Impaired Reproductive Performance of Waterbirds in Metal-Contaminated Tropical Rice Agroecosystems: Evidence from Little Egrets (Egretta garzetta)
Abstract
1. Introduction
2. Materials and Methods
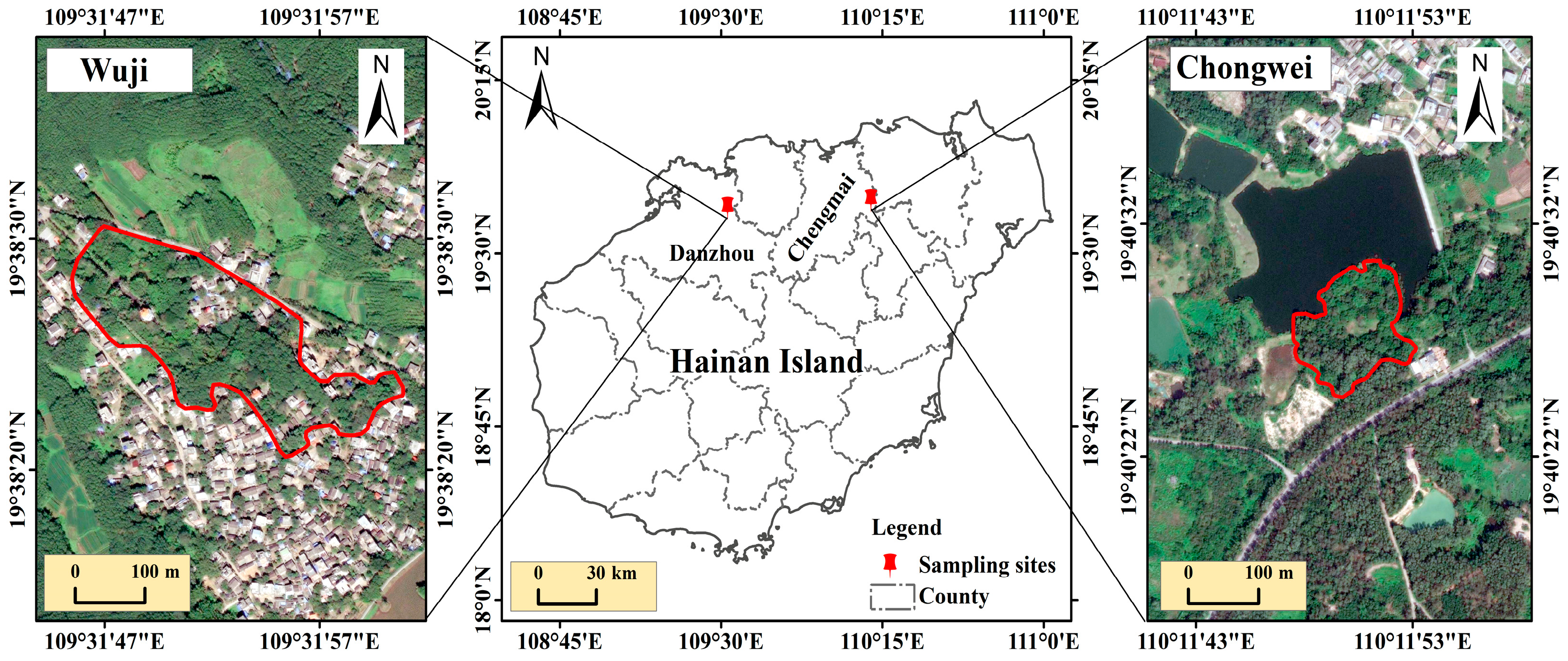
2.1. Study Area
2.2. Breeding Monitoring
2.3. Sample Collection
2.4. Laboratory Analysis, Quality Assurance, and Control
2.5. Calculation and Statistical Analysis
3. Results and Discussion
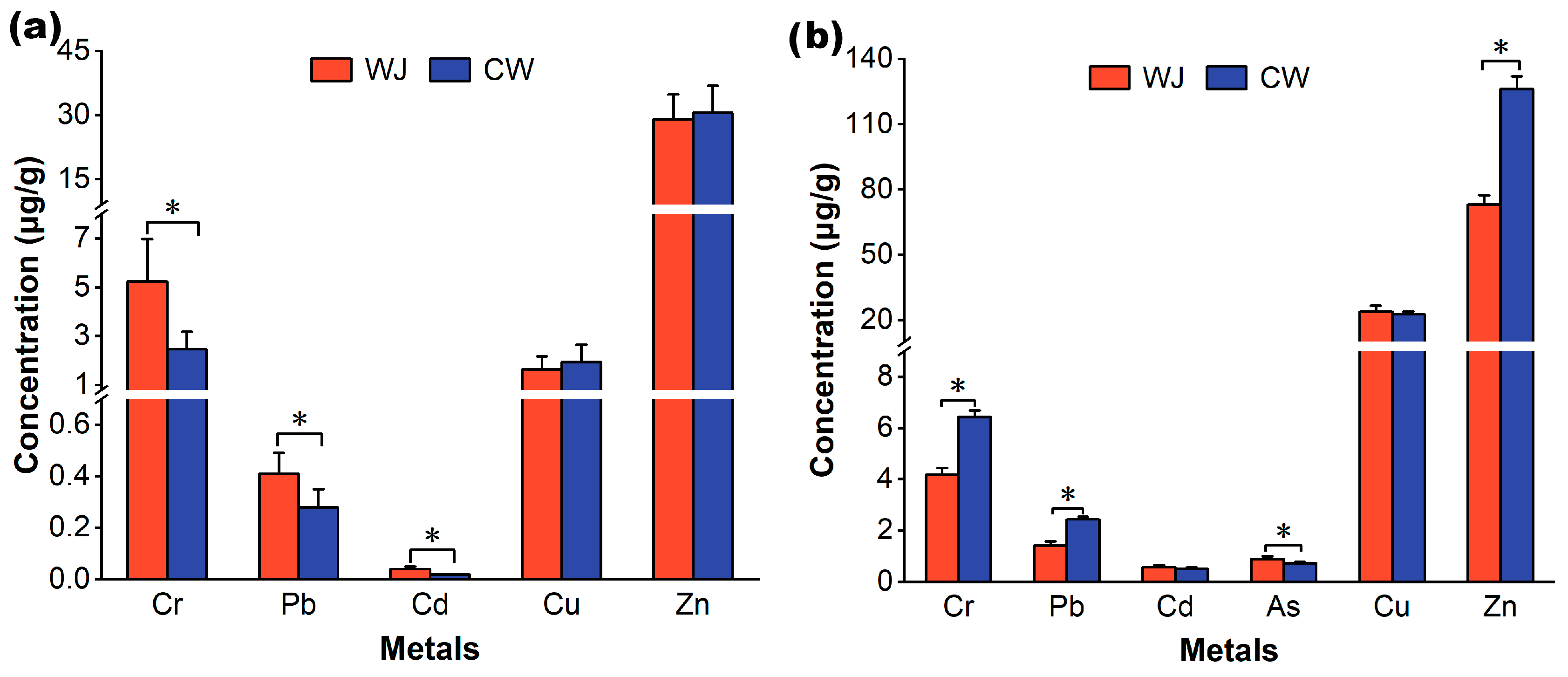
3.1. Heavy Metal Contamination in Foraging Habitats
3.2. Heavy Metals in Food and Feces of Little Egrets
3.3. Heavy Metals in Feathers and Eggshells of Little Egrets
3.4. Reproductive Performance of Little Egrets in Different Heronries
3.5. Limitations of the Study and Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sites | Number of Destroyed Nests | Number of Total Nests | Vandalism Rate (%) |
|---|---|---|---|
| Wuji Village | 4 | 72 | 5.56 |
| Chongwei Village | 11 | 95 | 11.58 |
| Sites | Mean ± SD (N) | p-Value |
|---|---|---|
| Min–Max | ||
| Wuji Village | 0.18 ± 0.02 (62) 0.12–0.22 | 0.217 |
| Chongwei Village | 0.19 ± 0.01 (59) 0.14–0.21 |
References
- Zabala, J.; Trexler, J.C.; Jayasena, N.; Frederick, P. Early breeding failure in birds due to environmental toxins: A potentially powerful but hidden effect of contamination. Environ. Sci. Technol. 2020, 54, 13786–13796. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhan, C.; Li, Y.; Zhou, D.; Yu, J.; Yang, J. A comparison of metal distribution in surface soil between wetland and farmland in the Sanjiang plain. HydroResearch 2023, 6, 65–72. [Google Scholar] [CrossRef]
- Liu, Z.; Bai, Y.; Gao, J.; Li, J. Driving factors on accumulation of cadmium, lead, copper, zinc in agricultural soil and products of the North China plain. Sci. Rep. 2023, 13, 7429. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Nam, H.K.; Son, S.J.; Do, M.S.; Yoo, J.C. Effects of Pesticide Use on the Distributions of Grey Herons (Ardea cinerea) and Great Egrets (Ardea alba) in Rice Fields of the Republic of Korea. Zool. Sci. 2021, 38, 162–169. [Google Scholar] [CrossRef]
- Herring, M.W.; Robinson, W.; Zander, K.K.; Garnett, S.T. Rice fields support the global stronghold for an endangered waterbird. Agric. Ecosyst. Environ. 2019, 284, 106599. [Google Scholar] [CrossRef]
- Hazen, E.L.; Abrahms, B.; Brodie, S.; Carroll, G.; Jacox, M.G.; Savoca, M.S.; Scales, K.L.; Sydeman, W.J.; Bograd, S.J. Marine top predators as climate and ecosystem sentinels. Front. Ecol. Environ. 2019, 17, 565–574. [Google Scholar] [CrossRef]
- Zaman, M.H.; Mustafa, G.; Sheikh, M.A.; Qadir, A.; Shahid, S.U.; Abbasi, N.A. A multi-tissue biomonitoring investigation of toxic trace elements and their trophic transfer potential in a semi aquatic bird species, the Cattle Egret (Bubulcus ibis). Chemosphere 2022, 300, 134582. [Google Scholar] [CrossRef] [PubMed]
- Neo, J.P.S.; Tan, B.H. The use of animals as a surveillance tool for monitoring environmental health hazards, human health hazards and bioterrorism. Vet. Microbiol. 2017, 203, 40–48. [Google Scholar] [CrossRef]
- Sievers, M.; Hale, R.; Parris, K.M.; Swearer, S.E. Impacts of human—induced environmental change in wetlands on aquatic animals. Biol. Rev. 2018, 93, 529–554. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Liang, J.; Yuan, X.Z.; Zeng, G.M.; Yuan, Y.J.; Wu, H.P.; Huang, X.L.; Liu, J.F.; Hua, S.S.; Li, F.; et al. An integrated model for assessing heavy metal exposure risk to migratory birds in wetland ecosystem: A case study in Dongting Lake Wetland, China. Chemosphere 2015, 135, 14–19. [Google Scholar] [CrossRef]
- Man, Y.B.; Chow, K.L.; Zhang, F.; Lei, K.M.; Leung, A.O.W.; Mo, W.Y.; Wong, M.H. Protecting water birds of wetlands: Using toxicological tests and ecological risk assessment, based on metal/loid (s) of water, sediment and biota samples. Sci. Total Environ. 2021, 778, 146317. [Google Scholar] [CrossRef]
- Xia, P.; Ma, L.; Yi, Y.; Lin, T. Assessment of heavy metal pollution and exposure risk for migratory birds—A case study of Caohai wetland in Guizhou Plateau (China). Environ. Pollut. 2021, 275, 116564. [Google Scholar] [CrossRef]
- Zhang, Y.; Ruan, L.; Fasola, M.; Boncompagni, E.; Dong, Y.; Dai, N.; Gandini, C.; Orvini, E.; Ruiz, X. Little egrets (Egretta garzetta) and trace-metal contamination in wetlands of China. Environ. Monit. Assess. 2006, 118, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Świsłowski, P.; Hebda, G.; Zinicovscaia, I.; Chaligava, O.; Isinkaralar, O.; Isinkaralar, K.; Rajfur, M. I believe I can fly… but in polluted air, why? Bird feathers as an example of environmental contaminant monitoring. Sci. Total Environ. 2025, 972, 179033. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, S.; Abbasi, N.A.; Chaudhry, M.J.I.; Ahmad, S.R.; Malik, R.N. Oxidative stress risk assessment through heavy metal and arsenic exposure in terrestrial and aquatic bird species of Pakistan. Environ. Sci. Pollut. Res. 2020, 27, 12293–12307. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, F.; Abbasi, N.A.; Chaudhry, M.J.I.; Mashiatullah, A.; Ahmad, S.R.; Qadir, A.; Malik, R.N. Dietary proxies (δ15N, δ13C) as signature of metals and arsenic exposure in birds from aquatic and terrestrial food chains. Environ. Res. 2020, 183, 109191. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.K.; Liang, Y.; Wang, H.; Dong, Y.H.; Leung, S.Y.; Wong, M.H. Bioaccumulation of heavy metals in fish and Ardeid at Pearl River Estuary, China. Ecotoxicol. Environ. Saf. 2014, 106, 62–67. [Google Scholar] [CrossRef]
- Bighetti, G.P.; Padilha, J.A.; Cunha, L.S.T.; Malm, O.; Mancini, P.L. Ventral feathers contained the highest mercury level in brown booby (Sula leucogaster), a pantropical seabird species. Chemosphere 2022, 298, 134305. [Google Scholar] [CrossRef]
- Fenstad, A.A.; Bustnes, J.O.; Lierhagen, S.; Gabrielsen, K.M.; Öst, M.; Jaatinen, K.; Hanssen, S.A.; Moe, B.; Jenssen, B.M.; Krøkje, Å. Blood and feather concentrations of toxic elements in a Baltic and an Arctic seabird population. Mar. Pollut. Bull. 2017, 114, 1152–1158. [Google Scholar] [CrossRef]
- Aziz, B.; Zubair, M.; Irshad, N.; Ahmad, K.S.; Mahmood, M.; Tahir, M.M.; Shah, K.H.; Shaheen, A. Biomonitoring of toxic metals in feathers of birds from North-Eastern Pakistan. Bull. Environ. Contam. Toxicol. 2021, 106, 805–811. [Google Scholar] [CrossRef]
- Zarrintab, M.; Mirzaei, R. Stress induced by heavy metals on breeding of magpie (Pica pica) from central Iran. Ecotoxicol. Environ. Saf. 2017, 143, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Brahmia, Z.; Scheifler, R.; Crini, N.; Maas, S.; Giraudoux, P.; Benyacoub, S. Breeding performance of blue tits (Cyanistes caeruleus ultramarinus) in relation to lead pollution and nest failure rates in rural, intermediate, and urban sites in Algeria. Environ. Pollut. 2013, 174, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Yang, W.; Wang, S.; Zhang, H.; Yang, Y.; Bao, X.; Zhang, Y. Effects of environmental metal pollution on reproduction of a free-living resident songbird, the tree sparrow (Passer montanus). Sci. Total Environ. 2020, 721, 137674. [Google Scholar] [CrossRef]
- Su, T.; He, C.; Jiang, A.; Xu, Z.; Goodale, E.; Qiu, G. Passerine bird reproduction does not decline in a highly-contaminated mercury mining district of China. Environ. Pollut. 2021, 286, 117440. [Google Scholar] [CrossRef] [PubMed]
- English, S.G.; Hess, H.; Bishop, C.A.; Porter, E.; Cheng, K.M.; Elliott, J.E. Bioaccumulation and effects of selenium from surface coal mining in an aquatic songbird. Environ. Res. 2022, 208, 112702. [Google Scholar] [CrossRef]
- Whitney, M.C.; Cristol, D.A. Impacts of sublethal mercury exposure on birds: A detailed review. Rev. Environ. Contam. Toxicol. 2017, 244, 113–163. [Google Scholar] [CrossRef]
- Ding, J.; Yang, W.; Yang, Y.; Ai, S.; Bai, X.; Zhang, Y. Variations in tree sparrow (Passer montanus) egg characteristics under environmental metal pollution. Sci. Total Environ. 2019, 687, 946–955. [Google Scholar] [CrossRef]
- Shahbaz, M.; Hashmi, M.Z.; Malik, R.N.; Yasmin, A. Relationship between heavy metals concentrations in egret species, their environment and food chain differences from two Headworks of Pakistan. Chemosphere 2013, 93, 274–282. [Google Scholar] [CrossRef]
- Qiu, H.X.; Xu, C.B.; Huang, X.; Wei, X.Y.; Pang, Z.L.; Du, L.F.; Jiang, L.; Zhang, J.L. Microplastic contamination in waterbirds and their habitats: Evidence from little egrets (Egretta garzetta) in tropical rice fields. Environ. Res. 2025, 285, 122376. [Google Scholar] [CrossRef]
- Wang, D.; Dang, Z.; Feng, H.; Wang, R. Distribution of anthropogenic cadmium and arsenic in arable land soils of Hainan, China. Toxicol. Environ. Chem. 2015, 97, 402–408. [Google Scholar] [CrossRef]
- Chen, W.X.; Li, Q.; Wang, Z.; Sun, Z.J. Spatial distribution characteristics and pollution evaluation of heavy metals in arable land soil of China. J. Environ. Sci. 2020, 41, 2822–2833. (In Chinese) [Google Scholar] [CrossRef]
- Hu, B.; Cui, R.; Li, J.; Wei, H.; Zhao, J.; Bai, F.; Song, W.; Ding, X. Occurrence and distribution of heavy metals in surface sediments of the Changhua River Estuary and adjacent shelf (Hainan Island). Mar. Pollut. Bull. 2013, 76, 400–405. [Google Scholar] [CrossRef]
- Statistical Bureau of Hainan Province (SBHP); Survey Office of National Bureau of Statistics in Hainan (SONBSH). Hainan Statistical Yearbook; China Statistics Press: Beijing, China, 2011. [Google Scholar]
- Ji, Q.; Xu, T.; Chen, H.Y.; Min, Y.; Fu, G.H. Study on the development of ecological agriculture in Hainan in the context of the free trade port construction. J. Anhui Agric. Sci. 2022, 50, 245–248. (In Chinese) [Google Scholar]
- Zabala, J.; Rodriguez-Jorquera, I.A.; Orzechowski, S.C.; Frederick, P. Mercury concentration in nestling feathers better predicts individual reproductive success than egg or nestling blood in a piscivorous bird. Environ. Sci. Technol. 2019, 53, 1150–1156. [Google Scholar] [CrossRef]
- Telesford-Checkley, J.M.; Mora, M.A.; Grant, W.E.; Boellstorff, D.E.; Provin, T.L. Estimating the contribution of nitrogen and phosphorus to waterbodies by colonial nesting waterbirds. Sc. Total Environ. 2017, 574, 1335–1344. [Google Scholar] [CrossRef]
- Turzańska-Pietras, K.; Chachulska, J.; Polechońska, L.; Borowiec, M. Does heavy metal exposure affect the condition of Whitethroat (Sylvia communis) nestlings? Environ. Sc. Pollut. Res. 2017, 25, 7758–7766. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, Z.; Fu, B.; Wu, D.; Wang, J.; Li, Y.; Tang, W. Distribution and fractionation of potentially toxic metals under different land-use patterns in suburban areas. Pol. J. Environ. Stud. 2022, 31, 475–483. [Google Scholar] [CrossRef]
- Jian, L.; Zhang, T.; Lin, L.; Xiong, J.; Shi, H.; Wang, J. Transfer and accumulation of trace elements in seawater, sediments, green turtle forage, and eggshells in the Xisha Islands, South China Sea. Environ. Sci. Pollut. Res. 2022, 29, 50832–50844. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, P.F.; O’hara, T.M.; Fisk, A.T.; Borga, K.; Solomon, K.R.; Muir, D.C.G. Trophic transfer of persistent organochlorine contaminants (OCs) within an Arctic marine food web from the southern Beaufort–Chukchi Seas. Environ. Pollut. 2003, 124, 509–522. [Google Scholar] [CrossRef]
- Olivares-Rieumont, S.; Rosa, D.D.L.; Lima, L.; Graham, D.W.; Alessandro, K.D.; Borroto, J.; Martínez, F.; Sanchez, J. Assessment of heavy metal levels in Almendares River sediments-Havana city, Cuba. Water Res. 2005, 39, 3945–3953. [Google Scholar] [CrossRef]
- Burton, G.A. Sediment quality criteria in use around the world. Limnology 2002, 3, 65–75. [Google Scholar] [CrossRef]
- WHO, World Health Organisation. Guidelines for Drinking-Water Quality, 4th ed; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Rani, M.; Ullah, R.; Alwahibi, M.S.; Elshikh, M.S.; AbdelGawwad, M.R.; Mahmood, A. Health risk assessment by toxic metals in little egrets (Egretta garzetta) and food chain contaminations. Saudi J. Biol. Sci. 2022, 29, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.E.; Preda, M. Trace metal distribution within marine and estuarine sediments of Western Moreton Bay, Queensland, Australia: Relation to land use and setting. Geogr. Res. 2005, 43, 173–193. [Google Scholar] [CrossRef]
- Shumilin, E.; Jimenez-Illescas, R.A.; Lopez-Lopez, S. Anthropogenic contamination of metals in sediments of the Santa Rosalıa Harbor, Baja California Peninsula. Bull. Environ. Contam. Toxicol. 2013, 90, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Duda, M.P.; Hargan, K.E.; Michelutti, N.; Kimpe, L.E.; Clyde, N.; Gilchrist, H.G.; Mallory, M.; Blais, J.M.; Smol, J.P. Breeding eider ducks strongly influence subarctic coastal pond chemistry. Aquat. Sci. 2018, 80, 40. [Google Scholar] [CrossRef]
- Boncompagni, E.; Muhammad, A.; Jabeen, R.; Orvini, E.; Gandini, C.; Sanpera, C.; Ruiz, X.; Fasola, M. Egrets as monitors of trace-metal contamination in wetlands of Pakistan. Arch. Environ. Contam. Toxicol. 2003, 45, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, C.A.; Bscendell-Young, L.I.; Elliott, J.E. Assessing trace-metal exposure to American dippers in mountain streams of southwestern British Columbia, Canada. Environ. Toxicol. Chem. 2005, 24, 836–845. [Google Scholar] [CrossRef]
- Berglund, Å.M.; Rainio, M.J.; Eeva, T. Temporal trends in metal pollution: Using bird excrement as indicator. PLoS ONE 2015, 10, e0117071. [Google Scholar] [CrossRef]
- Gann, G.L.; Powell, C.H.; Chumchal, M.M.; Drenner, R.W. Hg-contaminated terrestrial spiders pose a potential risk to songbirds at Caddo Lake (Texas/ Louisiana, USA). Environ. Toxicol. Chem. 2015, 34, 303–306. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Liu, F.; Lu, Y.; Zhou, X. Quantifying the bioaccumulation and trophic transfer processes of heavy metals based on the food web: A case study from freshwater wetland in northeast China. Sci. Total Environ. 2024, 928, 172290. [Google Scholar] [CrossRef]
- Kim, J.; Koo, T.H.; Oh, J.M. Monitoring of Heavy metal contamination using tissues of two Ardeids Chicks, Korea. Bull. Environ. Contam. Toxicol. 2010, 84, 754–758. [Google Scholar] [CrossRef]
- Sánchez-Virosta, P.; Espín, S.; García-Fernández, A.J.; Eeva, T. A review on exposure and effects of arsenic in passerine birds. Sci. Total Environ. 2015, 512, 506–525. [Google Scholar] [CrossRef]
- Kunito, T.; Kubota, R.; Fujihara, J.; Agusa, T.; Tanabe, S. Arsenic in marine mammals, seabirds, and sea turtles. Rev. Environ. Contam. Toxicol. 2008, 195, 31–69. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.A.; Williams, T.D.; Morrissey, C.A.; WM-Lai, V.; Cullen, W.R.; Elliott, J.E. Dose-dependent uptake, elimination, and toxicity of monosodium methanearsonate in adult zebra finches (Taeniopygia guttata). Environ. Toxicol. Chem. 2008, 27, 605–611. [Google Scholar] [CrossRef]
- Garcıa-Fernández, A.J. Ecotoxicology, avian. Encycl. Toxicol. 2014, 2, 289–294. [Google Scholar] [CrossRef]
- Vijver, M.G.; Van Gestel, C.A.M.; Lanno, R.P.; Van Straalen, N.M.; Peijnenburg, W.J.G.M. Internal metal sequestration and its ecotoxicological relevance: A review. Environ. Sci. Technol. 2004, 38, 4705–4712. [Google Scholar] [CrossRef]
- Nam, D.H.; Kim, E.Y.; Iwata, H.; Tanabe, S. Molecular characterization of two metallothionein isoforms in avian species: Evolutionary history, tissue distribution profile, and expression associated with metal accumulation. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrin. 2007, 145, 295–305. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Metals and radionuclides in birds and eggs from Amchitka and Kiska Islands in the Bering Sea/Pacific Ocean ecosystem. Environ. Monit. Assess. 2007, 127, 105–117. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, F.; Liu, K.; Chen, J.; Zheng, T.; Tang, M. Influence of heavy metals on Saunders’s Gull (Saundersilarus saundersi) reproduction in the Yellow River Estuary: Risk assessment and bioaccumulation. Environ. Sci. Pollut. Res. 2022, 29, 82379–82389. [Google Scholar] [CrossRef] [PubMed]
- Zarrintab, M.; Mirzaei, R.; Mostafaei, G.; Dehghani, R.; Akbari, H. Concentrations of metals in feathers of magpie (Pica pica) from Aran-O-Bidgol City in Central Iran. Bull. Environ. Contam. Toxicol. 2016, 96, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.N.; Zeb, N. Assessment of environmental contamination using feathers of Bubulcus ibis L.; as a biomonitor of heavy metal pollution, Pakistan. Ecotoxicology 2009, 18, 522–536. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Hashmi, M.Z.; Malik, R.N. Heavy-metal levels in feathers of cattle egret and their surrounding environment: A case of the Punjab province, Pakistan. Arch Environ. Contam. Toxicol. 2014, 66, 139–153. [Google Scholar] [CrossRef]
- Kaur, M.; Khera, K.S. Heavy metal contamination in feathers of house crow (Corvus splendens). J. Entomol. Zool. Stud. 2018, 6, 715–720. [Google Scholar]
- Pang, R.H.; Yu, T.L.; Busam, M. Low breeding success of the little egret (Egretta garzetta) near residential areas and in colonies exposed to gales: A comparison of colony in Sichuan, Southwest China, with literature. Anim. Cells Syst. 2019, 23, 235–240. [Google Scholar] [CrossRef]
- Berglund, Å.M.; Nyholm, N.E.I. Slow improvements of metal exposure, health-and breeding conditions of pied flycatchers (Ficedula hypoleuca) after decreased industrial heavy metal emissions. Sci. Total Environ. 2011, 409, 4326–4334. [Google Scholar] [CrossRef] [PubMed]
- Chevin, L.M.; Lande, R.; Mace, G.M. Adaptation, plasticity, and extinction in a changing environment: Towards a predictive theory. PLoS Biol. 2010, 8, e1000357. [Google Scholar] [CrossRef] [PubMed]

| Metals | Sediment (μg g−1) | Water (μg L−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Chongwei Village | Wuji Village | BV a | TEL c | Chongwei Village | Wuji Village | St b | WHO d | |
| Cr | 16.28 ± 7.67 | 78.28 ± 15.77 * | 61.00 | 37.30 | 0.74 ± 0.18 | 2.82 ± 0.81 * | 10.00 | 50.00 |
| Pb | 6.84 ± 2.58 | 11.80 ± 0.98 * | 26.00 | 35.00 | 4.69 ± 1.51 | 7.82 ±1.07 * | 10.00 | 10.00 |
| Cd | 0.68 ± 0.18 | 1.36 ± 0.32 * | 0.097 | 0.60 | 0.07 ± 0.02 | 0.06 ± 0.03 | 1.00 | 3.00 |
| As | 0.82 ± 0.30 | 11.36 ± 4.54 * | 11.20 | 5.90 | 0.63 ± 0.36 | 1.82 ± 0.66 * | 50.00 | 10.00 |
| Cu | 3.07 ± 0.70 | 4.62 ± 0.71 * | 22.60 | 35.70 | 2.33 ± 0.62 | 4.22 ± 1.22 * | 10.00 | 2000 |
| Zn | 9.51 ± 3.30 | 15.53 ± 1.82 * | 74.20 | 123.00 | 9.58 ± 1.81 | 17.66 ± 11.99 * | 50.00 | 3000 |
| Metals | Food | Feces | Feathers | Eggshells |
|---|---|---|---|---|
| Cr | 0.109 ± 0.054 | 0.224 ± 0.171 | 0.259 ± 0.152 | 0.103 ± 0.037 |
| Pb | 0.038 ± 0.009 | 0.238 ± 0.119 | 0.069 ± 0.015 | 0.014 ± 0.007 |
| Cd | 0.024 ± 0.007 | 0.552 ± 0.199 | 0.029 ± 0.012 | 0.011 ± 0.004 |
| As | ND | 0.471 ± 0.397 | ND | ND |
| Cu | 0.492 ± 0.222 | 6.301 ± 1.220 | 2.181 ± 0.340 | 0.579 ± 0.195 |
| Zn | 2.536 ± 0.845 | 8.980 ± 4.295 | 3.274 ± 0.453 | 0.235 ± 0.060 |
| Metals | Feces | Feather | Eggshell |
|---|---|---|---|
| Cr | 1.70 ± 0.91 | 2.18 ± 0.59 | 0.95 ± 0.31 |
| Pb | 6.07 ± 2.66 | 1.82 ± 0.31 | 0.37 ± 0.16 |
| Cd | 20.27 ± 5.38 | 0.96 ± 0.30 | 0.44 ± 0.15 |
| As | ND | ND | ND |
| Cu | 13.21 ± 1.90 | 4.64 ± 0.83 | 1.33 ± 0.67 |
| Zn | 3.33 ± 0.82 | 1.34 ± 0.21 | 0.10 ± 0.04 |
| Breeding Metric | Mean ± SD (N) | p-Value | |
|---|---|---|---|
| Wuji Village | Chongwei Village | ||
| Clutch size | 3.82 ± 0.77 (68) | 3.88 ± 0.73 (84) | 0.640 |
| Hatching success % | 0.77 ± 0.20 (68) | 0.88 ± 0.16 (84) | <0.001 |
| Fledging success % | 0.93 ± 0.13 (68) | 0.96 ± 0.11 (84) | 0.133 |
| Breeding success % | 0.71 ± 0.19 (68) | 0.84 ± 0.17 (84) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, H.; Huang, X.; Xu, C.; Zhang, J. Impaired Reproductive Performance of Waterbirds in Metal-Contaminated Tropical Rice Agroecosystems: Evidence from Little Egrets (Egretta garzetta). Toxics 2025, 13, 676. https://doi.org/10.3390/toxics13080676
Qiu H, Huang X, Xu C, Zhang J. Impaired Reproductive Performance of Waterbirds in Metal-Contaminated Tropical Rice Agroecosystems: Evidence from Little Egrets (Egretta garzetta). Toxics. 2025; 13(8):676. https://doi.org/10.3390/toxics13080676
Chicago/Turabian StyleQiu, Hanxun, Xin Huang, Chuanbiao Xu, and Jiliang Zhang. 2025. "Impaired Reproductive Performance of Waterbirds in Metal-Contaminated Tropical Rice Agroecosystems: Evidence from Little Egrets (Egretta garzetta)" Toxics 13, no. 8: 676. https://doi.org/10.3390/toxics13080676
APA StyleQiu, H., Huang, X., Xu, C., & Zhang, J. (2025). Impaired Reproductive Performance of Waterbirds in Metal-Contaminated Tropical Rice Agroecosystems: Evidence from Little Egrets (Egretta garzetta). Toxics, 13(8), 676. https://doi.org/10.3390/toxics13080676








