Exploring Different Toxic Effects of UV-Aged and Bio-Aged Microplastics on Growth and Oxidative Stress of Escherichia coli
Highlights
- UV-aged PS with a rougher surface and high degree of oxidation had a strong inhibitory effect on E. coli growth and induced strong oxidative stress.
- Bio-aged PS covered by a biological interface layer showed an enhanced effect on E. coli growth with attenuated oxidative stress.
- The main findings demonstrate the different toxicity-related effects of UV-aged and bio-aged microplastics on the bacterial growth and oxidative stress of E. coli.
- The main findings highlight the close correlation between changes in the properties of MPs driven by different aging processes and the toxicity towards E. coli.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Aged Microplastics
2.3. Characterization of Virgin and Aged PS
2.4. Bacteria Cultivation and Exposure
2.5. Determination of ATPase Activity, Production of ROS, and MDA Content
2.6. Statistical Analysis
3. Results and Discussion
3.1. Changes in Physicochemical Properties of Virgin and Aged PS
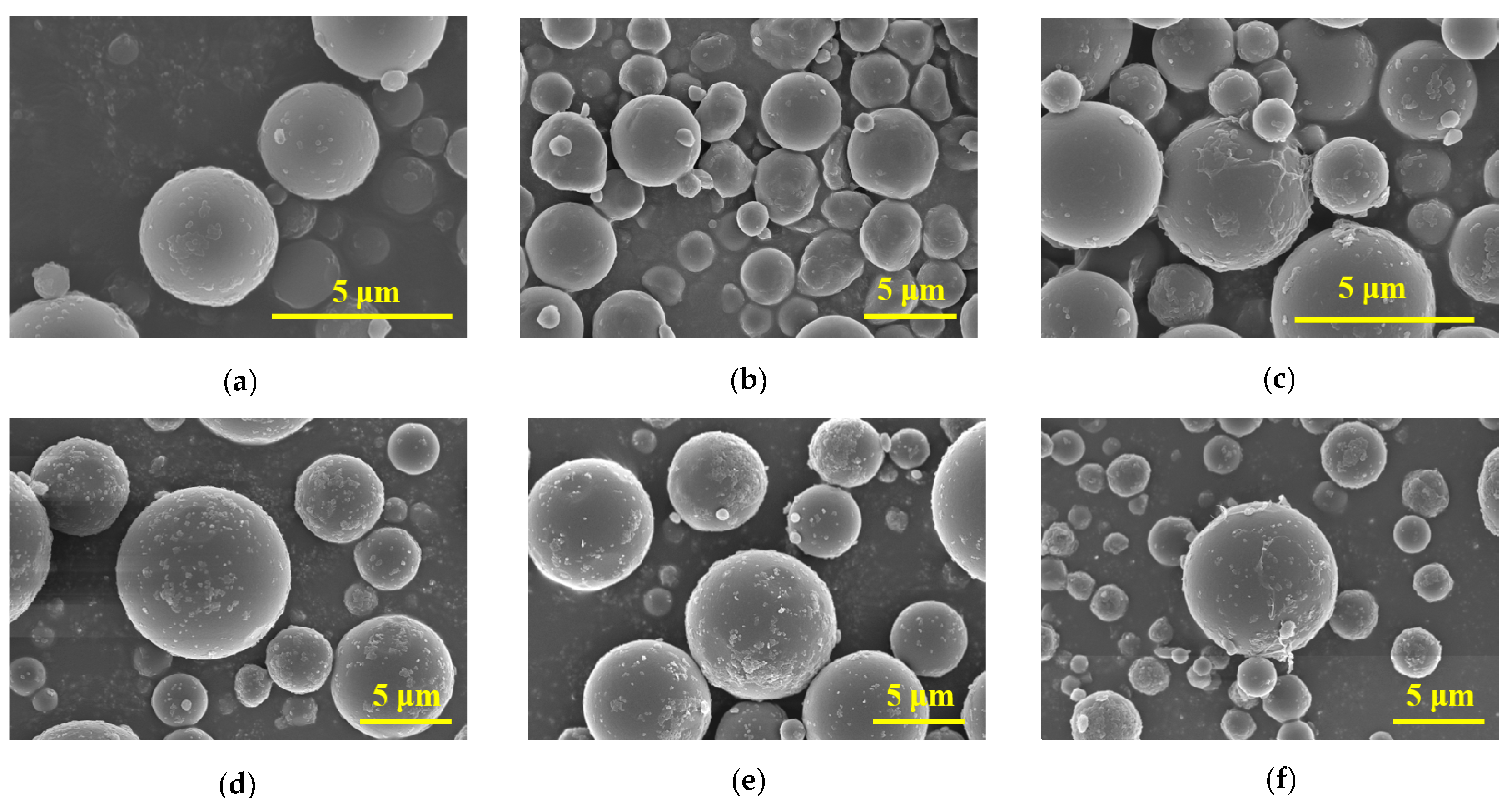
3.1.1. Surface Morphology
3.1.2. Functional Groups
3.1.3. Hydrophobicity
3.1.4. Fouling Biomass of Bio-PS
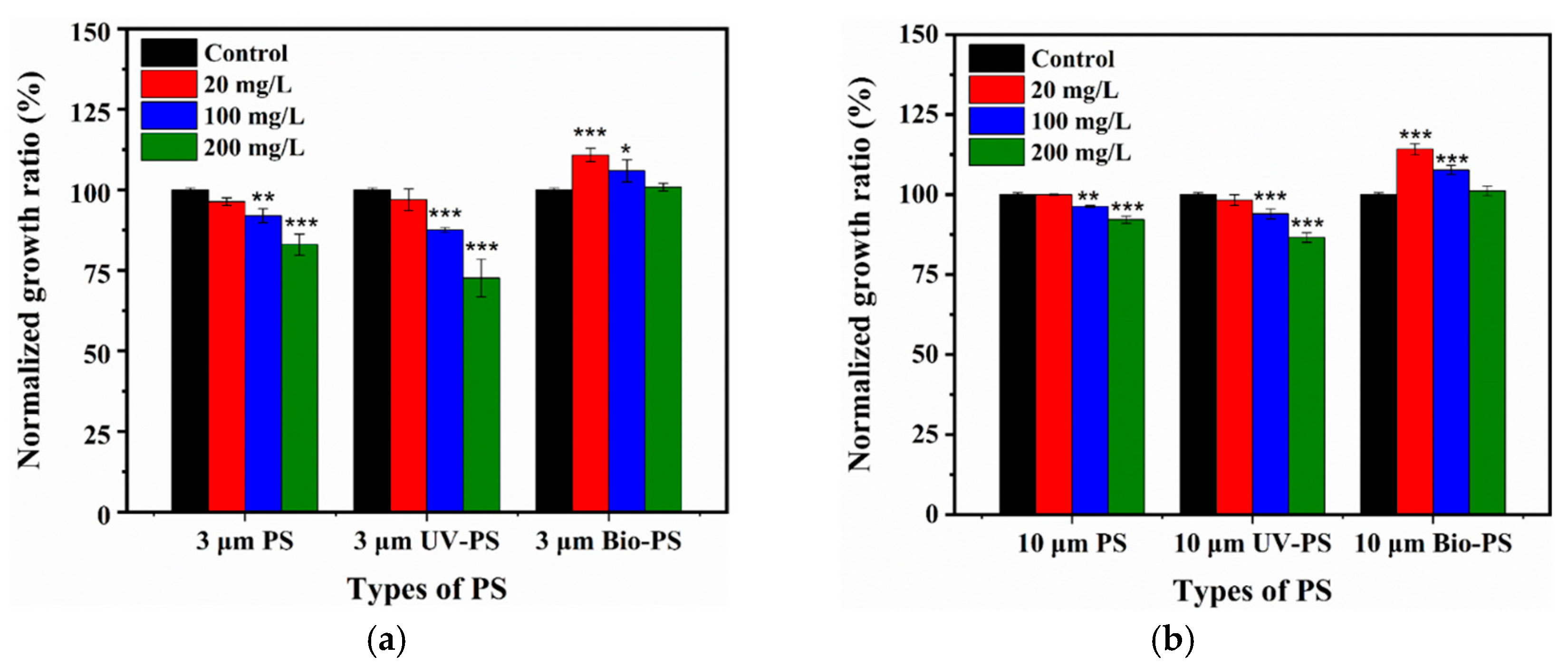
3.2. Effects of Virgin and Aged PS on Cell Growth of E. coli
3.2.1. Effects of Virgin PS on Cell Growth of E. coli
3.2.2. Effects of UV-PS on Cell Growth of E. coli
3.2.3. Effects of Bio-PS on Cell Growth of E. coli
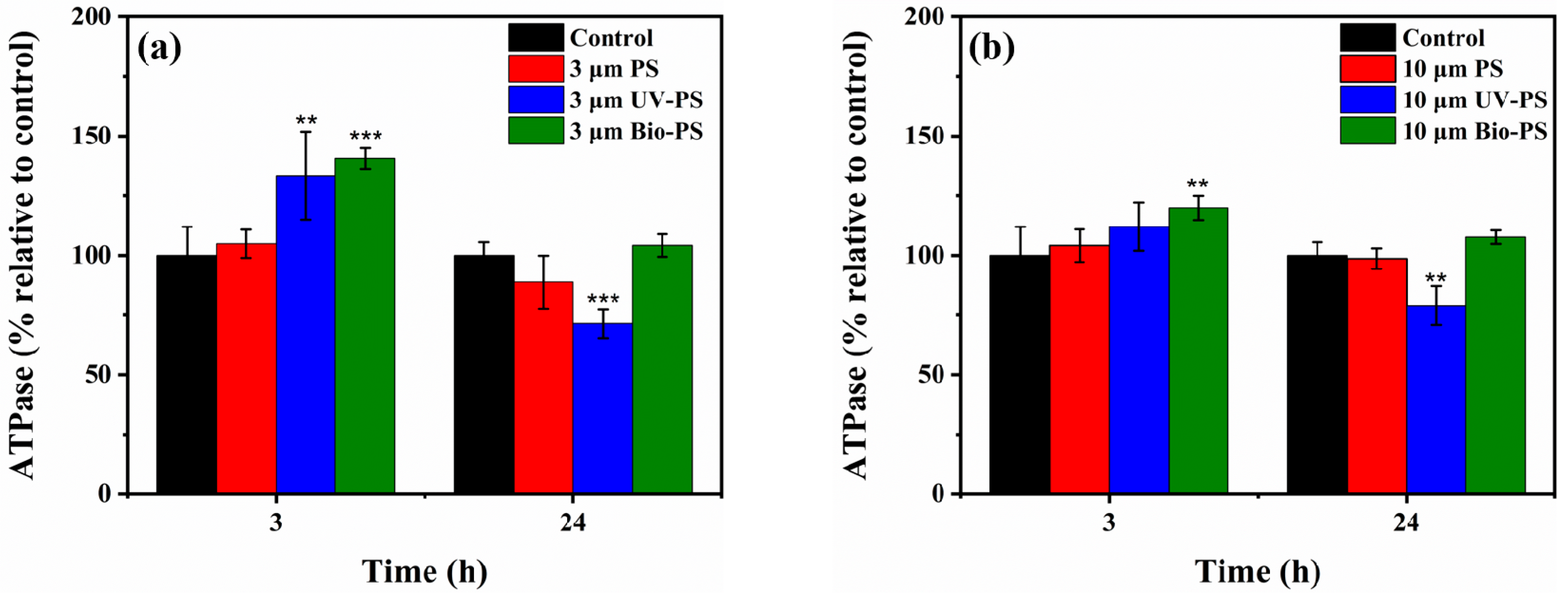
3.3. Effects of Virgin and Aged PS on ATPase Activity
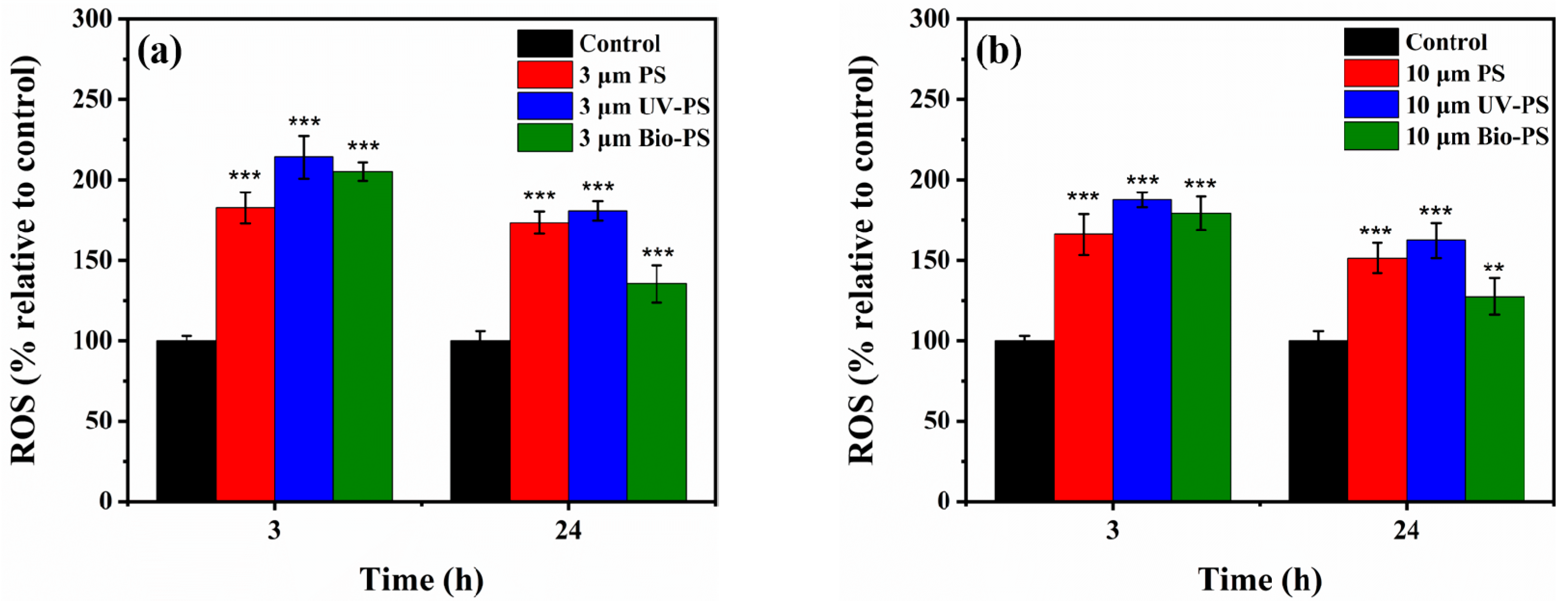
3.4. Effects of Virgin and Aged PS on Oxidative Stress
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rillig, M.C.; Lehmann, A. Microplastic in Terrestrial Ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics–The Facts 2022. 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022-2/ (accessed on 2 October 2022).
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in Freshwater Systems: A Review of the Emerging Threats, Identification of Knowledge Gaps and Prioritisation of Research Needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Alva, P.P.; Thomas, T.A. Microplastics: A Global Threat to Life and Living. Environ. Monit. Assess. 2025, 197, 725. [Google Scholar] [CrossRef]
- Lim, X. Microplastics Are Everywhere—but Are They Harmful? Nature 2021, 593, 22–25. [Google Scholar] [CrossRef]
- Law, K.L.; Thompson, R.C. Microplastics in the Seas. Science 2014, 345, 144–145. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, F.; Hassan Kazmi, S.S.U.; Zhao, Y.; Chen, M.; Wang, J. A Review of Microplastic Pollution in Seawater, Sediments and Organisms of the Chinese Coastal and Marginal Seas. Chemosphere 2022, 286, 131677. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, J.; Wang, J.; Yang, H.; Fu, Y.; Fu, M.; Li, S.; Zhao, H.; Wu, Y. A New Perspective on Understanding Soil Microplastics: Composition, Influencing Factors of the Soil Plastisphere, and Its Impacts on the Environmental Behavior of Co-Existing Contaminants. Chem. Eng. J. 2025, 518, 164640. [Google Scholar] [CrossRef]
- Munhoz, D.R.; Harkes, P.; Beriot, N.; Larreta, J.; Basurko, O.C. Microplastics: A Review of Policies and Responses. Microplastics 2023, 2, 1–26. [Google Scholar] [CrossRef]
- Salman, M.; Shaheen, A.; Ahmad, H.M.; Al-Hazmi, H.E.; Othman, M.H.D.; Aziz, F.; Anouzla, A.; Ali, I. Tackling Microplastics Pollution in Global Environment Through Integration of Applied Technology, Policy Instruments, and Legislation. J. Environ. Manag. 2023, 346, 118971. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Chen, T.; Zhou, Q.; Liu, Y.; Wei, J.; Waniek, J.J.; Luo, Y. Biofilm Formation and Its Influences on the Properties of Microplastics as Affected by Exposure Time and Depth in the Seawater. Sci. Total Environ. 2020, 734, 139237. [Google Scholar] [CrossRef]
- Qiu, X.; Ma, S.; Zhang, J.; Fang, L.; Guo, X.; Zhu, L. Dissolved Organic Matter Promotes the Aging Process of Polystyrene Microplastics under Dark and Ultraviolet Light Conditions: The Crucial Role of Reactive Oxygen Species. Environ. Sci. Technol. 2022, 56, 10149–10160. [Google Scholar] [CrossRef]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of Biofilm Formation on the Fate and Potential Effects of Microplastic in the Aquatic Environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef]
- Song, W.; Fu, C.; Fang, Y.; Wang, Z.; Li, J.; Zhang, X.; Bhatt, K.; Liu, L.; Wang, N.; Liu, F.; et al. Single and Combined Toxicity Assessment of Primary or UV-Aged Microplastics and Adsorbed Organic Pollutants on Microalga Chlorella pyrenoidosa. Environ. Pollut. 2023, 318, 120925. [Google Scholar] [CrossRef]
- Ainali, N.M.; Bikiari, D.N.; Lambropoulou, D.A. Physicochemical Alterations on UV Aged Polymers Leading to Microplastics Formation: A Multi-tiered Study of Polyester, Polycarbonate and Polyamide. Polym. Degrad. Stab. 2024, 222, 110692. [Google Scholar] [CrossRef]
- Andrady, A.L. The Plastic in Microplastics: A Review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.K.; Singh, R.P. UV-Irradiated Biodegradability of Ethylene-propylene copolymers, LDPE, and I-PP in Composting and Culture Environments. Biomacromolecules 2001, 2, 880–885. [Google Scholar] [CrossRef]
- Gardette, M.; Perthue, A.; Gardette, J.L.; Janecska, T.; Földes, E.; Pukánszky, B.; Therias, S. Photo- and Thermal-Oxidation of Polyethylene: Comparison of Mechanisms and Influence of Unsaturation Content. Polym. Degrad. Stab. 2013, 98, 2383–2390. [Google Scholar] [CrossRef]
- Mao, R.; Lang, M.; Yu, X.; Wu, R.; Yang, X.; Guo, X. Aging Mechanism of Microplastics with UV Irradiation and Its Effects on the Adsorption of Heavy Metals. J. Hazard. Mater. 2020, 393, 122515. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.H.; Wu, P.W.; Li, L.Q.; Yu, F.; Ma, J. Adsorption/desorption Behavior of Ciprofloxacin on Aged Biodegradable Plastic PLA under Different Exposure Conditions. J. Environ. Chem. Eng. 2023, 11, 109256. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wangjin, X.; Wang, Y.; Meng, G.; Chen, Y. The Adsorption Behavior of Metals in Aqueous Solution by Microplastics Effected by UV Radiation. J. Environ. Sci. 2020, 87, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, K.; Barrios, A.C.; Rajwade, K.; Kumar, A.; Oswald, J.; Apul, O.; Perreault, F. Aging of Microplastics Increases Their Adsorption Affinity towards Organic Contaminants. Chemosphere 2022, 298, 134238. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X.; Xue, J. Biofilm-Developed Microplastics as Vectors of Pollutants in Aquatic Environments. Environ. Sci. Technol. 2021, 55, 12780–12790. [Google Scholar] [CrossRef]
- Lee, C.E.; Messer, L.F.; Holland, S.I.; Gutierrez, T.; Quilliam, R.S.; Matallana-Surget, S. The Primary Molecular Influences of Marine Plastisphere Formation and Function: Novel Insights into Organism-Organism and -Co-Pollutant Interactions. Crit. Rev. Environ. Sci. Technol. 2024, 54, 138–161. [Google Scholar] [CrossRef]
- Flemming, H.C.; Neu, T.R.; Wozniak, D.J. The EPS Matrix: The “House of Biofilm Cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef]
- Tourney, J.; Ngwenya, B.T. The Role of Bacterial Extracellular Polymeric Substances in Geomicrobiology. Chem. Geol. 2014, 386, 115–132. [Google Scholar] [CrossRef]
- Elasri, M.O.; Miller, R.V. Study of the Response of a Biofilm Bacterial Community to UV Radiation. Appl. Environ. Microbiol. 1999, 65, 2025–2031. [Google Scholar] [CrossRef]
- Loeb, G.I.; Neihof, R.A. Marine Conditioning Films. Appl. Chem. Protein Interfaces 1975, 145, 319–335. [Google Scholar] [CrossRef]
- De Tender, C.; Devriese, L.I.; Haegeman, A.; Maes, S.; Vangeyte, J.; Cattrijsse, A.; Dawyndt, P.; Ruttink, T. Temporal Dynamics of Bacterial and Fungal Colonization on Plastic Debris in the North Sea. Environ. Sci. Technol. 2017, 51, 7350–7360. [Google Scholar] [CrossRef]
- Gao, J.; Ju, Z.; Yang, Q.; Zhou, X. Exploring Different Effects of Biofilm Formation and Natural Organic Matter Adsorption on the Properties of Three Typical Microplastics in the Freshwater. Sci. Total Environ. 2025, 958, 178156. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Gao, Y.; Adyel, T.M.; Huo, Z.; Liu, Z.; Wu, J.; Hou, J. Effects of Biofilm Colonization on the Sinking of Microplastics in Three Freshwater Environments. J. Hazard. Mater. 2021, 413, 125370. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, Y.; Li, J.; Wang, F.; Xia, S.; Zhao, J. Biofilm Alters Tetracycline and Copper Adsorption Behaviors onto Polyethylene Microplastics. Chem. Eng. J. 2020, 392, 123808. [Google Scholar] [CrossRef]
- Liu, S.; Fang, S.; Xiang, Z.; Chen, X.; Song, Y.; Chen, C.; Ouyang, G. Combined Effect of Microplastics and DDT on Microbial Growth: A Bacteriological and Metabolomics Investigation in Escherichia coli. J. Hazard. Mater. 2021, 407, 124849. [Google Scholar] [CrossRef]
- Jiang, A.; Pei, W.; Zhang, R.; Shah, K.J.; You, Z. Toxic Effects of Aging Mask Microplastics on E. Coli and Dynamic Changes in Extracellular Polymeric Matter. Sci. Total Environ. 2023, 899, 165607. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Xu, L.; Zhang, W.; Yang, G.; Zhao, Z.; Wang, Y.; Li, X. Comparative Toxic Effects of Microplastics and Nanoplastics on Chlamydomonas Reinhardtii: Growth Inhibition, Oxidative Stress, and Cell Morphology. J. Water Process. Eng. 2021, 43, 102291. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Wang, Y.; Sun, Y.; Xia, S.; Zhao, J. Effect of Biofilm Colonization on Pb(II) Adsorption onto Poly(Butylene Succinate) Microplastic during Its Biodegradation. Sci. Total Environ. 2022, 833, 155251. [Google Scholar] [CrossRef]
- Semerád, J.; Čvančarová, M.; Filip, J.; Kašlík, J.; Zlotá, J.; Soukupová, J.; Cajthaml, T. Novel Assay for the Toxicity Evaluation of Nanoscale Zero-Valent Iron and Derived Nanomaterials Based on Lipid Peroxidation in Bacterial Species. Chemosphere 2018, 213, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique Cellular Interaction of Silver Nanoparticles: Size-Dependent Generation of Reactive Oxygen Species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef]
- Zhong, X.; Downs, C.A.; Che, X.; Zhang, Z.; Li, Y.; Liu, B.; Li, Q.; Li, Y.; Gao, H. The Toxicological Effects of Oxybenzone, an Active Ingredient in Suncream Personal Care Products, on Prokaryotic Alga Arthrospira sp. and Eukaryotic Alga Chlorella sp. Aquat. Toxicol. 2019, 216, 105295. [Google Scholar] [CrossRef]
- Meng, F.; Tan, L.; Cai, P.; Wang, J. Effects of Polystyrene Nanoplastics on Growth and Hemolysin Production of Microalgae Karlodinium veneficum. Aquat. Toxicol. 2024, 266, 106810. [Google Scholar] [CrossRef]
- Davarpanah, E.; Guilhermino, L. Are Gold Nanoparticles and Microplastics Mixtures More Toxic to the Marine Microalgae Tetraselmis Chuii than the Substances Individually? Ecotoxicol. Environ. Saf. 2019, 181, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, T.; Wang, J.; Jiang, L.; Yin, Y.; Guo, H. Size-Dependent Toxic Effects of Polystyrene Microplastic Exposure on Microcystis Aeruginosa Growth and Microcystin Production. Sci. Total Environ. 2021, 761, 143265. [Google Scholar] [CrossRef]
- Kim, S.Y.; Woo, S.; Lee, S.-W.; Jung, E.-M.; Lee, E.-H. Dose-Dependent Responses of Escherichia Coli and Acinetobacter Sp. to Micron-Sized Polystyrene Microplastics. J. Microbioogy Biotechnol. 2025, 35, e2410023. [Google Scholar] [CrossRef]
- Ning, Q.; Wang, D.; An, J.; Ding, Q.; Huang, Z.; Zou, Y.; Wu, F.; You, J. Combined Effects of Nanosized Polystyrene and Erythromycin on Bacterial Growth and Resistance Mutations in Escherichia coli. J. Hazard. Mater. 2022, 422, 126858. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, T.; He, M.; Shah, K.J.; You, Z.; Zhang, T.; Zubair, M. Exploring the Toxicity of the Aged Styrene-Butadiene Rubber Microplastics to Petroleum Hydrocarbon-Degrading Bacteria under Compound Pollution System. Ecotoxicol. Environ. Saf. 2021, 227, 112903. [Google Scholar] [CrossRef]
- Chelomin, V.P.; Mazur, A.A.; Slobodskova, V.V.; Dovzhenko, N.V.; Kukla, S.P. Leachate from Weathered Face Masks Increases DNA Damage to Sperm of Sand Dollars Scaphechinus mirabilis. Toxics 2025, 13, 372. [Google Scholar] [CrossRef]
- Caruso, G. Microplastics as Vectors of Contaminants. Mar. Pollut. Bull. 2019, 146, 921–924. [Google Scholar] [CrossRef]
- Zhou, X.W.; Kang, F.X.; Qu, X.L.; Fu, H.Y.; Alvarez, P.J.J.; Tao, S.; Zhu, D.Q. Role of Extracellular Polymeric Substances in Microbial Reduction of Arsenate to Arsenite by Escherichia coli and Bacillus subtilis. Environ. Sci. Technol. 2020, 54, 6185–6193. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Zeng, Z.; Li, L.; Song, B.; Zhou, C.; Zeng, G.; Zhang, Y.; Xiao, R. Microplastics Act as an Important Protective Umbrella for Bacteria during Water/Wastewater Disinfection. J. Clean. Prod. 2021, 315, 128188. [Google Scholar] [CrossRef]
- Akdogan, Z.; Guven, B.; Kideys, A.E. Microplastic Distribution in the Surface Water and Sediment of the Ergene River. Environ. Res. 2023, 234, 116500. [Google Scholar] [CrossRef]
- Van Daele, M.; Van Bastelaere, B.; De Clercq, J.; Meyer, I.; Vercauteren, M.; Asselman, J. Mud and Organic Content are Strongly Correlated with Microplastic Contamination in a Meandering Riverbed. Commun. Earth Environ. 2024, 5, 453. [Google Scholar] [CrossRef]
- Hüffer, T.; Weniger, A.-K.; Hofmann, T. Sorption of Organic Compounds by Aged Polystyrene Microplastic Particles. Environ. Pollut. 2018, 236, 218–225. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, Z.; Yang, Y.; Sun, Y.; Yu, F.; Ma, J. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ. Pollut. 2018, 246, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Deng, J.Q.; Liang, J.; Xia, X.H. Comparison of lead adsorption on the aged conventional microplastics, biodegradable microplastics and environmentally-relevant tire wear particles. Chem. Eng. J. 2023, 460, 141838. [Google Scholar] [CrossRef]
- Kim, N.K.; Mao, N.; Lin, R.; Bhattacharyya, D.; Van Loosdrecht, M.C.M.; Lin, Y. Flame Retardant Property of Flax Fabrics Coated by Extracellular Polymeric Substances Recovered from Both Activated Sludge and Aerobic Granular Sludge. Water Res. 2020, 170, 115344. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, B.; Li, Q.; Liu, N.; Xia, B.; Zhu, L.; Qu, K. Toxicities of Polystyrene Nano- and Microplastics toward Marine Bacterium Halomonas alkaliphila. Sci. Total Environ. 2018, 642, 1378–1385. [Google Scholar] [CrossRef]
- Liu, G.; Jiang, R.; You, J.; Muir, D.C.G.; Zeng, E.Y. Microplastic Impacts on Microalgae Growth: Effects of Size and Humic Acid. Environ. Sci. Technol. 2020, 54, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Dai, J.; Bie, C.; Li, H.; Zhang, Z.; Guo, X.; Zhu, L. Bioaccessibility of Microplastic-Associated Antibiotics in Freshwater Organisms: Highlighting the Impacts of Biofilm Colonization via an In Vitro Protocol. Environ. Sci. Technol. 2022, 56, 12267–12277. [Google Scholar] [CrossRef]
- Richard, H.; Carpenter, E.J.; Komada, T.; Palmer, P.T.; Rochman, C.M. Biofilm Facilitates Metal Accumulation onto Microplastics in Estuarine Waters. Sci. Total Environ. 2019, 683, 600–608. [Google Scholar] [CrossRef]
- Mytych, J.; Wnuk, M.; Rattan, S.I.S. Low Doses of Nanodiamonds and Silica Nanoparticles Have Beneficial Hormetic Effects in Normal Human Skin Fibroblasts in Culture. Chemosphere 2016, 148, 307–315. [Google Scholar] [CrossRef]
- Bekiari, V.; Avramidis, P. Data Quality in Water Analysis: Validation of Combustion-infrared and Combustion-chemiluminescence Methods for the Simultaneous Determination of Total Organic Carbon (TOC) and Total Nitrogen (TN). Int. J. Environ. Anal. Chem. 2013, 94, 65–76. [Google Scholar] [CrossRef]
- Roig, B.; Gonzalez, C.; Thomas, O. Simple UV/UV-visible Method for Nitrogen and Phosphorus Measurement in Wastewater. Talanta 1999, 504, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Rozman, U.; Filker, S.; Kalčíková, G. Monitoring of Biofilm Development and Physico-chemical Changes of Floating Microplastics at the Air-water Interface. Environ. Pollut. 2023, 322, 121157. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Yang, Q.; Fan, X.; Zhou, X.; Ren, P. Exploring Different Toxic Effects of UV-Aged and Bio-Aged Microplastics on Growth and Oxidative Stress of Escherichia coli. Toxics 2025, 13, 706. https://doi.org/10.3390/toxics13090706
Gao J, Yang Q, Fan X, Zhou X, Ren P. Exploring Different Toxic Effects of UV-Aged and Bio-Aged Microplastics on Growth and Oxidative Stress of Escherichia coli. Toxics. 2025; 13(9):706. https://doi.org/10.3390/toxics13090706
Chicago/Turabian StyleGao, Juntong, Qimeng Yang, Xiarui Fan, Xinwei Zhou, and Peng Ren. 2025. "Exploring Different Toxic Effects of UV-Aged and Bio-Aged Microplastics on Growth and Oxidative Stress of Escherichia coli" Toxics 13, no. 9: 706. https://doi.org/10.3390/toxics13090706
APA StyleGao, J., Yang, Q., Fan, X., Zhou, X., & Ren, P. (2025). Exploring Different Toxic Effects of UV-Aged and Bio-Aged Microplastics on Growth and Oxidative Stress of Escherichia coli. Toxics, 13(9), 706. https://doi.org/10.3390/toxics13090706





