Lycium barbarum Polysaccharide Improves Iron Homeostasis in Spermatocytes and Sertoli Cells via NRF2 to Alleviate DEHP-Induced Male Reproductive Toxicity in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Treatment
2.2. Evaluation of Sperm Parameters
2.3. Organ Coefficient
2.4. Hematoxylin-Eosin (H&E) Staining
2.5. Immunohistochemical Staining
2.6. Cell Culture and Treatment
2.7. Cell Counting Kit-8 (CCK-8) Analysis
2.8. Malondialdehyde (MDA), Glutathione (GSH), and Total Antioxidant Capacity (T-AOC)
2.9. Fe2+ Content Determination
2.10. RNA Extraction and Quantitative Real-Time PCR (RT-qPCR)
2.11. Western Blotting Analysis
2.12. RNA Interference
2.13. Sample Size Determination
2.14. Statistical Analysis
3. Results
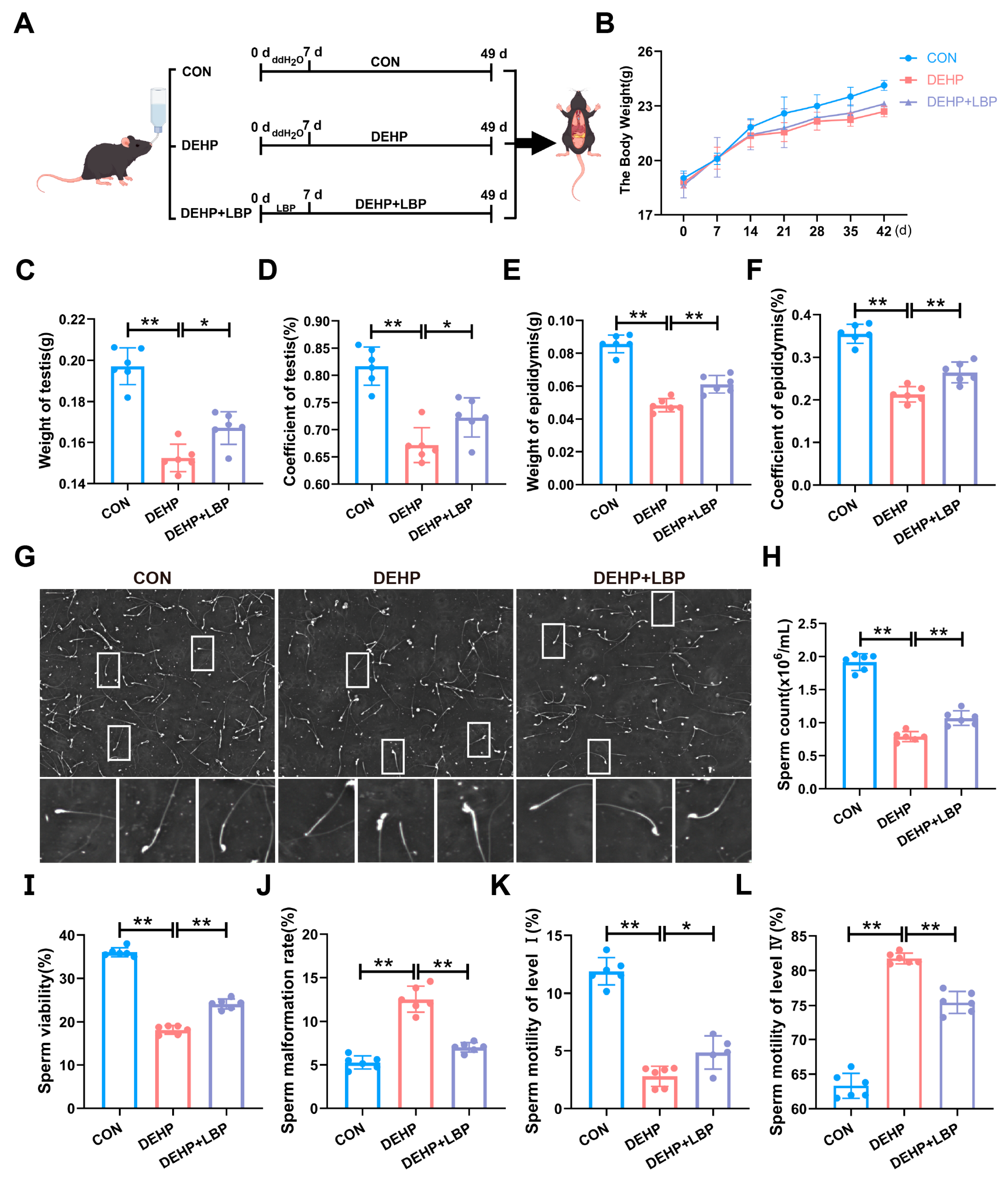
3.1. LBP Can Improve Testicular Injury Induced by DEHP
3.2. LBP Can Improve the Functional Damage of Mouse Testicular Spermatogenic Cells and Sertoli Cells Induced by DEHP
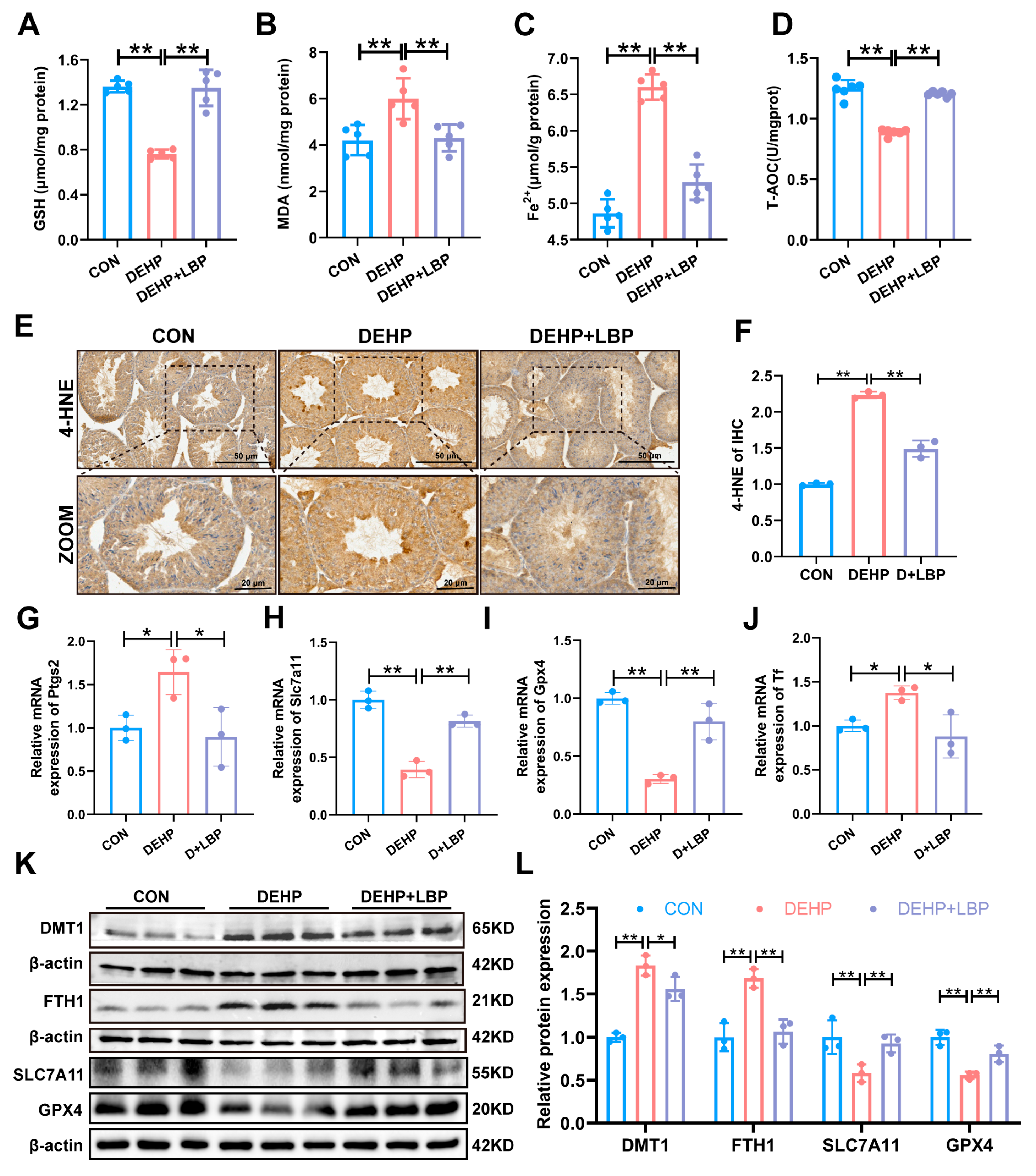
3.3. LBP Can Ameliorate Ferroptosis of Mouse Testicular Tissue Induced by DEHP
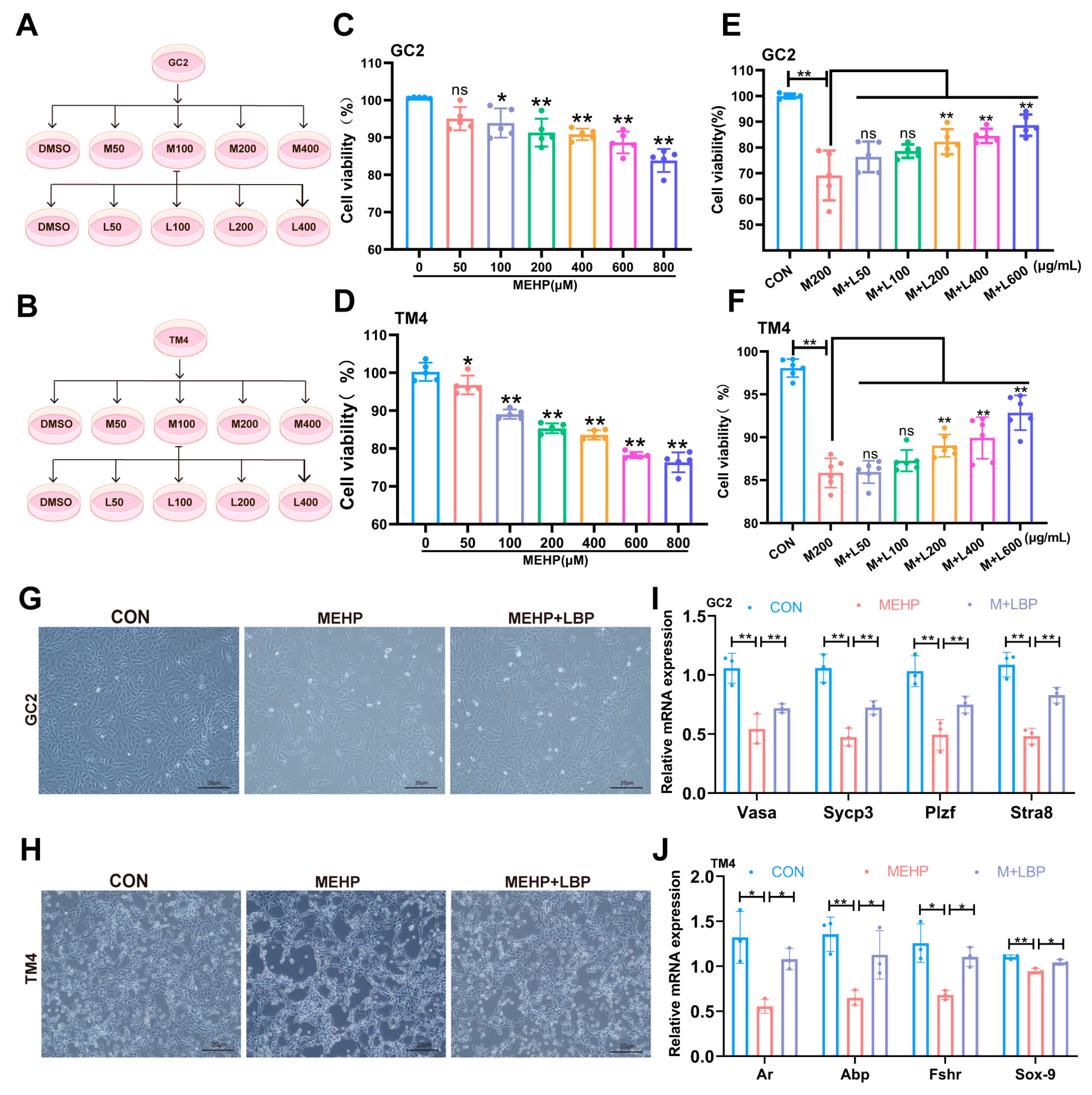
3.4. LBP Can Improve the Damage of Spermatocytes and Sertoli Cells Caused by MEHP
3.5. LBP Resists MEHP-Induced Ferroptosis in GC2 Cells and TM4 Cells
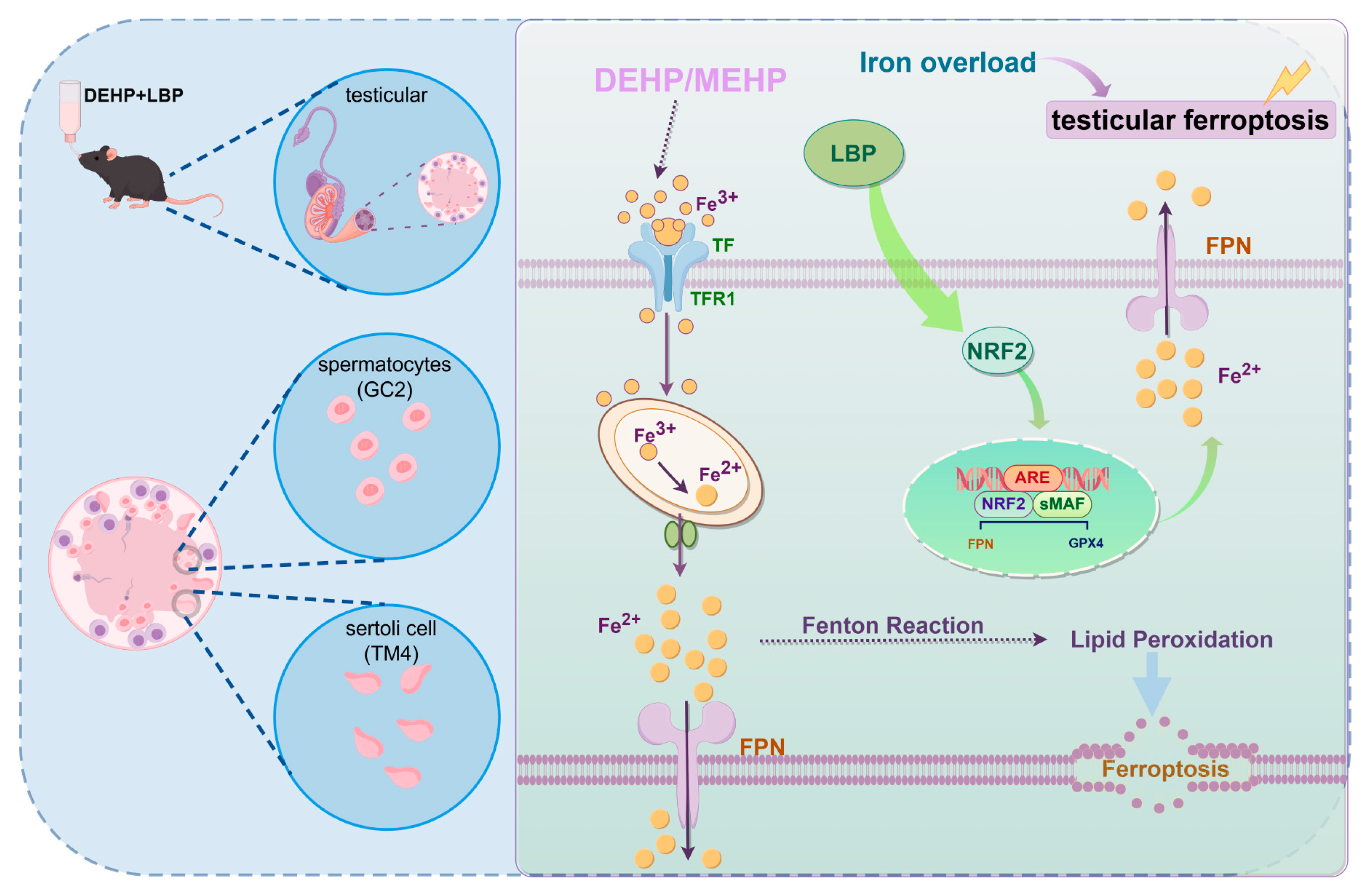
3.6. LBP Resists DEHP-Induced Ferroptosis of Testicular Spermatocytes and Sertoli Cells by Activating NRF2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barratt, C.L.R.; Björndahl, L.; De Jonge, C.J.; Lamb, D.J.; Osorio Martini, F.; McLachlan, R.; Oates, R.D.; Van Der Poel, S.; St John, B.; Sigman, M.; et al. The diagnosis of male infertility: An analysis of the evidence to support the development of global WHO guidance-challenges and future research opportunities. Hum. Reprod. Update 2017, 23, 660–680. [Google Scholar] [CrossRef] [PubMed]
- Beliles, R.; Salinas, J.A.; Kluwe, W.M. A review of di(2-ethylhexyl)phthalate (DEHP) risk assessments. Drug Metab. Rev. 1989, 21, 3–12. [Google Scholar] [CrossRef]
- Kamrin, M.A. Phthalate risks, phthalate regulation, and public health: A review. J. Toxicol. Environ. Health Part B 2009, 12, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Net, S.; Sempere, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, fate, behavior and ecotoxicological state of phthalates in different environmental matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, Z.; Zhang, Y. Adverse cardiovascular effects and potential molecular mechanisms of DEHP and its metabolites-A review. Sci. Total Environ. 2022, 847, 157443. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Xue, J.; Bai, C.; Guo, Y. Phthalate metabolites: Characterization, toxicities, global distribution, and exposure assessment. Environ. Pollut. 2021, 291, 118106. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Cui, K.; Xie, Z.; Liu, M.; Li, Y.; Lin, Y.; Zeng, Z.; Li, F. Occurrence of phthalate esters in water and sediment of urban lakes in a subtropical city, Guangzhou, South China. Environ. Int. 2008, 34, 372–380. [Google Scholar] [CrossRef]
- Kay, V.R.; Chambers, C.; Foster, W.G. Reproductive and developmental effects of phthalate diesters in females. Crit. Rev. Toxicol. 2013, 43, 200–219. [Google Scholar] [CrossRef]
- Rusyn, I.; Peters, J.M.; Cunningham, M.L. Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit. Rev. Toxicol. 2006, 36, 459–479. [Google Scholar] [CrossRef]
- Chen, Q.; Kong, Q.; Tian, P.; He, Y.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Lactic acid bacteria alleviate di-(2-ethylhexyl) phthalate-induced liver and testis toxicity via their bio-binding capacity, antioxidant capacity and regulation of the gut microbiota. Environ. Pollut. 2022, 305, 119197. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Zhao, T.; Chen, J.; Kang, L.; Wei, Y.; Han, L.; Shen, L.; Long, C.; Wu, S.; et al. Di-(2-ethylhexyl) phthalate exposure leads to ferroptosis via the HIF-1alpha/HO-1 signaling pathway in mouse testes. J. Hazard. Mater. 2022, 426, 127807. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, J.; Rylander, L.; Rignell-Hydbom, A.; Jönsson, B.A.G.; Lindh, C.H.; Giwercman, A. Phthalate exposure and reproductive parameters in young men from the general Swedish population. Environ. Int. 2015, 85, 54–60. [Google Scholar] [CrossRef]
- Tsukita, S.; Yamazaki, Y.; Katsuno, T.; Tamura, A.; Tsukita, S. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene 2008, 27, 6930–6938. [Google Scholar] [CrossRef]
- Smith, B.E.; Braun, R.E. Germ cell migration across Sertoli cell tight junctions. Science 2012, 338, 798–802. [Google Scholar] [CrossRef]
- Kaur, G.; Thompson, L.A.; Dufour, J.M. Sertoli cells--immunological sentinels of spermatogenesis. Semin. Cell Dev. Biol. 2014, 30, 36–44. [Google Scholar] [CrossRef]
- Xia, K.; Chen, H.; Wang, J.; Feng, X.; Gao, Y.; Wang, Y.; Deng, R.; Wu, C.; Luo, P.; Zhang, M.; et al. Restorative functions of Autologous Stem Leydig Cell transplantation in a Testosterone-deficient non-human primate model. Theranostics 2020, 10, 8705–8720. [Google Scholar] [CrossRef] [PubMed]
- Orth, J.M.; Gunsalus, G.L.; Lamperti, A.A. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology 1988, 122, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.-M.; Chan, E.; Kwok, C.-Y.; Lee, Y.-K.; Wu, J.-H.; Wan, C.-W.; Chan, R.Y.-K.; Yu, P.H.-F.; Chan, S.-W. A review of the anticancer and immunomodulatory effects of Lycium barbarum fruit. Inflammopharmacology 2012, 20, 307–314. [Google Scholar] [CrossRef]
- Mao, F.; Xiao, B.; Jiang, Z.; Zhao, J.; Huang, X.; Guo, J. Anticancer effect of Lycium barbarum polysaccharides on colon cancer cells involves G0/G1 phase arrest. Med. Oncol. 2011, 28, 121–126. [Google Scholar] [CrossRef]
- Chan, H.-C.; Chang, R.C.-C.; Ip, A.K.-C.; Chiu, K.; Yuen, W.-H.; Zee, S.-Y.; So, K.-F. Neuroprotective effects of Lycium barbarum Lynn on protecting retinal ganglion cells in an ocular hypertension model of glaucoma. Exp. Neurol. 2007, 203, 269–273. [Google Scholar] [CrossRef]
- Luo, Q.; Cai, Y.; Yan, J.; Sun, M.; Corke, H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004, 76, 137–149. [Google Scholar] [CrossRef]
- Su, C.-X.; Duan, X.-G.; Liang, L.-J.; Wang, F.; Zheng, J.; Fu, X.-Y.; Yan, Y.-M.; Huang, L.; Wang, N.-P. Lycium barbarum polysaccharides as an adjuvant for recombinant vaccine through enhancement of humoral immunity by activating Tfh cells. Vet. Immunol. Immunopathol. 2014, 158, 98–104. [Google Scholar] [CrossRef]
- Tang, Z.-Y.; Sun, D.; Qian, C.-W.; Chen, Q.; Duan, S.-S.; Sun, S.-Y. Lycium barbarum polysaccharide alleviates nonylphenol exposure induced testicular injury in juvenile zebrafish. Int. J. Biol. Macromol. 2017, 104, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; You, Z.; Gao, H.; Zhou, G.; Chen, Y.; Yu, J.; Xuan, Y. Protective effect of Lycium barbarum polysaccharides against doxorubicin-induced testicular toxicity in rats. Phytother. Res. 2012, 26, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Q.; Zheng, G.; Chen, Y.; Huang, M.; Zhang, L.; Lin, X. Protective effect of Lycium barbarum polysaccharides against cadmium-induced testicular toxicity in male mice. Food Funct. 2017, 8, 2322–2330. [Google Scholar] [CrossRef]
- Luo, Q.; Li, J.; Cui, X.; Yan, J.; Zhao, Q.; Xiang, C. The effect of Lycium barbarum polysaccharides on the male rats׳ reproductive system and spermatogenic cell apoptosis exposed to low-dose ionizing irradiation. J. Ethnopharmacol. 2014, 154, 249–258. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, H.; Jia, L.; Ma, Y.; Wang, X.; Zhu, L.; Wang, K.; Zhang, P.; Yang, H. Mechanism of 2,4-Dichlorophenoxyacetic acid-induced damage to rat testis via Fas/FasL pathway and the protective effect of Lycium barbarum polysaccharides. Environ. Toxicol. 2022, 37, 2764–2779. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ding, L.; Xu, B.; Zhang, Z.; Dai, W.; He, T.; Liu, L.; Du, X.; Fu, X. barbarum polysaccharide alleviates ferroptosis in Sertoli cells through NRF2/SLC7A11/GPX4 pathway and ameliorates DEHP-induced male reproductive damage in mice. Int. J. Biol. Macromol. 2024, 282, 137241. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, H.; Cui, J.-G.; Wang, J.-X.; Chen, M.-S.; Wang, H.-R.; Li, X.-N.; Li, J.-L. Ferroptosis is critical for phthalates driving the blood-testis barrier dysfunction via targeting transferrin receptor. Redox Biol. 2023, 59, 102584. [Google Scholar] [CrossRef]
- Wang, C.; Wang, W.; Dong, J.; Li, X.; Ye, T.; Zeng, F.; Jiang, M.; Shi, J.; Wang, X.; Zhang, L. Isatin improves oligoasthenospermia caused by busulfan by regulating GSH/GPX4 axis to inhibit ferroptosis. Front. Pharmacol. 2024, 15, 1489956. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Wang, C.; Chen, S.; Guo, H.; Jiang, H.; Liu, H.; Fu, H.; Wang, D. Forsythoside A Mitigates Alzheimer’s-like Pathology by Inhibiting Ferroptosis-mediated Neuroinflammation via Nrf2/GPX4 Axis Activation. Int. J. Biol. Sci. 2022, 18, 2075–2090. [Google Scholar] [CrossRef]
- Jiang, S.; Xiao, X.; Li, J.; Mu, Y. Lycium barbarum polysaccharide-glycoprotein ameliorates ionizing radiation-induced epithelial injury by regulating oxidative stress and ferroptosis via the Nrf2 pathway. Free. Radical. Biol. Med. 2023, 204, 84–94. [Google Scholar] [CrossRef]
- Fallarino, F.; Luca, G.; Calvitti, M.; Mancuso, F.; Nastruzzi, C.; Fioretti, M.C.; Grohmann, U.; Becchetti, E.; Burgevin, A.; Kratzer, R.; et al. Therapy of experimental type 1 diabetes by isolated Sertoli cell xenografts alone. J. Exp. Med. 2009, 206, 2511–2526. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhai, J.; Chen, Z.; Guo, Z.; Sun, X.; Li, J.; Wang, N.; Yao, X.; Zhang, C.; Deng, H.; et al. DEHP regulates ferritinophagy to promote testicular ferroptosis via suppressing SIRT1/PGC-1alpha pathway. Sci. Total Environ. 2024, 954, 176497. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Fu, X.; Han, H.; Yang, H.; Xu, B.; Dai, W.; Liu, L.; He, T.; Du, X.; Pei, X. Nrf2-mediated ferroptosis of spermatogenic cells involved in male reproductive toxicity induced by polystyrene nanoplastics in mice. J. Zhejiang Univ. B 2024, 25, 307–323. [Google Scholar] [CrossRef]
- Kerins, M.J.; Ooi, A. The Roles of NRF2 in Modulating Cellular Iron Homeostasis. Antioxid. Redox Sign. 2018, 29, 1756–1773. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, W.; Yang, L.; Chen, Z.; Zhai, J.; Zhu, Q.; Guo, Z.; Wang, N.; Zhang, C.; Deng, H.; et al. Mechanism of testicular injury induced by Di-ethylhexyl phthalate and its protective agents. Chem. Biol. Interact. 2023, 381, 110575. [Google Scholar] [CrossRef]
- Pan, Y.; Jing, J.; Dong, F.; Yao, Q.; Zhang, W.; Zhang, H.; Yao, B.; Dai, J. Association between phthalate metabolites and biomarkers of reproductive function in 1066 Chinese men of reproductive age. J. Hazard. Mater. 2015, 300, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A.; Mehrzadi, S.; Siahpoosh, A.; Basir, Z.; Bahrami, N.; Goudarzi, M. The ameliorative effect of ellagic acid on di-(2-ethylhexyl) phthalate-induced testicular structural alterations, oxidative stress, inflammation and sperm damages in adult mice. Reprod. Biol. Endocrin. 2021, 19, 146. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, N.; Goudarzi, M.; Hosseinzadeh, A.; Sabbagh, S.; Reiter, R.J.; Mehrzadi, S. Evaluating the protective effects of melatonin on di(2-ethylhexyl) phthalate-induced testicular injury in adult mice. Biomed. Pharmacother. 2018, 108, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z. Extraction, Structural Characterization, and Biological Functions of Lycium Barbarum Polysaccharides: A Review. Biomolecules 2019, 9, 389. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium Barbarum: A Traditional Chinese Herb and A Promising Anti-Aging Agent. Aging Dis. 2017, 8, 778–791. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Kannan, K. A Review of Biomonitoring of Phthalate Exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef]
- Latini, G. Monitoring phthalate exposure in humans. Clin. Chim. Acta 2005, 361, 20–29. [Google Scholar] [CrossRef]
- Silva, M.J.; Barr, D.B.; Reidy, J.A.; Kato, K.; Malek, N.A.; Hodge, C.C.; Hurtz, D.; Calafat, A.M.; Needham, L.L.; Brock, J.W. Glucuronidation patterns of common urinary and serum monoester phthalate metabolites. Arch. Toxicol. 2003, 77, 561–567. [Google Scholar] [CrossRef]
- Dostalova, P.; Zatecka, E.; Ded, L.; Elzeinova, F.; Valaskova, E.; Kubatova, A.; Korenkova, V.; Langerova, L.; Komrskova, K.; Peknicova, J. Gestational and pubertal exposure to low dose of di-(2-ethylhexyl) phthalate impairs sperm quality in adult mice. Reprod. Toxicol. 2020, 96, 175–184. [Google Scholar] [CrossRef]
- Liu, R.-J.; He, Y.-J.; Liu, H.; Zheng, D.-D.; Huang, S.-W.; Liu, C.-H. Protective effect of Lycium barbarum polysaccharide on di-(2-ethylhexyl) phthalate-induced toxicity in rat liver. Environ. Sci. Pollut. Res. 2021, 28, 23501–23509. [Google Scholar] [CrossRef]
- Li, Y.; Takizawa, H.; Azuma, A.; Kohyama, T.; Yamauchi, Y.; Kawada, T.; Kudoh, S.; Sugawara, I. The effects of oxidative stress induced by prolonged low-dose diesel exhaust particle exposure on the generation of allergic airway inflammation differ between BALB/c and C57BL/6 mice. Immunopharmacol. Immunotoxicol. 2009, 31, 230–237. [Google Scholar] [CrossRef]
- Yao, H.; Edirisinghe, I.; Rajendrasozhan, S.; Yang, S.; Caito, S.; Adenuga, D.; Rahman, I. Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L1174–L1186. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, J.; Wang, W.; Zeng, Y.; Jiang, J. Obesity and cancer: Mouse models used in studies. Front. Oncol. 2023, 13, 1125178. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.-J.; Zheng, J.; Han, X.-X.; Jiang, Y.-P.; Li, Z.-M.; Wu, J.; Chang, Q.; Niu, Y.; Sun, T.; Li, Y.-X.; et al. Lycium barbarum polysaccharide attenuates diabetic testicular dysfunction via inhibition of the PI3K/Akt pathway-mediated abnormal autophagy in male mice. Cell Tissue Res. 2018, 374, 653–666. [Google Scholar] [CrossRef]
- Lei, X.; Huo, P.; Wang, Y.; Xie, Y.; Shi, Q.; Tu, H.; Yao, J.; Mo, Z.; Zhang, S. Lycium barbarum Polysaccharides Improve Testicular Spermatogenic Function in Streptozotocin-Induced Diabetic Rats. Front. Endocrinol. 2020, 11, 164. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, A.; Sun, X.; Li, X.; Zhao, X.; Li, S.; Ma, A. Protective Effects of Lycium barbarum Polysaccharides on Testis Spermatogenic Injury Induced by Bisphenol A in Mice. Evid. Based Complement. Altern. Med. 2013, 2013, 690808. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Liu, D.; Liu, S.; Li, C.; Guo, J.; Foyet, H.S. Lycium barbarum Polysaccharide Ameliorates Heat-Stress-Induced Impairment of Primary Sertoli Cells and the Blood-Testis Barrier in Rat via Androgen Receptor and Akt Phosphorylation. Evid. Based Complement. Altern. Med. 2021, 2021, 5574202. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Fu, G.; Dai, J.; Zhang, D.; Zhu, L.; Tang, X.; Zhang, L.; Zhou, T.; Duan, P.; Quan, C.; Zhang, Z.; et al. Di(2-ethylhexyl) phthalate induces apoptosis through mitochondrial pathway in GC-2spd cells. Environ. Toxicol. 2017, 32, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.-F.; Zou, T.; Tuo, Q.-Z.; Xu, S.; Li, H.; Belaidi, A.A.; Lei, P. Ferroptosis: Mechanisms and links with diseases. Signal Transduct. Target. Ther. 2021, 6, 49. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, L.; Zhu, T.; Xu, S.; He, J.; Mao, N.; Liu, Z.; Wang, D. Neuroprotective effects of Lycium barbarum polysaccharide on light-induced oxidative stress and mitochondrial damage via the Nrf2/HO-1 pathway in mouse hippocampal neurons. Int. J. Biol. Macromol. 2023, 251, 126315. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Kang, R.; Tang, D. Iron Metabolism in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 590226. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, M. PM2.5 induces ferroptosis in human endothelial cells through iron overload and redox imbalance. Environ. Pollut. 2019, 254 Pt A, 112937. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 2016, 63, 173–184. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Zhao, X.; Li, Y.; Lv, S.; Zhou, W.; Wang, J.; Sun, Z.; Li, Y.; Guo, C. Ferroptosis contributing to cardiomyocyte injury induced by silica nanoparticles via miR-125b-2-3p/HO-1 signaling. Part. Fibre Toxicol. 2024, 21, 17. [Google Scholar] [CrossRef]
- Dong, H.; Qiang, Z.; Chai, D.; Peng, J.; Xia, Y.; Hu, R.; Jiang, H. Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1. Aging 2020, 12, 12943–12959. [Google Scholar] [CrossRef]
- Duarte, T.L.; Talbot, N.P.; Drakesmith, H. NRF2 and hypoxia-inducible factors: Key players in the redox control of systemic iron homeostasis. Antioxid. Redox Signal. 2021, 35, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Kanayama, M.; Maruyama, A.; Yoshida, A.; Tazumi, K.; Hosoya, T.; Mimura, J.; Toki, T.; Maher, J.M.; Yamamoto, M.; et al. Nrf2 regulates ferroportin 1-mediated iron efflux and counteracts lipopolysaccharide-induced ferroportin 1 mRNA suppression in macrophages. Arch. Biochem. Biophys. 2011, 508, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhao, W.; Yang, R.; Xu, S.Q.; Wang, S.Y.; Li, M.M.; Jiang, Y.K.; Hao, Z.C.; Guan, W.; Kuang, H.X.; et al. Lignans from Schisandra chinensis (Turcz.) Baill ameliorates cognitive impairment in Alzheimer’s disease and alleviates ferroptosis by activating the Nrf2/FPN1 signaling pathway and regulating iron levels. J. Ethnopharmacol. 2025, 341, 119335. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Shang, Y.; Yang, H.; Ding, L.; Deng, Y.; Xu, B.; Fu, X. Lycium barbarum Polysaccharide Improves Iron Homeostasis in Spermatocytes and Sertoli Cells via NRF2 to Alleviate DEHP-Induced Male Reproductive Toxicity in Mice. Toxics 2025, 13, 677. https://doi.org/10.3390/toxics13080677
Zhang Z, Shang Y, Yang H, Ding L, Deng Y, Xu B, Fu X. Lycium barbarum Polysaccharide Improves Iron Homeostasis in Spermatocytes and Sertoli Cells via NRF2 to Alleviate DEHP-Induced Male Reproductive Toxicity in Mice. Toxics. 2025; 13(8):677. https://doi.org/10.3390/toxics13080677
Chicago/Turabian StyleZhang, Zhen, Yitong Shang, Hong Yang, Liyang Ding, Yu Deng, Bo Xu, and Xufeng Fu. 2025. "Lycium barbarum Polysaccharide Improves Iron Homeostasis in Spermatocytes and Sertoli Cells via NRF2 to Alleviate DEHP-Induced Male Reproductive Toxicity in Mice" Toxics 13, no. 8: 677. https://doi.org/10.3390/toxics13080677
APA StyleZhang, Z., Shang, Y., Yang, H., Ding, L., Deng, Y., Xu, B., & Fu, X. (2025). Lycium barbarum Polysaccharide Improves Iron Homeostasis in Spermatocytes and Sertoli Cells via NRF2 to Alleviate DEHP-Induced Male Reproductive Toxicity in Mice. Toxics, 13(8), 677. https://doi.org/10.3390/toxics13080677






