Nudix Hydrolase with NAD(P)H Pyrophosphohydrolase Activity. Protein J. 2017, 36, 425–432. [Google Scholar] [CrossRef]
- Shand, H.; Patra, S.; Ghorai, S. Fatty acid binding protein as a new age biomarker. Clin. Chim. Acta Int. J. Clin. Chem. 2025, 565, 120029. [Google Scholar] [CrossRef]
- Lapenna, D. Glutathione and glutathione-dependent enzymes: From biochemistry to gerontology and successful aging. Ageing Res. Rev. 2023, 92, 102066. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.Z.; Wang, Z.G. Gossypol: A potential antifertility agent for males. Annu. Rev. Pharmacol. Toxicol. 1984, 24, 329–360. [Google Scholar] [CrossRef]
- Waites, G.M.; Wang, C.; Griffin, P.D. Gossypol: Reasons for its failure to be accepted as a safe, reversible male antifertility drug. Int. J. Androl. 1998, 21, 8–12. [Google Scholar] [CrossRef]
- Wang, H.; Piao, Z.; Ma, H.; Cao, L.; Liu, J.; Wu, J. Short-term exposure to gossypol causes reversible reproductive toxicity and nephrotoxicity in mice. Nan Fang Yi Ke Da Xue Xue Bao [J. South. Med. Univ.] 2023, 43, 251–256. [Google Scholar] [CrossRef]
- Hoage, T.R.; Cameron, I.L. Folliculogenesis in the ovary of the mature mouse: A radioautographic study. Anat. Rec. 1976, 184, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.L.; Fang, X.; Li, J.X.; Chen, Z.W.; Wu, W.K.; Guo, X.X.; Liu, N.J.; Huang, J.F.; Chen, F.Y.; Wang, L.J.; et al. Dirigent gene editing of gossypol enantiomers for toxicity-depleted cotton seeds. Nat. Plants 2023, 9, 605–615. [Google Scholar] [CrossRef] [PubMed]

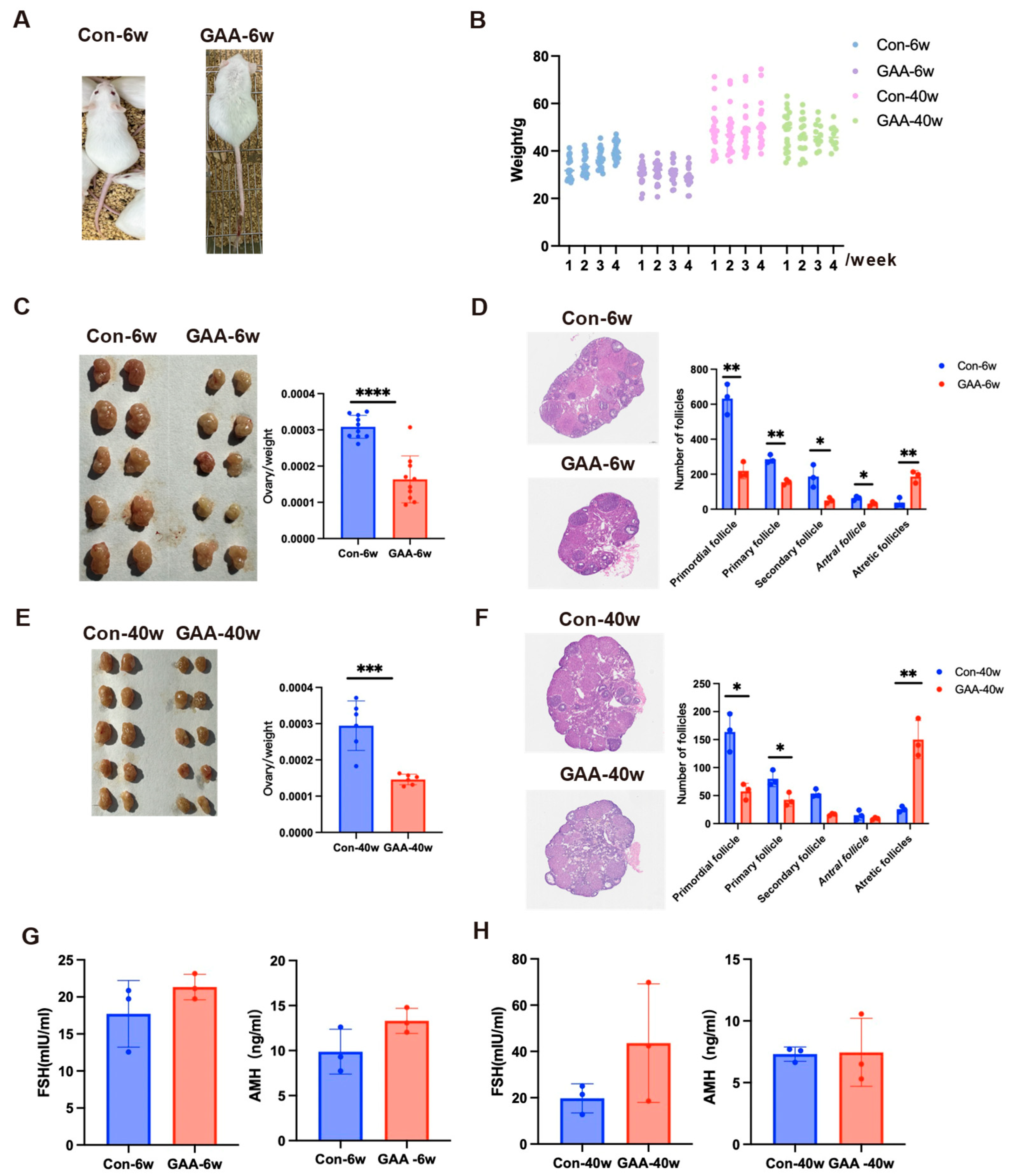
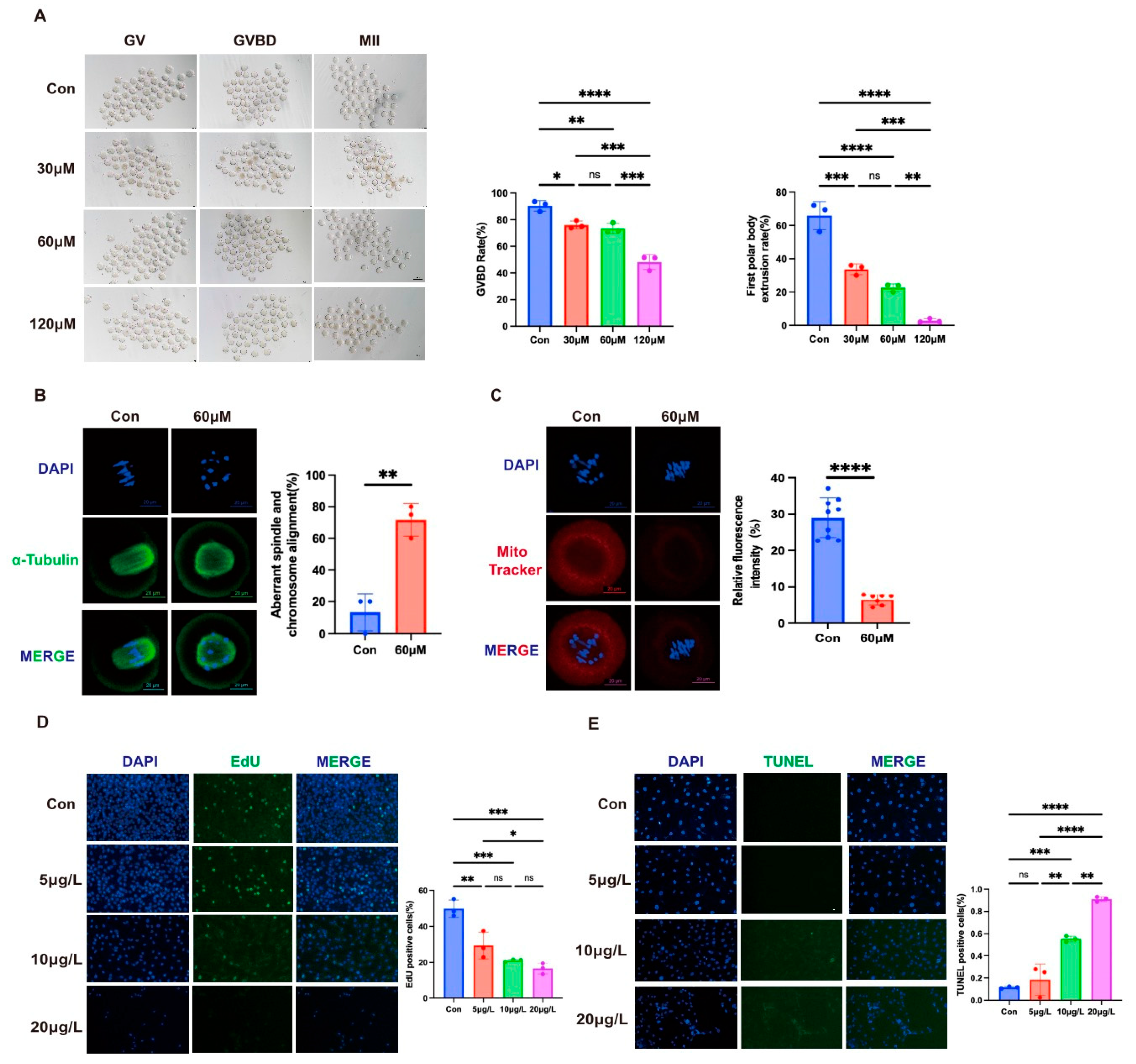
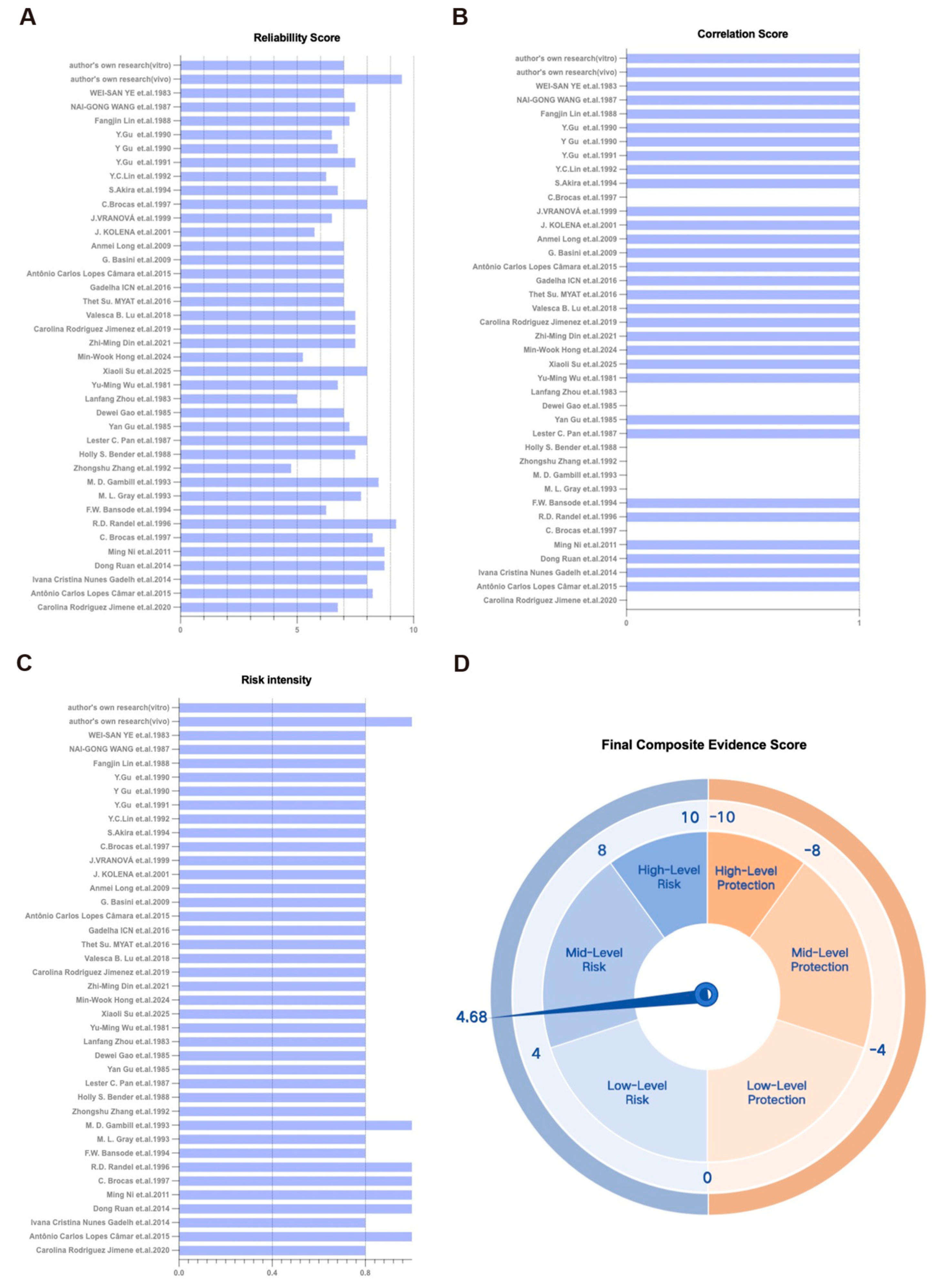
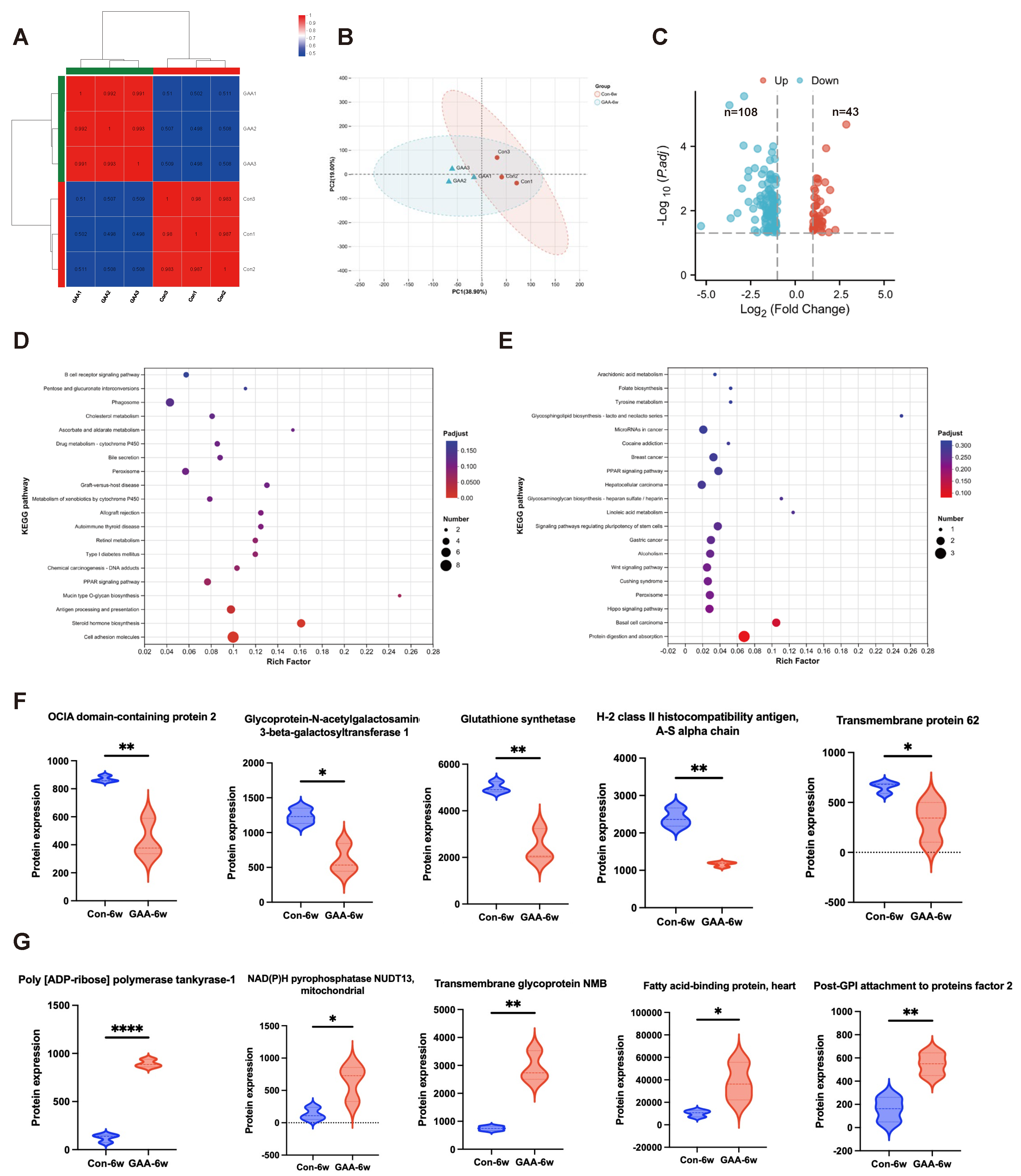
| ID | Study | Location | Species | Exposure Period | Age | Type of Gossypol | Exposure Dose | Exposure Methods | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Jimenez et al., 2021 [18] | Brazil | Santa Inês ewes | 10 months | / | Cottonseed | / | Feeding | Cottonseed had no effect on maternal–offspring follicular dynamics but may alter the future steroidogenic response in offspring of dams exposed to cottonseeds during their reproductive period. |
| 2 | Câmara et al., 2015 [19] | Brazil | Santa Inês crossbred ewes | 63 days | / | Cottonseed cake | Feed was offered at 1.5% of the animal’s body weight. | Feeding | Gossypol-fed sheep exhibited a significantly reduced proportion of viable ovarian follicles (20.6%) and an increased proportion of atretic follicles (79.4%). |
| 3 | Gadelha et al., 2014 [20] | Brazil | Wistar rats | 15 days | 60 to 70 days old | Gossypol acetic acid (Fluka, G4382) | 25 mg gossypol/kg/day | Subcutaneous injection | Gossypol reduced the number of viable follicles and altered hormone levels, thereby disrupting the estrous cycle. |
| 4 | Ruan et al., 2014 [21] | China | Fujian Longyan mountain hemp duck | 3 months | 19 weeks | Cottonseed meal | 14.82, 28.03, 32.81, 39.18, and 50.16 mg/kg free gossypol | Feeding | Dominant follicle integrity was significantly compromised at a dietary free gossypol level of 28.03 mg/kg, characterized by deformation, rupture, partial dissolution, and hemorrhagic spots on follicular vascular walls. |
| 5 | Ni et al., 2011 [22] | China | Kunming mice | 24 h, 48 h | 25 to 30 days old | Gossypol acetic acid | 1.6 and 8.40 mg/L, 0.5 mL | Intraperitoneal injection | Gossypol can effectively induce apoptosis in mouse luteal cells. |
| 6 | Brocas et al., 1997 [9] | USA | Nonlactating Holstein cow | 9 weeks | / | Cottonseed meal | 6.84 g free gossypol/day | Feeding | No significant differences (p > 0.10) were observed between cottonseed meal-fed and control cows regarding oocyte yield, cleavage rate, or blastocyst development. |
| 7 | Randel et al., 1996 [23] | USA | Brangus heifers | 65 days | 2 years old | Cottonseed meal; whole cottonseed | 5 g free gossypol/day; 15 g free gossypol/day | Feeding | Ovarian and stromal weights, as well as total follicle counts per heifer, did not differ among the three treatment groups. However, CSM heifers had fewer follicles >5 mm compared with WCS and control heifers. |
| 8 | Bansode, 1994 [24] | India | Rhinopoma kinneari | 2 days, 4 days, 6 days, 8 days | / | Gossypol acetate | 10 mg/day | Feeding | Gossypol acetate exerts cytotoxic and antifolliculogenic effects, inducing oocyte and follicle degeneration and inhibiting their development. |
| 9 | Gray et al., 1993 [25] | USA | Postpubertal beef heifers; mature cows | 62 days; 33 weeks | / | Cottonseed meal and whole cottonseeds | 0, 0.5, 2.5, 5, 10, and 20 g free gossypol/day; 20 mg free gossypol/kg/day | Feeding | The gossypol levels used in these studies are unlikely to impair reproductive performance in beef heifers or cows. |
| 10 | Gambill and Humphrey, 1993 [26] | USA | Crossbred beef heifers | 64 days | / | Cottonseed meal and whole cottonseeds | 6.1 g free gossypol/day; 13.7 g free gossypol/day | Feeding | Ovarian metrics, corpus luteum characteristics, and follicle number and size were comparable across treatments. |
| 11 | Zhang et al., 1992 [27] | China | ICR mice | / | 8–13 weeks | Gossypol acetic acid | 5, 20, and 50 mg/kg | / | Gossypol acetate at varying doses did not affect oocyte aneuploidy rates in mice. |
| 12 | Bender et al., 1988 [28] | USA | Sprague Dawley rats | 30 days | / | Gossypol acetic acid | 40 and 60 mg gossypol/kg/day | Intragastric administration | Despite fewer estrous cycles, gossypol-treated rats showed no histopathological changes in their ovaries, uterus, or vagina. |
| 13 | Pan et al., 1987 [29] | USA | Rats | 60 days | / | Gossypol acetic acid | 20 mg gossypol/kg/day | Orally | Changes included increased ooplasmic lysosomes and underdeveloped smooth endoplasmic reticulum in granulosa cells. |
| 14 | Gu and Anderson, 1985 [30] | USA | Long–Evans strain rats | 15 days; 20 days | / | Gossypol acetate | 10 mg gossypol/kg/day; 1, 5, and 10 mg gossypol/kg/day | Subcutaneous injection | At male-effective doses, gossypol halted estrous cycles and significantly reduced ovarian weight. |
| 15 | Gao et al., 1985 [31] | China | Rats | Day 1–5 of gestation | / | Gossypol | 100, 81, 66, 53, 43, 35, 28, and 23 mg gossypol/kg/day | Intragastric administration | Ovarian tissues showed no significant changes; corpus luteum and follicles at all stages appeared normal across all doses. |
| 16 | Zhou and Lei, 1984 [32] | China | Wistar rats | Six times a week for 8 consecutive weeks | / | Gossypol acetic acid | 30 mg/kg gossypol | Intragastric administration | Ovarian morphology did not differ significantly between gossypol and control groups. |
| 17 | Wu et al., 1981 [33] | USA | Hamsters | 76 days; 40 days; 20 days | / | Gossypol | 5 mg gossypol/kg/day; 10 mg gossypol/kg/day; 10 and 20 mg gossypol/kg/day | Intragastric administration | Gossypol altered pituitary and ovarian hormones in proestrus and estrus but did not affect estrous length, ovulation count, or pregnancy rate. |
| ID | Study | Location | Exposure Period | Cell Type | Cell Source | Type of Gossypol | Exposure Concentration | Outcomes |
|---|---|---|---|---|---|---|---|---|
| 1 | Su et al., 2025 [34] | China | 44 h | Oocytes | Porcine ovaries | Gossypol (purity >99%, MedChemExpress, Princeton, NJ, USA) | 10, 20, and 40 μM gossypol | Gossypol impaired porcine oocyte maturation in vitro by reducing PB1 extrusion, inhibiting cumulus expansion, and disrupting meiosis. |
| 2 | Hong et al., 2024 [35] | Korea | 72 h | Granulosa cells | Swine ovaries | / | 6.25 and 12.5 μM gossypol | Gossypol is cytotoxic to porcine granulosa cells, inhibiting proliferation and impairing oocyte maturation. |
| 3 | Ding et al., 2021 [36] | China | 2 h; 8 h; 14 h | Oocytes | Kunming mouse ovaries (3–4 weeks old) | Gossypol (Yirui Biotech, Hangzhou, China) | 20, 40, and 60 μM gossypol | Gossypol impairs polar body extrusion, disrupts spindle structure, induces mitochondrial dysfunction and oxidative stress, and triggers early apoptosis. |
| 4 | Jimenez et al., 2019 [11] | Brazil | 24 h; 96 h | Granulosa cells and oocytes | Santa Inês ewe ovaries (1-year-old) | Gossypol acetic acid (G4382, Sigma-Aldrich, São Paulo, Brazil) | 5, 10, and 20 μg/mL gossypol | Gossypol impairs granulosa cell development and preantral follicle integrity in sheep. |
| 5 | Luz et al., 2018 [8] | Brazil | 24 h; 7 days | Ovaries | Rat, mouse, and goat ovaries | Gossypol acetic acid (G4382, Fluka, Buchs, Switzerland) | 5, 10, and 20 μg/mL gossypol | Gossypol may directly impair follicular maturation and female fertility. |
| 6 | Myat and Tetsuka, 2017 [37] | Japen | 24 h at 1 day and 7 days | Theca cells | Bovine ovaries | Gossypol (Sigma-Aldrich, St. Louis, MO, USA) | 0.2, 1, 5, and 25 μg/mL gossypol | Gossypol inhibits thecal steroidogenesis by downregulating steroidogenic enzyme genes without impacting cell viability in cattle. |
| 7 | Gadelha et al., 2016 [38] | Brazil | 24 h; 7 days | Ovarian follicles | Adult Bantam chicken ovaries | Gossypol acetic acid (G4382, Fluka, Buchs, Switzerland) | 5, 10, and 20 μg/mL gossypol | Gossypol increased atresia across all follicular stages in cultured chicken ovaries, indicating impaired follicle viability and maturation. |
| 8 | Câmara et al., 2015 [19] | Brazil | 24 h; 7 days | ovaries | Santa Inês ewe ovaries (3–5 years old) | Gossypol acetic acid (G4382, Fluka, Buchs, Switzerland) | 5, 10, and 20 μg/mL gossypol | Gossypol in cottonseed directly induces follicular atresia in sheep. |
| 9 | Basini et al., 2009 [39] | Italy | 48 h | Granulosa cell | Large White cross-bred gilt swine ovaries | Gossypol (Sigma-Aldrich, St. Louis, MO, USA) | 5 and 25 μg/mL gossypol | Gossypol markedly impairs porcine granulosa cell proliferation, steroidogenesis, and angiogenesis. |
| 10 | Long et al., 2009 [40] | China | 24 h; 48 h | Luteal cells | Landrace–Yorkshire hybrid sow corpora lutea | Gossypol (Zhejiang Institute of Light Industry, Hangzhou, China) | 0.4, 2, 10, and 50 mg/L gossypol | Gossypol dose-dependently inhibits luteal cell proliferation and induces apoptosis, with effects increasing over time at certain concentrations. |
| 11 | Kolena et al., 2001 [41] | Czech Republic | Oocyte–cumulus complexes | Porcine ovaries | / | 10−4, 10−5, and 10−6 M gossypol | Gossypol suppresses FSH- and EGF-induced OCC expansion, reduces progesterone secretion, and decreases EGF receptor levels in granulosa cells. | |
| 12 | Vranová et al., 1999 [42] | Slovak Republic | 3 days | Granulosa cells | Porcine small follicles | Gossypol (Sigma Chemical Company, St. Louis, MO, USA) | 10−5, 5 × 10−5, 10−4, and 5 × 10−4 M gossypol | Gossypol inhibits large follicles or conditioned media from stimulating progesterone production in cultured small follicles. |
| 13 | Brocas et al. (1997) [9] | USA | Oocytes | Cow ovaries | Gossypol (Sigma Chemical Company, St. Louis, MO, USA; Lot 93H4014) | 2.5, 5, and 10 μg/mL gossypol | Gossypol addition during in vitro maturation did not affect oocyte cleavage rate (p > 0.10). | |
| 14 | Akira et al., 1994 [43] | USA | 48 h | Granulosa cells | Porcine ovaries | / | 1–4 μM gossypol | Gossypol inhibits FSH-induced aromatase activity in cultured porcine granulosa cells. |
| 15 | Lin et al., 1992 [44] | USA | 3 h | Luteal cells | Bovine | 3H-gossypol acetic acid (prepared in-house following Stipanovic’s method) | 2.15 and 3.4 μM 3H-gossypol acetic acid (3H-gossypol) | The cell membrane showed the highest gossypol binding, with most localized in particulate fractions. |
| 16 | Gu et al., 1991 [45] | USA | 3 h | Luteal cells | Bovine ovaries containing corpora lutea | Gossypol acetic acid (Sigma-Aldrich, St. Louis, MO, USA) | 4.25, 8.5, 12.75, and 17.00 μM gossypol or gossypolone | Gossypol inhibits both 3β-hydroxysteroid and cytochrome P450scc enzyme activities. |
| 17 | Gu et al., 1990a [46] | USA | 3 h | Luteal cells | Dairy cow corpora lutea | Gossypol acetic acid (Sigma-Aldrich, St. Louis, MO, USA) | 10, 20, and 40 μg/mL gossypol acetic acid | Gossypol dose-dependently inhibits hCG- and forskolin-induced progesterone secretion and intracellular cAMP formation. |
| 18 | Gu et al., 1990b [47] | USA | 3 h | Luteal cells | Bovine ovaries containing corpora lutea | Gossypol (Sigma-Aldrich, St. Louis, MO, USA) | 4.25, 8.5, 17, and 34 μM gossypol; 170 μM gossypol | Gossypol suppresses progesterone synthesis in bovine luteal cells by inhibiting steroidogenic enzymes. |
| 19 | Lin and Zheng, 1988 [48] | China | 4 h; 24 h | Granulosa cells | Rats (30-day-old) | Gossypol (Shanghai Oil No. 2 Factory, Shanghai, China) | 33, 44, and 55 μg/50 μL gossypol | Gossypol selectively inhibits progesterone synthesis in granulosa cells without significantly affecting aromatase activity. |
| 20 | Wang et al., 1987 [49] | China | 3 h | Luteal cells | Wistar rats | Gossypol acetic acid (Sigma-Aldrich, St. Louis, MO, USA) | 10, 20, and 30 μg/mL gossypol | GAA inhibits luteal steroidogenesis by suppressing gonadotropin-stimulated cAMP formation via adenylate cyclase inhibition. |
| 21 | Ye et al., 1983 [50] | USA | 24 h; 48 h | Ovary cells | Chinese hamster | Gossypol acetate (Sigma-Aldrich, St. Louis, MO, USA) | 5, 10, 50, and 100 μg/mL gossypol | Gossypol dose-dependently reduces survival and mitotic index of Chinese hamster ovary cells. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Ying, J.; Ma, X.; Zhong, Y.; Huo, R.; Meng, Q. Effects of Gossypol Exposure on Ovarian Reserve Function: Comprehensive Risk Assessment Based on TRAEC Strategy. Toxics 2025, 13, 763. https://doi.org/10.3390/toxics13090763
Sun X, Ying J, Ma X, Zhong Y, Huo R, Meng Q. Effects of Gossypol Exposure on Ovarian Reserve Function: Comprehensive Risk Assessment Based on TRAEC Strategy. Toxics. 2025; 13(9):763. https://doi.org/10.3390/toxics13090763
Chicago/Turabian StyleSun, Xiaoyan, Jia Ying, Xuan Ma, Yunong Zhong, Ran Huo, and Qingxia Meng. 2025. "Effects of Gossypol Exposure on Ovarian Reserve Function: Comprehensive Risk Assessment Based on TRAEC Strategy" Toxics 13, no. 9: 763. https://doi.org/10.3390/toxics13090763
APA StyleSun, X., Ying, J., Ma, X., Zhong, Y., Huo, R., & Meng, Q. (2025). Effects of Gossypol Exposure on Ovarian Reserve Function: Comprehensive Risk Assessment Based on TRAEC Strategy. Toxics, 13(9), 763. https://doi.org/10.3390/toxics13090763







